Abstract
Water stress management is one of the most significant issues in agricultural and crop research. Water stress affects more than 42 million hectares of rice cultivation lands in Asian countries. The current scenario constraints are the production of rice with higher quality to improve global food security and meet the food demands of the growing world’s population. The present study investigates the effects of water stress on two rice varieties, (Aduthurai-ADT) ADT-45 and ADT-49, and characterizes the growth, physiological, biochemical and antioxidant activities of the rice cultivars. Histochemical staining with 3,3-diaminobenzidine (DAB) and Nitro Blue Tetrazolium (NBT) was used to detect drought-induced ROS species. FTIR (Fourier-transform infrared spectroscopy) and ESR (Electron Spin Resonance) spectra were performed on the control and drought samples. The results showed a decrease in shoot and root length, fresh and dry weight, RWC and decreasing photosynthetic pigments such as chlorophyll a and b. The content of carotenoids, reducing sugars, starch, proline and Malondialdehyde (MDA) were increased in drought-tolerant plants, while the protein and amino acid content dropped. A high level of free radical accumulation has been detected in drought-imposed samples in both rice varieties by histochemical staining. Enzymatic antioxidants such as Catalase (CAT), Peroxidase (POX), Ascorbate Peroxidase (APX) and Superoxide dismutase (SOD) activities were increased in drought-induced plants compared to the control of two rice varieties, scavenging Reactive Oxygen Species (ROS) species. Besides, (ESR was used to confirm that the remarkable signals indicating free radical accumulation were higher water-deficit plants than in control plants. ESR spectroscopy is a reliable approach for distinguishing between tolerant and sensitive rice types. FTIR spectroscopy was used to identify various functional groups in drought-imposed and control samples. FTIR was used in the study to get significant insights into a plant stress response and to find novel drought tolerance features. The present study reveals that the rice variety ADT-49 was more drought resistant than ADT-45. Our findings demonstrated that drought affects growth and physiological metabolism, giving a significant basis for drought escape or tolerance in developing new varieties. In order to increase the drought tolerance mechanism in rice crops, further investigation is needed on the application and function of plant hormones under drought stress circumstances.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice is a cereal crop in India and South Asia, with 43 million hectares under production (NRRI, 2019). Global rice production is expected to reach 493.79 million tons annually, with India contributing 112.91 million tons (Childs Nathan, 2019). Furthermore, on 1.8 million hectares, Tamil Nadu produces 28.1 percent of the world’s rice. Tamil Nadu has 37 rice varieties, the most productive of which are ADT-45 and ADT-49, yielding 6100 and 6173 kg ha−1, respectively (Government of India report, 2018). In contrast, the water deficit has a substantial influence on rice development. A key issue for sustaining enough food for the world’s expanding population while dealing with unforeseen global climate change fluctuations (rainfall, temperature, drought, etc.,) (Naithani, 2016).Water deficits are a significant biological limitation induced by the uneven distribution of rainfall (Ramachandran et al., 2017). Drought stress causes physiological and metabolic changes in plants, which significantly alter their growth and development (Ramya & Arulbalachandran, 2019; Ramya et al., 2022a). Under drought stress, the osmotic pressure of plant cells is essential for sustaining turgidity (Osakabe et al., 2014). Compatible osmolyte solutes protect plants from osmotic stress while preserving cytosolic osmotic equilibrium. During drought conditions, cell division and elongation may be reduced at the cellular level (Kumar et al., 2014). Abiotic stressors affect physiological processes, causing decreased germination, seedling vigour and photosynthesis in plants (Jothimani & Arulbalachandran, 2020; Pandey & Shukla, 2015). Water deficit-affected plants have been discovered to change their metabolic processes through ion and nutrient translocation (Ramya et al., 2022b). FTIR spectroscopy generates a spectrum used to determine plant materials’ metabolic fingerprints. It is a cutting-edge tool to understand better how plants respond to drought (Amir et al., 2013).
Reactive oxygen species (ROS) are essential in plant production because they promote healthy metabolism by controlling cell growth. ROS is an unpaired electron oxygen species produced during drought stress (abiotic stress) indicating the cellular environment is under threat due to water deficit and damaging cellular membranes. It triggers the defense of cells by various alternate physiological and biochemical mechanisms (Arulbalachandran et al., 2016; Foyer & Noctor, 2011; Ramya et al., 2022a). The formation of free radicals in the biological system could be calculated using a spin—trapping method in electron spin resonance spectroscopy (Garlick et al., 1987). The ESR method calculates estimates the ability of biological systems to scavenge superoxide and hydroxyl radicals and detects ROS that is formed chemically (Dunnick et al., 2014; Sato et al., 2008) Drought tolerance of two rice varieties, ADT-45 and ADT-49, was studied using soil drought stress to evaluate morphological characteristics, photosynthetic pigments, biochemical alterations, enzymatic antioxidant function, and FTIR analysis. This study determines the degree of free radical accumulation appropriate for selecting rice varieties for drought tolerance; ESR measurements were performed on the plant samples.
Materials and methods
Plant materials and Sterilization of seeds
The two rice varieties (Aduthurai 45 & 49) were obtained from Tamil Nadu Agricultural University (TNAU), CBE, India. The seeds were sterilized with 0.1% mercury chloride solution (5 min) after washing with deionised H2O. The seeds were imbibed in distilled water for 48 h at room temperature (25 ± 2 °C). Twenty seeds were sown in each pot with triplicates.
In-vivo study
The investigation was carried out in the greenhouse at the Department of Botany (Periyar University, TN, India). The drought treatment was employed in the pot culture method, adopted by the International Rice Research Institute (IRRI, 2002). The pots (6 × 10 × 12 cm dimension) were filled with soil manure 3:1 for the drought experiment. The plants were maintained in well-watered condition for up to 40 days and simulation of water deficit was employed for 5 days (41st–45th day) by withheld of water. The soil moisture content (Fractional Water Index (FWI) during water stress was observed by a soil moisture probe (Soil Moisture Meter). The moisture of the soil was measured from 0 to 10 (FWI) soil depth in the pots of both control and water deficit conditions. We considered the soil to be in drought because the soil moisture level continuously fell until it reached zero, and our soil meter indicated a dry condition in the range of 0–3 (FWI). In the control plants, the soil moisture was maintained at a consistent level (8–10 FWI). The treated plants were collected in triplicate on the 46th day to examine physiological and biochemical parameters, antioxidant activities, histochemical detection, biometabolites, and ROS species, which were detected by FTIR and ESR respectively.
Growth characteristics
Shoots and roots length (cm)
The shoot and root lengths of the drought-stressed and control seedlings were measured on the 46th day. Three plants from each replication were used to compute the mean value for all drought and control plants.
Plant weight (fresh and dry mass) (g)
The shoot and root of the plants were examined in triplicates, after drought imposition (well-watered plants were used as control), and the fresh and dry weight was registered. The samples were dried in an oven at 60 °C until all the plants.
