Abstract
The rapid ripening and fruit size are two important factors, which significantly influence the economy and marketability of apricot. Generally, the postharvest quality of fresh crops depends on the quality achieved at harvest time, which is influenced considerably by pre-harvest factors. Therefore, this study aimed to investigate the effect of foliar application of calcium chloride and potassium nitrate on the physical and biochemical attributes of apricot fruit. We evaluated the effect of foliar sprays of 0.5% or 1% calcium chloride (CaCl2) and 1% or 2% potassium nitrate (KNO3) on the yield and at harvest quality of apricot fruits cv. ‘Shahroudi’ during the 2019 and 2020 growing seasons. The experiment was conducted in a commercial apricot orchard located in Birjand, Iran. The results indicated that twice foliar spray of both chemicals at the pre-harvest stage affected most of the evaluated traits in both years. KNO3 application at both concentrations significantly increased the length, width, and weight of apricot fruit and maintained total phenol content, vitamin C, and titratable acidity compared to the control. Interestingly, treated fruit with KNO3 solution at both concentrations increased fruit width (150%), fruit length (160%), and fresh fruit weight (180%), compared to the control. Besides, KNO3 (1% or 2%) and 1% CaCl2 treatments significantly increased fruit firmness (25%) and calcium content (35%) of fruit pulp compared to the control. Overall, the results suggest practical multiple practical application of both chemicals (particularly KNO3) at proper concentration and time during the growth season to improve the quality and increase the yield of apricot fruit ‘Shahroudi’ cultivar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Foliar fertilization is an important tool for the sustainable and productive management of crops. Foliar spray is a way to reduce the use of chemical fertilizers and their environmental hazards (Niu et al. 2021). Besides, by foliar feeding, nutrients can be delivered directly and quickly to the plant or fruit if needed. Some of the plant organs, like fruits, need nutrients such as calcium and potassium more than the whole plant; or in early spring, when the roots are still unable to absorb nutrients due to low soil temperatures, foliar spraying is vital (Fernández et al. 2013). In fruit trees, the primary periods of nitrogen (N) accumulation coincide with the vegetative growth in spring (Barker and Pilbeam 2015). Potassium (K) accumulation followed the same pattern as N accumulation. This demand for nutrients can be supplied from redistribution or uptake (Barker and Pilbeam 2015). The high demand for K and N during fruiting years, particularly during early growth, suggests that any reduction of root nutrient uptake during those periods could result in impaired fruit growth and yield (Barker and Pilbeam 2015). In many perennial high-value crops, foliar fertilizers should be applied during the period of highest nutrient demand under the premise that soil supply and root uptake may be inadequate to meet needs even with adequate soil-applied fertilizer (Fernández et al. 2013).
Foliar application of potassium salts is one of the most important practices of the new strategies applied in the integrated fruit production systems, improving fruit quality (Solhjoo et al. 2017). Among the different sources of potassium fertilizers, potassium nitrate (KNO3) is used almost exclusively for foliar spray. KNO3 fertilizer contains 38.5% K and 13% N (Fernández et al. 2013). Previous studies have revealed that the application of K involves in many physiological processes, such as where it impacts the mechanism of stomatal opening and closing by affecting cell water potential and turgor, photosynthesis, assimilate transport and enzyme activation, which have direct impact on crop productivity and fruit quality (Kanai et al. 2011). Prasad et al. (2015) showed that foliar spray of pear trees with 1.5 or 2% KNO3 increases soluble solids, total sugar, and other biochemical properties such as vitamin C and total acid of treated pears and improved quality compared to the control. Aksoy et al. (1993) investigated the effect of multiple foliar application of 2% KNO3 on apricot fruit of cv. ‘Tokaloglu’ in Turkey from late winter. They found that fruit weight and soluble solids significantly increased compared to control. In addition, they showed that spraying with 2% KNO3 solution every 2 weeks performed better than spraying with 8% KNO3 just once.
Pre-harvest spraying of fruit trees with different calcium (Ca) salts solution is one of the most useful strategies to improve the postharvest quality characteristics of fruits and reduce the use of fungicides because Ca increases the resistance of fruits pathogens (Martins et al. 2020; Öztürk et al. 2019). Calcium chloride (CaCl2) application is a type of Ca source suggested by many researchers. Lal et al. (2011) reported that foliar spraying of apricot trees cv. ‘Harcot’ with 1.5% CaCl2 effectively minimized weight loss in treated fruits during storage compared to control. Fruit quality traits (total soluble solids, titratable acidity, and vitamin C) were also better after 8 days of storage at the ambient condition compared to control. Yousefi et al. (2015) also indicated that the foliar application of 0.5% CaCl2 in the apricot cultivar ‘Jahangiri’ improved some biochemical (protein, free amino acid, and vitamin C) and fruit quality attributes. Ca salt foliar sprays during fruit growth and development provide a safe mode of supplementing endogenous Ca in fresh fruits (Sinha et al. 2019).
Apricot (Prunus armeniaca L.) is a soft, delicious fruit, climacteric, and highly perishable (Moradinezhad and Jahani 2019). Apricot is generally considered to be a rich source of carotenoids, ascorbic acid, polyphenols, iron, potassium, polysaccharides, fiber, and minerals (Muzzaffar et al. 2018). Iran is one of the leading producers of apricot fruit globally, like Turkey, China, Uzbekistan, and Pakistan (Li et al. 2020). Among different apricot cultivars in Iran, there is a high demand for the Shahroudi cultivar for fresh consumption due to its great taste and attractive appearance and color. Apricot is a valuable temperate fruit, with high nutritional value, health benefits, and tasty flavor as fresh fruit and cultivated widely in the world. However, depending on the cultivar, full mature apricots lose their postharvest quality and commercial acceptability within 1 week after harvest due to rapid ripening. Storing fruits at low temperatures may help extend the shelf life, but it is not enough for long-term storage or transportation of apricots (Pretel et al. 2000), as postharvest quality of fresh crops generally depends on the quality achieved at the time of harvest. Therefore, complementary techniques such as pre-harvest foliar fertilization using different nutrients and particularly K and Ca fertilizers during growth season are required to improve the quality at harvest (Alexa et al. 2018) and also prolong the shelf-life of fresh fruits (Aslantürk et al. 2021).
The positive effects of foliar fertilization with various N, K, and Ca fertilizers such as urea, potassium nitrate, calcium nitrate, and potassium sulfate have been proven on different fruit trees (Hegazi et al. 2011; Hosseini et al. 2021; Madani et al. 2016; Mandal et al. 2012; Sud and Bhutani 1988; Zeraatgar et al. 2018). However, little information has been reported about the pre-harvest application of KNO3 or CaCl2 on apricot (Aksoy et al. 1993; Mohit and Thakur 2017; Ozturk et al. 2001). Besides, the review of the literature showed no report regarding the foliar spray of CaCl2 and KNO3 on apricot tree ‘Shahroudi’ cultivar, which is one the most important commercial cultivars widely cultivated in Iran due to its tasty flavor and attractiveness as fresh fruit. Therefore, this study aimed to investigate the effect of foliar application of CaCl2 and KNO3 on the physical and biochemical attributes of apricot fruit ‘Shahroudi’ cultivar.
2 Material and Methods
2.1 Plant Material
The experiment was conducted in a commercial orchard located in Birjand (32° 86′ N latitude, 59° 22′ E longitude, 1480 m above sea level), South Khorasan province, Iran. Trees (10 years old) were selected for vegetative and crop load uniformity at random within the orchard. The trees were spaced 3 m between rows and 2.5 m within rows in the north-south orientation. These experiments were conducted on apricot trees cv. ‘Shahroudi,’ during the 2019 and 2020 growing seasons. The experimental trees received similar cultural practices including, fertilizers, irrigation, and plant protection, except for the experimental treatments. To determine the study site’s soil texture, samples were taken from a depth of 0–60 cm, and the soil properties were examined in the laboratory, as shown in Table 1.
2.2 Pre-harvest Treatments of Calcium Chloride and Potassium Nitrate
A total of 25 trees were selected for this experiment (5 trees per treatment). The apricot trees were either treated by solutions of CaCl2 (Merck, Germany) at 0.5 and 1% concentrations or KNO3 (Merck, Germany) at 1 and 2% concentrations. Also, we considered spraying with distilled water as a control. We sprayed the solutions in the morning with a hand sprayer (on the shoots, leaves, and fruits). The trees were sprayed twice at the pre-harvest stage. The first foliar spraying was done on May 4 (about 45 days after fruit set), and the second spraying was done 2 weeks before the commercial harvest on May 24. Tween 20 (Merck, Germany) surfactant at 0.01% was added to all used solutions for foliar application. Apricots were harvested at a stage when the peel had light green color with yellow spots and with total soluble solids of approximately 10 °Bx. Harvested fruits were then transferred to the postharvest laboratory of the Department of Horticultural Science, University of Birjand.