Relative water content (RWC) (%)
After water drought imposition, relative water content was used, determined according to Pieczynski et al. (2013). The shoot and root of the plants were measured for fresh weight and then left saturated in water for three hours anf their turgid weights (TW) were calculated. The samples were then dehydrated at 70 °C for 48 h for measuring the dry weight was determined.
Photosynthetic pigments (mg −1 fr.wt)
Two hundred milligrams (200 mg) of fresh young leaves were mashed with 80% acetone in a pestle and mortar and separated at 4000 g for 15 min in a centrifuge. The step was repeated until the green residue obtained faded colour and made up to 20 ml with 80 percent acetone. Chlorophyll a (663 nm) and b (645) contents were estimated given by Arnon (1949), and carotenoids were recorded at 480 nm, according to Krik & Allen (1965).
Analysis of biochemical metabolites (mg −1 fr.wt)
Reducing sugars
The shoot and root samples (0.2 g) were ground with (10 ml/80%) and the homogenate was centrifuged (8000 g) for fifteen minutes. In a new fresh tube, the upper phase was transferred. The sample aliquot was read at 420 nm according to the procedure given by Nelson (1944), the glucose was used as standard.
Soluble carbohydrate (starch)
The soluble starch was determined using the residue of reducing sugar, the extract was washed with ethanol with 5 ml D·H2O. After it was mixed with 6.5 ml of HCIO4 (52%) prior to the water bath (80 °C for 30 min). After 15 min, it was centrifuged (8000 g) with 10 ml of DH2O. The step was repeated for re-extraction to make the volume 10 ml with distilled water. The extract was filtered through Whatmann No.1 filter paper and soluble sugar was determined according to Clegg (1956).
Protein content
Five hundred milligrams of fresh plant samples were ground with 5 ml of trichloroacetic acid (10%) using a pestle and mortar. The macerated homogenate was centrifuged (8000 g) for 15 min. After discarding the upper phase, the residue phase was collected in a new test tube aliquot with 0.1 normality of NaOH and this aliquot was used as a substrate of soluble protein and protein was estimated, the method of Lowry et al. (1951).
SDS-PAGE separation of proteins
Using TCA and acetone, the protein was extracted from both varieties along with control plants. The well of SDS PAGE was filled there with 30 µl of sample protein. The protein marker (Prestained) range of 14.4–116 kDa was used as standard. The PAGE was prepared with 5% stacking gel and 10% separation gel in Mini Vertical Slab Cassette Electrophoresis (Medox) according to the method Laemmli (1970).
Total amino acids
Fresh shoot and root (500 mg) were crushed in 5 ml of ethanol (80%) and the homogenate was centrifuged (8000 g) for 10 min. The supernatant was aliquot and made up of 10 ml of 80% ethanol. The supernatant (extract) was saturated with 0.1 normality NaOH using the indicator methyl red. The mixture was boiled with 1 ml of ninhydrin reagent for twenty minutes and dissolved in 20 ml of DH2O. The amino acids were determined by the method of Moore and Stein (1948).
Proline
Ten ml of sulphosalicylic acid (3%) was used to homogenise the plant tissue (0.5 g). The homogenate was allowed to react with 2 ml of ninhydrin reagent and boiled at 100 °C for an hour protein content was determined using the mixture with 4 ml of toluene by vigorous mixing and determined according to the method by Bates et al. (1973).
Malondialdehyde (MDA)
Along with 2 ml of enzyme extract, 1 ml of TCA (20% V/V) was mixed and boiled at 95 °C (30 min). The homogenate mixture was centrifuged at 14,000 g for (10 min). With an extinction coefficient of 155 mM-1 cm-1, the MDA was measured according to Health & Packer (1968). Lipid peroxidation was determined by Dhindsa et al. (1981).
Enzymatic antioxidant enzymes
The lyophilized plant tissue (0.5 g) was homogenized with 5 ml of phosphate buffer (50 mM; pH 7.0). The homogenate was centrifuged at 5000 g (20 min). The supernatant (extract) was used for the enzymatic assay.
Catalase activity (CAT-EC 1.11.1.6)
40 µl of enzyme extract was mixed with 2.6 ml of 50 mM phosphate buffer. The mixture was allowed to initiate using 0.4 ml of H2O2 (15 mM) by the method of Chandlee and Scandalios (1984). The activity of catalase was measured (240 nm) and expressed Unit mg-1 protein without extract, the buffer was used as a standard.
Peroxidase activity (POX-EC 1.11.1.7)
To begin the reaction, 500 µl of the extract was mixed with 2 ml of potassium buffer (50 mM; pH 7.0), 0.01% pyrogallol, and 1 ml of hydrogen peroxide (50 mM) by the method of Chance and Maehly (1955). The reaction mixture kept 25°C (5 min) and to stopping with one ml of 2.5 N H2SO4 after reading the reaction mixture at 420 nm, the result was recorded as U mg−1 protein.
Ascorbate peroxidase activity (APX-EC 1.11.1.1)
The enzyme extract (200 µl) was mixed with 1 ml of potassium buffer (50 mM; pH 7.0) along with 10 µl of acerbate acid and 10 µl of H2O2 (0.1 mM). The mixture was measured at 290 nm against a blank and using the method of Asada and Takahashi (1987). The blank was used in the reaction mixture without extract.
Superoxide dismutase activity (SOD-EC 1.15.1.1)
1 ml of enzyme extract was mixed with 3 ml of potassium buffer (50 mM; pH 7.0), and the reaction was initiated by adding 0.028 mM riboflavin, 0.01 mM methionine, and 0.02 mM potassium cyanide along with 0.15% NBT salt by illumination. The mixture was read at 560 nm to determine the enzymatic activity according to the method of Beauchamp and Fridovich (1971).
DAB and NBT (histochemical detection)
The accumulation of free radicals such as H2O2 and O2·− was detected by histochemical staining analysis such as DAB and NBT respectively by the method of Kumar et al. (2014). The treated leaves were collected and imbibed for 12 h DAB stain in the dark. The accumulation of superoxide anion (O2·−) in the leaves was detected by the NBT staining method. To detect the O2·− in leaves were immersed in NBT solution (0.5 mg/ml; 10 mM phosphate buffer; pH 7.8) at room temperature for 8 h and kept in dark conditions. The stained leaves were treated with ethanol (95%) and boiled in a water bath for 15 min and leaves were stored in 40% (v/v) glycerol for detection to observe to accumulation of ROS.
FTIR analysis
Lyophilized shoot and root tissue (500 mg) was macerated with potassium bromide (sample/KBr; 1:99) and the aliquot was in a hot air oven (60 °C). The dry samples of the mixture were loaded in FTIR spectroscopy (Perkin Elmer; Model RX I) in the scanning range between 400 and 4000 cm−1 with 4 cm−1 resolution. The technique was conducted by Archbishop Casimir Instrumentation Centre (ACIC), at St. Joseph’s College, Tiruchirapalli, TN, India.