2.3 Measurement of Fruit Physical Properties
To evaluate the traits, 10 fruits were used in each treatment. Fruit length and width were measured using a digital caliper (LINEAR, 49–923) to the nearest hundredth of a millimeter and expressed in millimeters. Also, the fruit weight was obtained using an accurate digital scale (A&D FX-5000i, Japan) with an accuracy of 0.01 g, and the data were expressed in grams. The firmness of apricot fruit was measured by a digital penetrometer (Fruit Hardness Tester, Model FHT 200, Extech Co., USA) with a 2-mm probe. Data were presented in Newton. The color components include L* (brightness), a* (redness and greens), b* (yellowness and blue color), chroma (purity of color), and hue (color angle) of apricot fruit skin measured using a colorimeter (TES, 135-TAIWAN).
2.4 Measurement of Fruit Chemical Properties
Total soluble solids in apricot juice were measured using a handheld refractometer (RF 10, Brix, 0–32%, Extech Co., USA), and data were expressed as °Bx. To check titratable acidity, the apricot pulp was homogenized in 40-ml distilled water and filtered to extract the juice. A 10-ml apricot juice was taken in a titration flask and titrated against 0.1 N NaOH in the presence of phenolphthalein, till permanent light pink color appeared. The TA is expressed as the percentage of malic acid. Total phenolic content was determined using the Folin-Ciocalteu method (Emmons et al. 1999), by mixing 8.25 ml of deionized water, 0.5 ml of extract (fresh weight), 0.75 ml of 20% Na2CO3, and 0.5 ml of reagent Folin-Ciocalteu. After a 40-min reaction in a water bath at 40 °C, the absorbance at 755 nm was measured using a spectrophotometer (Bio Quest, CE 2502). The final results were expressed as mg of gallic acid per gram of fresh apricot (Chuah et al. 2008). To determine the calcium content of fruit tissue, pulp samples were separately put in tap water for 10 min, then washed in distilled water, and dried at 60 °C in an air-circulating oven; 0.25 g of the dried pulp was then digested in 5-mL H2SO4 on a hot plate at 450 °C in a fume chamber for 7 min. Then, 10-mL H2O2 was added into the mixtures, and the heating was continued for another 4 min. The solution mixtures were brought up to 100-mL with distilled water. Calcium ion concentration was measured with an atomic absorption spectrophotometer, and the results were expressed as mg Ca g−1 dry weight (DW). Vitamin C content was determined using 2,6-dichlorophenol indophenols (Nielsen 2010). The results were expressed as mg 100 g−1 of fresh weight (FW).
2.5 Experimental Design and Statistical Analysis
The experiment was conducted using a randomized complete block design (RCBD). Three replicates were used for each treatment, and all results were expressed as mean ± standard error (SE). Analysis of variance was performed with the Genstat program (Discovery Edition, version 9.2, 2009, VSN, International, UK) to evaluate significance. The LSD test at the level of 1% (P ≤ 0.01) was also used to identify the significant differences between the means.
3 Results
The obtained results of the analysis of variance for all evaluated traits were similar in the 2019 and 2020 growing seasons. In other words, the data of experiments in both years were not significantly different, and therefore, they were combined in the tables.
3.1 Length, Width, Length to Width Ratio, and Fruit Weight
The data analyzed showed that foliar application of apricot tree significantly affected the physical properties evaluated of fruit such as length, width, weight, firmness, and length to width ratio. As shown in Table 2, foliar application of KNO3 (1 or 2%) had the most significant effect on the diameter, length, and size of apricot fruit (Fig. 1), while the lowest amount of diameter, length, and size was obtained from control and CaCl2 treatment (0.5 or 1%). The diameter of fruits treated with KNO3 at a concentration of 2% was 50.3 mm, while the average diameter of fruit in control samples was 18.9 mm. Also, foliar application of KNO3 increased the fruit length by 22.6 mm compared to the control and significantly decreased the fruit length to width ratio. Also, the results showed that the lowest fruit weight (22.71 g) was obtained in control. Meanwhile, KNO3 treatment increased the fruit weight twofold than the control, and the highest fruit weight (51.08 g) was related to 2% KNO3 treatment. However, no significant difference was observed between 2% and 1% KNO3 treatments.
3.2 Firmness
CaCl2 1% and KNO3 at both applied concentrations significantly increased firmness compared to the control. According to Table 2, the highest average tissue firmness of apricot fruit was obtained in treated fruits with CaCl2 1% (15.98 N), followed by KNO3 treatments at concentrations of 1 and 2%, and the lowest was related to the control (11.67 N) and CaCl2 0.5% (11.85 N).