Measurements of electron spin resonance (ESR) spectroscopy
The ESRs of drought-induced and control plants were determined using lyophilized materials (shoot and root). The materials were loaded into ESR quartz tubes to produce paramagnetic species, according to Filek et al. (2015). The unstable atoms of the sample are trapped in Electron Spin Resonance spectroscopy (JEOL; Model-JES FA200, Japan) in the (X-band (9.1 GHz; 100-kHz frequency), using a spin trapping reagent named 5,5-dimethyl-1pyrroline-N-oxide (five-membered nitrogen derivatives). As a quantitative norm for the radicals, the g-factor standard (g = 2.0036) was calculated using a sample with a known number of spins. The milligram of the plant samples was proportional to the number of spins measured. The experiment was studied at the Indian Institute of Technology in Madras. The hyperfine splitting constant (g values) reported in the ESR spectral data were used to determine ESR characteristics accuracy.
Statistics analysis
One way ANOVA was used to analyse the results statistically with, Pearson correlations using the software SPSS (version 21.0), which correlated with a significance p < 0.05.
Results
Growth characteristics
During water deficit conditions, both rice varieties’ growth characteristics deteriorated. Drought-imposed plants of the ADT-45 (13.56 percent) and ADT-49 (5.45 percent) varieties showed a decrease in shoot length (Fig. 1a). In contrast, drought-treated plants’ root lengths became reduced by 13.88 percent and 8.9 percent, respectively, as compared to respective controls (Fig. 4a). The fresh weight of ADT-45 and ADT-49 (26.66 percent and 22.86 percent) and dry weight (24.83 percent and 10.33 percent) of the shoot were reduced (Fig. 1b). The root weight decreased significantly in drought-stressed ADT-45 and ADT-49 plants (24.41 percent and 15.69 percent, respectively) relative to non-stressed rice plants (26.5 percent and 16.55 percent) (Fig. 4b). Plant length was found to have a strong positive association with morphological and biochemical characteristics (F = 1876.63, p < 0.001). In drought-induced plants of ADT-45 and ADT-49, the RWC of the shoot (36.75 percent and 23.82 percent decreased, respectively (Fig. 1c). In drought-treated plants, the RWC of the root was also decreased (40.26 percent and 23.68 percent, respectively) as compared to well-watered plant (Fig. 4c). The RWC of drought-stricken rice varieties was found to have a strong positive association with root dry weight (p < 0.05) and root protein (r = 0.008, p < 0.01). The activity of CAT, on the other hand, showed a negative correlation (p < 0.05).
Effect of drought on ADT-45 and ADT-49 rice variety at 46th day. a Plant length b Plant weight c RWC d Reducing sugar e Starch f protein (n = 3) CS-Control Shoot DS-Drought Shoot. The value are expressed the mean ± SEM different letters are indicate significance difference (p < 0.05) according to Turkey post hoc test
Measurement of photosynthetic pigments
Water deficit decreases chlorophyll-a and b in the present study rice varieties, with relative Chl-a and Chl-b values of 33.08 percent and 29.95 percent in the drought-stressed ADT-45 variety. The drought-treated ADT-45 plants had a 13.83 percent improvement in carotenoid content. The Chl-a and b values were 30.54 percent and 13.72 percent, respectively, in drought-treated ADT-49, compared to the control plant, The carotenoid content increased by 12.84 percent (Fig. 2a). Drought-imposed on both varieties of rice such as ADT-45 and ADT-49 revealed a strong positive correlation (p < 0.001) in different fractions of photosynthetic pigments. In both varieties of drought-treated plants, one-way ANOVA revealed significant variation in chlorophyll (F = 342.75) and carotenoid (F = 682.16) content (Fig. 3).
Biochemical analysis
In the drought-treated ADT-45 and ADT-49 varieties, the sugar content was reduced to14.31 percent and 15.90 percent respectively in the shoot (Fig. 1d), the highest value was recorded in the root (23.70 percent and 26.24 percent), rather than to control (Fig. 4d). Compared to control rice plants, drought-treated plants had a higher level of starch in the shoot (6.69 percent and 7.14 percent, respectively) of ADT-45 and ADT-49 (Fig. 1e). Compared to control plants, the starch content of the roots was slightly higher (12.36 percent and 23.08 percent) compared to control (Fig. 4e). Reduced sugar content was found to e strongly associated with the starch content of shoots in drought plants (p < 0.001). Soluble protein content in the shoot was decreased by 8.3 percent and 4.16 percent, respectively, and the root by 11.62 percent and 8.63 percent (Figs. 1f, 4f). The amino acid content of the shoot was relatively low in the droughts ADT-45 and ADT-49 (6.66 percent and 4.21 percent, respectively; Fig. 3a). Similarly, it was lower in the roots of drought-affected rice plants (16.6 percent and 13.52 percent, respectively) than in control (Fig. 5a). According to ANOVA, protein (F = 3.68) and amino acids (F = 16.48) showed a substantial difference in response to treatment. On the other hand, the shoot protein and amino acid levels showed no association, whereas, the roots showed a strong correlation. On SDS-PAGE, the protein profile patterns of both varieties were shown in Fig. 6. The ADT-49 control plants had more polypeptide bands in the 45–14.4 kDa range than the ADT-45 control plants. Six polypeptides in the 26–14.4 kDa range were expressed in the control plants, however, none were found in drought-treated plants. The drought plant of ADT-45, expressed four polypeptides of 25–14.4 kDa, while the ADT-45 control plant showed two bands (22–14 kDa). Three polypeptides ranging in size from 22 to 18 kDa were found in drought-stressed ADT-49 plants but not in drought-stressed ADT-45 plants.
Effect of drought on ADT-45 and ADT-49 rice variety at 46th day. a Plant length b Plant weight c RWC d Reducing sugar e Starch f protein (n = 3) CR-Control Root DR-Drought Root. The value are expressed the mean ± SEM different letters are indicate significance difference (p < 0.05) according to Turkey post hoc test
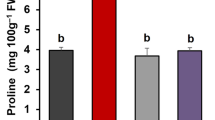
The proline content of shoots was increased by 21.94 percent in ADT-45 and 24.66 percent in ADT-49, respectively, in both drought-induced rice varieties (Fig. 3b). The proline content of ADT-45 and ADT-49 roots was higher by 13.38 percent and 15.38 percent, respectively, than control rice plants (Fig. 5b). As compared to the control plant, the MDA content of the shoot increased in both rice varieties (35.79 percent and 42.12 percent) (Fig. 2b). Drought-induced plants had a strong negative association for MDA content between morphological and biochemical traits (F = 1641.49, p < 0.01).
Enzymatic antioxidant activity (Umg-1 protein)
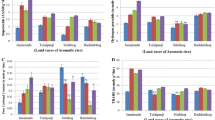
Under water deficit conditions, the shoot and root’s enzymatic antioxidant activities (CAT, POX, APX, and SOD) were progressively increased. ADT-45 and ADT-49 drought plants had increased CAT operation by 34.74 percent and 40.8 percent respectively in their shoots (Fig. 3c). Drought induced plants had 40.72 percent and 46.32 percent higher CAT activity in their roots respectively than non-treated plants (Fig. 5c). Activity of POX in the shoot increased in ADT-45 and ADT-49 (12.29 percent and 39.88 percent, respectively) compared to control plants, (Fig. 3d). In ADT-45 and ADT-49, the root control plants had the highest POX occurrence (21.98 percent and 41.05 percent) compared to drought-induced plants (Fig. 5d). Compared to drought, the APX activities of shoots (19.27 percent and 20.72 percent) were higher in the control plant of ADT-45 and ADT-49 (Fig. 3e). Compared to drought treated plants, ADT-45 and ADT-49 had the highest APX activity (8.45 percent and 18.29 percent, respectively) (Fig. 5e). Stressed rice plants’ shoots had higher levels of SOD operation than non-stressed rice plants’ shoots by 37.5 percent and 55.5 percent for ADT-45 and ADT-49, respectively (Fig. 3f). As compared to non treated plants, SOD activity was higher in the roots of ADT-45 (5.81%) and ADT-49 (29.33%) (Fig. 5f). Drought-induced rice varieties enzymatic antioxidants showed a strong positive association between morphological and biochemical characters (p < 0.01).
Reactive oxygen species (ROS)
To detect the ROS accumulation (H2O2 and O2·−), DAB and NBT staining methods were used in both rice varieties (Fig. 7). The appearance of brown (H2O2) and dark blue (O2·−) spots in drought stress-induced rice shoots in ADT-49 compared to ADT-45 and untreated plants indicates the presence of ROS accumulation.
Fourier-transform infrared spectroscopy (FTIR)
The FT-IR spectra peaks and their probable functional groups are shown in control; drought in both rice varieties (shoot and root). The FTIR spectra peaks in control shoot and root in ADT 45, the peak at 3410 cm−1, 2925 cm−1 and 2854 cm−1 1654 cm−1, 1462 cm−1, 1421 cm−1, 1384 cm−1, 1320 cm−1,1232 cm−1,1059 cm−1, corresponding to carbohydrates, lipids, methylene group, amides, liglin, alkyl groups, protein, carbohydrates phospholipids, carbohydrates and 800–600 range to indicated the halogen compounds respectively. The specific functional groups observed in drought stress imposed shoot and root samples of ADT-45 rice variety 3697 cm−1, 1544 cm−1, 915 cm−1 corresponding to alcohol, protein, and alkenes respectively, however some functional groups are not present in drought stressed rice compared to control (Supplementary Table 1; Fig. 8).
The FTIR spectra peaks in control shoot and root in ADT 49, the peak at 3389 cm−1, 2923 cm−1, 2853 cm−1, 2004 cm−1,1877 cm−1, 1646 cm−1,1547 cm−1,1455 cm−1,1423 cm−1, 1384 cm−1, 1319 cm−1, 1235 cm−1, 1156 cm−1,1055 cm−1 corresponding to carbohydrates, lipids, methylene group, organic compounds, carbonyl group, amides, protein, liglin, alkyl groups, protein, carbohydrates, phospholipids, primary and secondary aliphatic ethers, carbohydrates and 800–600 range to indicated the halogen compounds respectively. The specific functional groups observed in drought stress imposed shoot and root samples of ADT-49 rice variety 3697 cm−1, 2114 cm−1, 1545 cm−1, and 919 cm−1 corresponding to alcohol, nitrogen components, protein, and alkenes respectively, however some functional groups are not present in drought stressed rice compared to control (Supplementary Table 2; Fig. 8).
Electron spin resonance (ESR) spectroscopy measurement
In this study, ESR detected unpaired electrons from ROS signals. The ESR signal in the shoots and roots (ADT-45 and ADT-49) was higher in drought-induced plant samples than in control plant samples (Figs. 9 and 10). By correlating the peak area obtained from stable radicals, the ESR bands of spin adducts were used for the computable analysis of unpaired electrons. Drought stress is imposed on each free radical exhibited in the specific spectrum of ESR and the intensity of an ESR signal is proportional to the concentration of free radicals present in the shoot and root samples. Compared to control samples, which exhibited an oxidation rate persisted in the variety ADT-45, the strong signal was recorded in the variety ADT-49 rather than ADT-45.
Furthermore, compared to the respective controls, the signal was more robust in the shoots of both varieties than in the root samples in the drought condition. Peaks with a narrow signal in the g factor range of 1.69–10 revealed that the drought sample generates unpaired electrons. On the other hand, the low-intensity signals were also recorded in control plants, which produced fewer free radicals in rice varieties. A small signal was also detected at g = 2.0, between the fourth and fifth hyperfine lines.
Discussion
Water deficit causes a significantly losing of yield in crop production, which is a major agronomic issue (Bray et al., 2000). It revealed a crop yield reduction when subjected to continuous droughts (Somerville, 2001). This study found that plant length was significantly reduced in the varieties ADT-45 and ADT-49 when compared to the respective controls. ADT-49 inhibited plant shoot growth when compared to ADT-45. The decrease in shoot growth observed in Helianthus annus during water deficit conditions could be attributed to cell shrinkage and leaf senescence (Manivannan et al., 2007), peanut (Sankar et al., 2016). When non-treated rice plants were compared to drought-induced ADT-45 and ADT-49 shoot and root lengths, they showed a significant reduction in growth characteristics (Ramachandran et al., 2021).
The fresh and dry weights of the rice plant were reduced by (26.66 percent and 22.86 percent) and dry weight (24.83 percent and 10.33 percent) in drought-induced ADT-45 the control and decreased by (24.41 percent and 15.69 percent) (26.5 percent and 16.55 percent) in ADT-49. The current findings corroborated Fischer’s (1980) findings that water stress reduces growth rate, which is reflected in total plant length, fresh and dry weight, and other growth characteristics of wheat cultivars. According to Mohammadian et al. (2005), drought-induced plants were gradually affected, and shoot and root weights were reduced in sugar beet. According to Chutipaijit (2016), water deficit stress significantly reduced drought-stressed rice plants’ relative growth rate stability. RWC was found to be lower in ADT-45 compared to ADT-49, according to our findings. Inadequate water levels cause a significant drop in RWC. Under drought-stress, the rice plants, which is attributed to the plant's relative growth rates combined with unstable cell membranes (Ramachandran & Arulbalachandran, 2018).
ADT-45 reduced chlorophyll a and b by (33.08 percent and 29.95 percent, respectively), while ADT-49 was 30.54 percent and 13.72 percent. The reduction of photosynthetic pigments in drought-stressed plants was linked to leaf morphology, which revealed discolouration and ROS formation, resulting in chlorophyll destruction (Bouchemal, 2017). Ha (2014) recorded that photosynthetic pigments in rice plants were decreased under water stress conditions. Under drought stress reactive oxygen species increased causing oxidative damage, which carotenoid pigments avoid, and it has many roles in drought tolerance in wheat (Loggini, 1999), black gram (Ramya et al., 2022b).