3.3 Color Attributes of Fruit Skin
Foliar spraying with CaCl2 and KNO3 had no significant effect on the skin color characteristics of apricot fruit (Table 3). The results revealed that the applied treatments had no negative impact on the control and did not reduce the lightness of the skin.
3.4 Total Soluble Solids (TSS), Titratable Acidity (TA), and TSS/TA Ratio
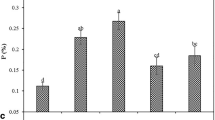
The results showed that foliar application of CaCl2 and KNO3 had no significant effect on TSS (Fig. 2a). However, the TA (Fig. 2b) and TSS/TA (Fig. 2c) values were affected by foliar application. The lowest TA (0.80%) was obtained from control samples. However, there was no significant difference between the control and 0.5% CaCl2 treatment. Also, the highest TA value (1.46%) was recorded in treated fruits with 2% KNO3, which had no significant difference with 1% KNO3 treatment. The results indicated that the highest TSS/TA ratio (14.31) was related to the control samples while the lowest TSS/TA ratio (7.98) was found in treated trees with 1% KNO3. No significant difference was found between 1%, 2% KNO3, and 1% CaCl2 treatments.
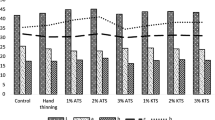
The effect of foliar application with calcium chloride (CaCl2) and potassium nitrate (KNO3) at different concentrations on total soluble solids (TSS) (a), titratable acidity (TA) (b), TSS/TA (c), total phenol content (TPC) (d), vitamin C (e), and calcium content (Ca) (f) of apricot fruit at harvest. Error bars represent the standard error deviation. Symbols with the same letter are not significantly different at P ≤ 0.01 (LSD test)
3.5 Total Phenolic Compounds (TPC)
All treatments used in the experiment showed an increase in phenolic compounds compared to the control (Fig. 2d). The results showed that the lowest phenol (60.81 mg GAE g−1 FW) was obtained from control fruits, whereas the highest phenol value (62.97 mg GAE g−1 FW) was related to treated fruits with 2% KNO3. However, there was no significant difference between 1%, 2% KNO3, and 1% CaCl2 treatments. Also, no significant difference was observed between 0.5% and 1% CaCl2 treatments.
3.6 Ascorbic Acid Content
All treatments used in the experiment showed a significant increase in the ascorbic acid (vitamin C) content of apricot fruit compared to the control (Fig. 2e). There was no significant difference between different levels of CaCl2 and KNO3 treatments. Nevertheless, the highest ascorbic acid value was obtained in treated fruit with KNO3 2% (18.89 mg g−1), and, the lowest amount was observed in control (12.43 mg g−1).
3.7 Calcium Content of Fruit Pulp
The results showed that all treatments increased the calcium content of apricot fruit compared to the control. As expected, the highest average calcium content (96.77 mg −1) was obtained from fruits treated with 1% CaCl2, whereas the lowest calcium content value (50.44 mg g−1 DW) was recorded in the control. Also, there was no significant difference between the treatments of KNO3 (1 or 2%) and CaCl2 0.5% (Fig. 2f).
4 Discussion
Fruit size is considered to be one of the important external factors in determining fruit quality in apricot as it dramatically influences consumers’ appeal. Nitrogen (N) is the most important factor in plant growth and development. The form of N (nitrate or ammonium) is one of the important factors on the growth, development, and the yield of plants. N has been shown to increase leaf area and increase photosynthesis (Atkinson et al. 2015). Carbohydrates produced in the process of photosynthesis are transported to the fruits (as main sinks) through the phloem, which increases the fruit size (both length and diameter) (Barker and Pilbeam 2015). The results of the present study showed that foliar application of KNO3 fertilizer caused a significant increase in apricot fruit size. This increase is probably due to the presence of N in KNO3 fertilizer. However, the role of K in this case cannot be ignored. Although, K is not a component of organic molecules or plant structure and is involved as a free ion in several physiological and biochemical processes (Atkinson et al. 2015). However, K increases the photosynthetic intensity of chloroplasts and the rate at which leaf material is transported through the phloem to storage tissues, thereby improving fruit yield and quality (Atkinson et al. 2015). Improving fruit yield and quality can be attributed to the effect of K on the entry of carbohydrates or the synthesis of plant regulators into young fruits (Fernández et al. 2013). Mohit and Thakur (2017) reported that the application of different nitrogen fertilizers such as urea and calcium nitrate significantly increased the yield and quality of apricot fruit. They stated that the maximum fruit width, length, and weight were obtained where the highest concentration of calcium nitrate was applied. Nitrogen has been shown to increase fruit size by increasing cell division and cell elongation (Sušin et al. 2006). Also, previous studies have shown that adequate nitrogen is essential for leaf growth, fruit formation, and fruit size, which ultimately leads to the production of a high-yield crop (Ben-Oliel et al. 2005; Zhang et al. 2019). Besides, previous researches indicated that foliar spraying of different fruit trees with potassium fertilizers such as KNO3 in olive (Hegazi et al. 2011) and potassium sulfate in fig (Soliman et al. 2018) increased fruit length, weight, and yield. Fruits with the larger size in the present study in KNO3-treated trees may be a result of the fact that potassium and nitrogen are involved in several physiological, biological, and biochemical processes, such as stimulating cell division, cell elongation, as well as biosynthesis and the transport of organic nutrients and carbohydrate accumulation in the plant (Cronje et al. 2009). Increased vegetative growth and fruit quality are associated with potassium and nitrogen in cell growth, sugar transport, and tourniquet pressure (Solhjoo et al. 2017). Similarly, other researchers have stated that KNO3 application increases the yield of barberry (Hosseini et al. 2021) and apple fruit (Mosa et al. 2015). The significant reduction in fruit length to width ratio in KNO3 treated fruits may be related to increased fruit pit and or pulp size (Ramezani and Shekafandeh 2011).