Interestingly, our results revealed that the carotenoid content was increased in both rice plant varieties, with ADT-49 having a significantly higher level of carotenoids than ADT-45. The findings are consistent with Luchi et al. (2001), who discovered that endogenous ABA derived from carotenoid precursors is essential for plant survival during stress periods in Arabidopsis thaliana, black gram (Jothimani & Arulbalachandran, 2020). Chlorophyll and protein degradation are regulated during chloroplast senescence. Chlorophyll pigment decolourization appears to be needed for the degeneration of proteins associated with the mediation of membrane proteases (Thompson, 1987).
During chloroplast senescence, chlorophyll and protein degradation were highly regulated (Thompson, 1987). Furthermore, the accumulation of sugar content in cotton plants can be responsible for transpiration and the maintenance of leaf water content (Ackerson, 1981). Maisura et al. (2014) discovered that osmotic adjustment was effectively accomplished by the accumulation of reducing sugar from the soil in the rice plants. In our research, the reducing sugar content of ADT-49 increased and was significantly positively associated with the proline content. Wang et al. (2005) discovered that as sugar content increased, protein structure stabilized to respond to osmoregulation under water stress conditions.
When the ADT-45 rice variety was subjected to a water deficit, the association between protein and amino acid decreased compared to the ADT-49 rice variety. In water deficit conditions, Ashraf et al. (2003) found that soluble protein content was lower in Vigna radiata plants than in control plants. Wang et al. (2016) found that the total free amino acid concentration of Camellia sinensis decreased in response to water deficiency. ROS levels can rise during a drought, causing the photosynthetic apparatus to close and oxidation of proteins, lipids, and carbohydrates in some woody species (Liu, 2011). The number of bands that appeared per lane in the leaf proteins of rice varieties ADT-45 and ADT-49 on SDS-PAGE ranged from 45 to 14.4 kDa. When these were compared to control plants, both varieties of drought-induced rice produced fewer bands. Thus, the drought-stressed leaves of ADT-45 and ADT-49 are likely to have lower protein levels, which is confirmed by a previous observation of elevated levels of hydroxyl radical products in these rice varieties’ chloroplasts (Salekdeh, 2002).
Under water deficit, the proline content of the drought-tolerant variety ADT-49 was significantly higher than that of ADT-45. Osmotic control, detoxification, membrane stability, and protein incorporation may all involve proline accumulation (Liu, 2011; Lokhande, 2010). According to Deivanai et al. (2011), proline increases the resistance of water-stressed rice plants. In addition, the RWC had a negative association with proline material in ADT-45 and ADT-49. The drought-treated plant results indicate that osmotic adjustment occurred, resulting in water retention and the avoidance of cellular dehydration. During water deficit stress, MDA levels in ADT-49 were significantly higher than in ADT-45. The increase in MDA content in rice plants was caused by water stress conditions, indicating the extent of damage when a plant is exposed to adverse conditions (Farooq, 2009; Lou et al., 2017).
Under water deficit, catalase activity in rice shoots and roots increased 1.34 and 1.4 fold in ADT-45, respectively, and 1.4 and 1.46 fold in ADT-49. Increased CAT activity in plants can protect chloroplasts from stress, and it may have electron flows and target the ROS portion (Foyer & Noctor, 2011). After the drought, ADT-45 shoot and root POX activity increased by 1.12 and 1.21 fold, respectively; 1.3 and 1.41 fold in ADT-49. Similarly, under water deficit stress conditions, POX activity increased in soybeans (Xiong, 2002). These may be caused by rising H2O2 levels, affecting photorespiration and dissipating from other cell compartments (Abogadallah, 2010).
Furthermore, POX operation aids in maintaining tissue damage by scavenging harmful materials (Uzilday, 2012). The primary role of the ascorbate–glutathione pathway is to avoid excessive H2O2 in cells (Halliwell & Foyer, 1976). Drought increases the activity of APX in shoots and roots by 1.19 and 1.0 fold in ADT-45, respectively; 1.2 and 1.18 fold in ADT-49. The APX activity was higher in drought induced plants than in other concentrations and control rice plants (Lou et al., 2017; Sharma et al., 2012). The activity of SOD was increased by 1.3 and 1.05 fold in ADT-45 and 1.5 and 1.29 fold in ADT-49 during water deficit stress, respectively. Arabzadeh et al. (2013) investigated SOD activity in two Haloxylon species, finding that drought stress altered the synthesis and deposition of active enzymes. Our findings are consistent with previous data indicating that SOD activity increased during water stress in sunflowers (Gunes, 2008).
Histochemical detection
The histochemical reaction using DAB and NBT stains revealed that under drought stress conditions, ROS accumulation, such as brown spots and O2·− (dark blur spots), was increased. The RoS species accumulation was higher in variety ADT-49 compared to variety ADT-45 and control plants. Histochemical detection of oxidative stress as brown spots of H2O2 and dark blue spots of O2·− in rice plants under drought stress conditions was found to be more than generation H2O2 and O2·− accumulation of water deficit stress-induced in rice variety ADT-49 compared to respective control and other rice variety ADT-45 treated and respective control plants in the current study. Drought-stressed rice plant cells produced O2·−, which hampered electron transport in the chloroplast, and O2·− may have been converted into H2O2, which may have caused metabolic abnormalities in drought-stressed plants (Price 1989; Wang et al., 2005). Jiang et al. (2013) and Pyngrope et al. (2013) observed H2O2 and O2·− as brown patches and dark blue patches, respectively, in water stress-affected plants.
The FTIR band absorption peak at 1545.45 cm−1 was attributed to N–H stretching in this study and assigned to the protein band. While determining the protein secondary structure, primarily through hydrogen bonding between amide I and amide II moieties, was dependent on the absorption of the amide group’s amide I stretching vibrations (Surewicz and Mantsch (1988). FTIR spectroscopy revealed that the peak value of the ADT-49 drought-stressed rice plants differed the most from the control and ADT-45 rice varieties. Surewicz and Mantsch (1988), Ramya et al. (2022a) discovered that protein structure was changed in Arabidopsis and maize under abiotic stress, but that this reaction did not last after sustained stress, and the protein refolded slowly. Spectral variations with water deficit were observed in the FTIR data. This research discovered that FTIR spectroscopy is yet another tool for quickly obtaining high-throughput information on drought stress.
When comparing the control and drought-induced plants, the changes in lipids, amides, and carbohydrates was observed. Data from FTIR spectra were normalised at a wave number of 3745 cm−1. The peaks in the spectra that correspond to lipids, amides, and carbohydrates are absorption peaks. Our findings are consistent with Ogbaga et al. (2016), who found that these metabolites were involved in sorghum's prolonged drought. Control plants had higher intensities of the 3410.56, 2925.06, 1642.27, 1384.80, and 1320.23 cm−1 bands corresponding to lipids, amides, and carbohydrates than stressed plants. Furthermore, a decrease in the intensities of the bands observed with increasing drought suggested a decrease in lipid and carbohydrate content as well as changes in the proteome composition (Athar et al., 2016; Lahlali et al., 2014). The spectra may thus be used to extract information concerning drought-specific responses (Fig. 8).
Drought-induced changes in cellular function decreased the growth characteristics in both tested varieties compared to control plants. The ESR simulation procedure was determined, and spectroscopy revealed that the ESR signals distinguished between varieties and their control samples. The hyperfine of the ESR signals revealed that free radical accumulation was greater in the ADT-49 variety than in the ADT-45 and power. The ESR data revealed that drought-induced rice had a lower g value than control plants. Drought stress increased the ESR signal compared to non-treated samples (Fig. 9 and 10) because it causes free radicals and the activation of antioxidant enzymes (Xie et al., 2008). Fair conditions, according to Witwicki et al. (2009), had decreased electron spin concentration, resulting in the lowest g value due to hydrogen bonds between oxygen and water molecules (Filek et al., 2015). Water stress caused oxidative damage through the development of activated oxygen species O2•– and subsequent reactions with membrane-bound organelles of proteins and lipids. The generation of free radicals is frequently associated with oxidative stress during tissue reoxygenation (Halliwell & Gutteridge, 1990). Our findings show that the chlorophyll and protein degradation in drought-stressed rice leaves reflects the high level of free radicals generated by the Fenton reaction from H2O2. It plays a role in the non-enzymatic subunit of rubisco in wheat chloroplasts (Ishida et al., 1999). According to He et al. (2014), the basic ESR spectrum obtained for each free radical and the strength of an ESR signal are proportional to the concentration of free radicals. The ESR method was used in this study to demonstrate that drought-induced plants defend themselves against free radicals through antioxidant enzymes (Filek et al., 2015). The ESR signal of antioxidant enzymes showed high band strength in the drought-induced plant variety compared to the control in this analysis. The drought-to-ESR signal intensity relationship was found to be proportional. The ESR signal increased during the stress-induced rice plant, indicating that the drought phase influenced the formation of radicals previously reported in soybean seeds (Priestley et al., 1985).
Conclusion
The current scenario constraints are the production of rice with higher-quality to improve global food security and meet the food demands of the growing world’s population. In this study, the physiological and metabolic investigations of rice varieties ADT-45 and ADT-49 showed alterations at a certain level compared to control plants. The study depicts that the induced drought negatively impacted growth parameters, physiological metabolisms such as RWC, photosynthetic pigments, and biochemical contents. Besides, the electron spins resonance showed higher band intensity, which was directly proportion to antioxidant enzymes and histochemical analysis which confirms the formation of free radicals during drought conditions. The ESR spectroscopy is an assured method for identifying tolerant and sensitive rice varieties. Besides, Further studies focus on the application and the role of plant hormones under drought conditions to enhance sustainable or tolerant rice varieties.
References
Abogadallah, G. M., Serag, M., & Quick, W. P. (2010). Fine and coarse regulation of reactive oxygen species in the salt tolerant mutants of barnyard grass and their wild type parents under salt stress. Physiologia Plantarum, 138(1), 60–73.
Ackerson, R. C. (1981). Osmoregulation in cotton in response to water stress. II. Leaf carbohydrate status in relation to osmotic adjustment. Plant Physiology, 67(3), 489–493.
Amir, R. M., Anjum, F. M., Khan, M. I., Khan, M. R., Pasha, I., & Nadeem, M. (2013). Application of Fourier transform infrared (FTIR) spectroscopy for the identification of wheat varieties. Journal of Food Science and Technology, 50(5), 1018–1023.
Arabzadeh, N., & Khavari-Nejad, R. A. (2013). Effect of drought stress on superoxide dismutase activity in two species of Haloxylon aphyllum and Haloxylon persicum. Pakistan Journal of Biological Sciences, 16(8), 351–361.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24(1), 1.
Arulbalachandran, D., Yasmin, K., Jothimani, K., Ramachandran, M., & Pradeepkumar, R. (2016). Role of ABA on antioxidant mechanism under drought crops. International Journal for Species, 17, 48–55.
Asada, K., & Takahashi, M. (1987). Production and scavenging of active oxygens in chloroplasts (pp. 227–287). Elsevier.
Ashraf, M., Mueen-Ud-Din, M., & Warraich, N. H. (2003). Production efficiency of mungbean (Vigna radiata L.) as affected by seed inoculation and NPK application. International Journal of Agriculture and Biology, 5(2), 179–180.
Athar, H. U. R., Ambreen, S., Javed, M., Hina, M., Rasul, S., Zafar, Z. U., Manzoor, H., Ogbaga, C. C., Afzal, M., Al-Qurainy, F., & Ashraf, M. (2016). Influence of sub-lethal crude oil concentration on growth, water relations and photosynthetic capacity of maize (Zea mays L.) plants. Environmental Science and Pollution Research, 23(18), 18320–18331.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry, 44(1), 276–287.
Bouchemal, K., Bouldjadj, R., Belbekri, M. N., Ykhlef, N., & Djekoun, A. (2017). Differences in antioxidant enzyme activities and oxidative markers in ten wheat (Triticum durum Desf.) genotypes in response to drought, heat and paraquat stress. Archives of Agronomy and Soil Science, 63(5), 710–722.
Bray, E. A. (2000). Response to abiotic stress. Biochemistry and Molecular Biology of Plants, 1158–1203.
Chance, B., & Maehly, A. C. (1955). Assay of catalases and peroxidases. Methods in Enzimology, 2, 764–775.
Chandlee, J. M., & Scandalios, J. G. (1984). Analysis of variants affecting the catalase developmental program in maize scutellum. Theoretical and Applied Genetics, 69(1), 71–77.
Childs Nathan, R.C.S., 19J, U.S. Department of Agriculture, Economic Research Service, October (15), 2019, 1–11.
Chutipaijit, S. (2016). Changes in physiological and antioxidant activity of indica rice seedlings in response to mannitol-induced osmotic stress. Chilean Journal of Agricultural Research, 76(4), 455–462.
Clegg, K. M. (1956). The application of the anthrone reagent to the estimation of starch in cereals. Journal of the Science of Food and Agriculture, 7(1), 40–44.
Deivanai, S., Xavier, R., Vinod, V., Timalata, K., & Lim, O. F. (2011). Role of exogenous proline in ameliorating salt stress at early stage in two rice cultivars. Journal of Stress Physiology and Biochemistry, 7(4), 157–174.
Dhindsa, R. S., Plumb-Dhindsa, P. A. M. E. L. A., & Thorpe, T. A. (1981). Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32(1), 93–101.
Dunnick, K. M., Badding, M. A., Schwegler-Berry, D., Patete, J. M., Koenigsmann, C., Wong, S. S., & Leonard, S. S. (2014). The effect of tungstate nanoparticles on reactive oxygen species and cytotoxicity in raw 264.7 mouse monocyte macrophage cells. Journal of Toxicology and Environmental Health, Part A, 77(20), 1251–1268.
Farooq, M., Wahid, A., Kobayashi, N. S. M. A., Fujita, D. B. S. M. A., & Basra, S. M. A. (2009). Plant drought stress: effects, mechanisms and management. In Sustainable Agriculture (pp. 153–188). Springer.
Filek, M., Labanowska, M., Koscielniak, J., Biesaga-Koscielniak, J., Kurdziel, M., Szarejko, I., & Hartikainen, H. (2015). Characterization of barley leaf tolerance to drought stress by chlorophyll fluorescence and electron paramagnetic resonance studies. Journal of Agronomy and Crop Science, 201(3), 228–240.
Fischer, R. A. (1980). Influence of water stress on crop yield in semiarid regions (No. CIS-511. CIMMYT.).
Foyer, C. H., & Noctor, G. (2011). Ascorbate and glutathione: The heart of the redox hub. Plant Physiology, 155(1), 2–18.
Garlick, P. B., Davies, M. J., Hearse, D. J., & Slater, T. F. (1987). Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circulation Research, 61(5), 757–760.
GOI, D. (2018). Agricultural Statistics at a glance 2018. Government of India, Ministry of Agriculture & Farmers Welfare, Department of Agriculture, Cooperation & Farmers Welfare, Directorate of Economics and Statistics.
Gunes, A., Pilbeam, D. J., Inal, A., & Coban, S. (2008). Influence of silicon on sunflower cultivars under drought stress, I: Growth, antioxidant mechanisms, and lipid peroxidation. Communications in Soil Science and Plant Analysis, 39(13–14), 1885–1903.
Ha, P. T. T. (2014). Physiological responses of rice seedlings under drought stress. Journal of Scientific Research and Development, 12(5), 635–640.
Halliwell, B., & Foyer, C. H. (1976). Ascorbic acid, metal ions and the superoxide radical. Biochemical Journal, 155(3), 697–700.
Halliwell, B., & Gutteridge, J. M. (1990). Role of free radicals and catalytic metal ions in human disease: An overview. Methods in Enzymology, 186, 1–85.
He, W., Liu, Y., Wamer, W. G., & Yin, J. J. (2014). Electron spin resonance spectroscopy for the study of nanomaterial-mediated generation of reactive oxygen species. Journal of Food and Drug Analysis, 22(1), 49–63.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1), 189–198.
International Rice Research Institute (IRRI). Standards evaluation system for rice, Available online: http://www.knowledgebank.irri.org/images/docs/rice-standard-evaluation-system.pdf 2002 (Accessed 20 September 2018).
Ishida, H., Makino, A., & Mae, T. (1999). Fragmentation of the large subunit of ribulose-1, 5-bisphosphate carboxylase by reactive oxygen species occurs near Gly-329. Journal of Biological Chemistry, 274(8), 5222–5226.
Jiang, J., Su, M., Chen, Y., Gao, N., Jiao, C., Sun, Z., Li, F., & Wang, C. (2013). Correlation of drought resistance in grass pea (Lathyrus sativus) with reactive oxygen species scavenging and osmotic adjustment. Biologia, 68(2), 231–240.
Jothimani, K., & Arulbalachandran, D. (2020). Physiological and biochemical studies of black gram (Vigna mungo (L.) Hepper) under polyethylene glycol induced drought stress. Biocatalysis and Agricultural Biotechnology, 29, 101777.
Kirk, J. T. O., & Allen, R. L. (1965). Dependence of chloroplast pigments synthesis on protein effects on actilione. Biochemical and Biophysical Research Communication, 27, 523–530.
Kumar, S., Dwivedi, S. K., Singh, S. S., Bhatt, B. P., Mehta, P., Elanchezhian, R., Singh, V. P., & Singh, O. N. (2014). Morpho-physiological traits associated with reproductive stage drought tolerance of rice (Oryza sativa L.) genotypes under rain-fed condition of eastern Indo-Gangetic Plain. Indian Journal of Plant Physiology, 19(2), 87–93.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685.
Lahlali, R., Jiang, Y., Kumar, S., Karunakaran, C., Liu, X., Borondics, F., Hallin, E., & Bueckert, R. (2014). ATR–FTIR spectroscopy reveals involvement of lipids and proteins of intact pea pollen grains to heat stress tolerance. Frontiers in Plant Science, 5, 747.
Liu, C., Liu, Y., Guo, K., Fan, D., Li, G., Zheng, Y., Yu, L., & Yang, R. (2011). Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environmental and Experimental Botany, 71(2), 174–183.
Loggini, B., Scartazza, A., Brugnoli, E., & Navari-Izzo, F. (1999). Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiology, 119(3), 1091–1100.
Lokhande, V. H., Nikam, T. D., & Penna, S. (2010). Differential osmotic adjustment to Iso-osmotic NaCl and PEG stress in the in vitro cultures of Sesuvium portulacastrum (L.) L. Journal of Crop Science and Biotechnology, 13(4), 251–256.
Lou, D., Wang, H., Liang, G., & Yu, D. (2017). OsSAPK2 confers abscisic acid sensitivity and tolerance to drought stress in rice. Frontiers in Plant Science, 8, 993.
Lowry, O. H., Rosenbrough, N. L., Parr, A. L., & Fandall, R. J. (1951). Protein measurement with folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Luchi, S., Kobayashi, M., Taji, T., Naramoto, M., Seki, M., Kato, T., Tabata, T., Kakubari, Y., Yamaguchi-Shinozaki, K., & Shinozaki, K. (2001). Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. The Plant Journal, 27(4), 325–333.
Maisura, M. A., Lubis, I., Junaedinand, A., & Ehara, H. (2014). Some physiological character responses of rice under drought conditions in a paddy system. Journal of International Society for Southeast Asian Agricultural Sciences, 20(1), 104–114.
Manivannan, P., Jaleel, C. A., Sankar, B., Kishorekumar, A., Somasundaram, R., Lakshmanan, G. A., & Panneerselvam, R. (2007). Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids and Surfaces B: Biointerfaces, 59(2), 141–149.
Mohammadian, R., Moghaddam, M., Rahimian, H., & Sadeghian, S. Y. (2005). Effect of early season drought stress on growth characteristics of sugar beet genotypes. Turkish Journal of Agriculture and Forestry, 29(5), 357–368.
Moore, S., & Stein, W. H. (1948). Photometric ninhydrin method for use in the chromatography of amino acids. Journal of Biological Chemistry, 176(1), 367–388.
Naithani, S. (2016). Plants and global climate change: A need for sustainable agriculture. Current Plant Biology, 6(1).
National Rice Research Institute (NRRI), for the ICAR, Annual report (2019) ISBN 81-88409-11-1.
Nelson, N. (1944). A photometric adaptation of the Somogyi method for the determination of glucose. Journal of Biological Chemistry, 153(2), 375–380.
Ogbaga, C. C., Stepien, P., Dyson, B. C., Rattray, N. J., Ellis, D. I., Goodacre, R., & Johnson, G. N. (2016). Biochemical analyses of sorghum varieties reveal differential responses to drought. PLoS ONE, 11(5), e0154423.
Osakabe, Y., Osakabe, K., Shinozaki, K., & Tran, L. S. P. (2014). Response of plants to water stress. Frontiers in Plant Science, 5, 86.
Pandey, V., & Shukla, A. (2015). Acclimation and tolerance strategies of rice under drought stress. Rice Science, 22(4), 147–161.
Pieczynski, M., Marczewski, W., Hennig, J., Dolata, J., Bielewicz, D., Piontek, P., Wyrzykowska, A., Krusiewicz, D., Strzelczyk-Zyta, D., Konopka-Postupolska, D., & Krzeslowska, M. (2013). Down-regulation of CBP80 gene expression as a strategy to engineer a drought-tolerant potato. Plant Biotechnology Journal, 11(4), 459–469.
Price, A. H., Atherton, N. M., & Hendry, G. A. (1989). Plants under drought-stress generate activated oxygen. Free Radical Research Communications, 8(1), 61–66.
Priestley, D. A., Werner, B. G., Leopold, A. C., & McBride, M. B. (1985). Organic free radical levels in seeds and pollen: The effects of hydration and aging. Physiologia Plantarum, 64(1), 88–94.
Pyngrope, S., Bhoomika, K., & Dubey, R. S. (2013). Reactive oxygen species, ascorbate–glutathione pool, and enzymes of their metabolism in drought-sensitive and tolerant indica rice (Oryza sativa L.) seedlings subjected to progressing levels of water deficit. Protoplasma, 250(2), 585–600.
Ramachandran, M., & Arulbalachandran, D. (2018). Exogenous abscisic acid mediated morphological characteristics, photosynthetic pigments and antioxidant metabolism under drought stress in rice (Oryza sativa L.). Madras Agricultural Journal, 105.
Ramachandran, M., Arulbalachandran, D., Dilipan, E., & Ramya, S. (2021). Comparative analysis of abscisic acid recovery on two varieties of rice (Oryza sativa L.) under drought condition. Biocatalysis and Agricultural Biotechnology, 33, 102006.
Ramachandran, M., Arulbalachandran, D., & Jothimani, K. (2017). ABA-mediated drought stress resistance in crops for sustainable agriculture. Arulbalachandran D. (Eds) Sustainable agriculture towards food security. (pp. 69–83). Springer.
Ramya, S., & Arulbalachandran, D. (2019). Climate Change and Impact of Drought on Crops: A Review. S. Anbazhagan, A. Jothibasu, G. Balamurugan (Eds.), Climate change in water resources, 45, Allied Publishers.
Ramya, S., & Arulbalachandran, D. (2022). Effect of exogenous Abscisic acid on growth and biochemical changes, antioxidant enzyme activities in black gram (Vigna mungo (L.) Hepper) under drought. Research Journal of Biotechnology, 17(4), 1–10.
Ramya, S., Arulbalachandran, D., & Ramachandran, M. (2022). Influence of exogenous abscisic acid on morpho-physiological and yield of maize (Zea mays L.) under drought stress. Plant Science Today, 9(2), 288–300.
Salekdeh, G.H., Siopongco, J., Wade, L.J., Ghareyazie, B., & Bennett, J., (2002). Proteomic analysis of rice leaves during drought stress and recovery, Proteomics. International (Eds.) (2nd Ed., pp. 1131–1145).
Sankar, B., Gopinathan, P., Karthishwaran, K., & Somasundaram, R. (2016). Variation in growth of peanut plants under drought stress condi-tion and in combination with paclobutrazol and ABA. Current Botany., 5(5), 14–21.
Sato, K., Tashiro, Y., Chibana, S., Yamashita, A., Karakawa, T., & Kohrogi, H. (2008). Role of lipid-derived free radical in bleomycin-induced lung injury in mice: Availability for ESR spin trap method with organic phase extraction. Biological and Pharmaceutical Bulletin, 31(10), 1855–1859.
Sharma, P., Jha, A. B., Dubey, R. S., & Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany1–26.
Somerville, C., & Briscoe, J. (2001). Genetic Engineering and Water. Science, 292(5525), 2217–2217.
Surewicz, W. K., & Mantsch, H. H. (1988). New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochimica Et Biophysica Acta -Protein Structure and Molecular Enzymology, 952, 115–130.
Thompson, J. E., Legge, R. L., & Barber, R. F. (1987). The role of free radicals in senescence and wounding. New Phytologist, 105(3), 317–344.
Uzilday, B., Turkan, I., Sekmen, A. H., Ozgur, R. E. N. G., & Karakaya, H. C. (2012). Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Science, 182, 59–70.
Wang, F. Z., Wang, Q. B., Kwon, S. Y., Kwak, S. S., & Su, W. A. (2005). Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. Journal of Plant Physiology, 162(4), 465–472.
Wang, W., Xin, H., Wang, M., Ma, Q., Wang, L. N. A., Kaleri, Y., Wang, Y., & Li, X. (2016). Transcriptomic analysis reveals the molecular mechanisms of drought-stress-induced decreases in Camellia sinensis leaf quality. Frontiers in Plant Science, 7, 385.
Witwicki, M., Jezierska, J., & Ozarowski, A. (2009). Solvent effect on EPR, molecular and electronic properties of semiquinone radical derived from 3,4-dihydroxybenzoic acid as model for humic acid transient radicals: High-field EPR and DFT studies. Chemical Physics Letters, 473, 160–166.
Xie, Y., Ling, T., Han, Y., Liu, K., Zheng, Q., Huang, L., Yuan, X., He, Z., Hu, B., Fang, L., & Shen, Z. (2008). Carbonmonoxide enhances salt tolerance by nitricoxide-mediated maintenance of ion home-ostasis and up-regulation of antioxidant defense in wheat seedling roots. Plant Cell Environment, 31(12), 1864–1881.
Xiong, L., Schumaker, K. S., & Zhu, J. K. (2002). Cell signaling during cold, drought and salt stress. The Plant Cell, 14, 165–183.
Acknowledgements
The author’s express gratitude to Periyar University, Salem, TN, India for providing infrastructure and resources to carry out the investigation. M. Ramachandran could like to express thankfulness to Periyar University for financial supporting under the Scheme of University Research Fellowship (URF– PU/AD-3/URF/2016).
Author information
Authors and Affiliations
Contributions
MR, DA designed the experiments. MR, DA and SR carried out the experiments and data collection. MR, DA and SG wrote the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marimuthu, R., Gurunathan, S., Sellamuthu, R. et al. Physio-biochemical characterizations in the drought induced rice (Oryza sativa L.): pathway to understand the drought tolerance mechanisms. Plant Physiol. Rep. 28, 388–404 (2023). https://doi.org/10.1007/s40502-023-00737-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-023-00737-5