The texture of fresh fruit is an essential factor in determining quality. Various factors, including Ca, affect the fluidity of cell membranes. It is proven that Ca can prevent ripening and senescence by cross-linking with pectic polymers in the cell wall and protecting cell membrane integrity (Mohebbi et al. 2020). Ghorbani et al. (2017) reported that CaCl2 (5 g L−1) maintained the firmness of apple fruit tissue because calcium serves as an intermolecular binding agent that stabilizes pectin-protein complexes of the middle lamella. Ca also plays an important role in the cell membrane by inducing rigidification at the membrane surface of apple fruit tissue. Thus, the maintenance of higher firmness in treated fruits might be due to the thickening of the middle lamella of fruit cells owing to increased formation of deposition of calcium pectate (Ortiz et al. 2011). CaCl2 is more effective in improving the firmness of fruits by delaying senescence, preserving the cellular structure, and retarding respiration rate (Ekinci et al. 2016). The enzyme polygalacturonase (PG) degrades pectin in most fruits, leading to the softening of fruit tissue. Pectin methylesterase (PME) is also a pectin-degrading enzyme, which causes pectin to depolymerize by activating polygalacturonase. Ethylene has been shown to synthesize PG and PME enzymes. In this regard, Tzoutzoukou and Bouranis (1997) showed that foliar application of apricot trees (cv. Bebekou) with CaCl2 significantly reduces ethylene production, which leads to a decrease in cell wall-destroying enzymes (PG and PME) and reduces the rate of fruit respiration rate. They also reported that foliar application of apricot trees with 0.5% CaCl2 at 13, 17, and 21 days before harvest maintains tissue firmness greater than control at harvest. They stated that the higher firmness of apricot fruit in CaCl2-treated samples is because CaCl2 treatment reduced ethylene production more than three times compared to control and also inhibited the rate of respiration. There are also similar reports on different fruits such as peaches (Manganaris et al. 2007), plums (Kirmani et al. 2013), and strawberries (Langer et al. 2019). Besides, the results showed that not only CaCl2 (1%) but also KNO3 (2% and 1%) treatments significantly maintained the firmness of fruit tissue compared to the control. It has been shown that K makes high osmotic pressure and turgor pressure, which can provide power for cell division, cell wall extension, and cell expansion and finally, reduce fruit splitting (Fernandes et al. 2017). Therefore, it can be concluded that the K in KNO3 has prevented the softening of apricot fruit tissue by increasing the flexibility and thickness of the cell membrane. Similarly, EL-Seginy (2006) reported that soil fertilization of K (120 kg K2O/fed/year) of apricot trees (cv. Canino) in Egypt increased the firmness. Besides, EL-Seginy (2006) showed that the amount of potassium is directly related to water content in the fruit. Potassium caused water storage in the tissues, thus increasing the freshness and strength of the fruit. However, with increasing fruit size, Ca accumulation time decreases, and fruit firmness reduces compared to samples treated with CaCl2 (Atkinson et al. 2015).
The results revealed that the applied treatments in the foliar application had no negative impact on the color attributes and did not change the lightness of the skin. The results of the current study were in line with the findings of Hernández-Muñoz et al. (2006) on strawberry and Moradinezhad et al. (2019) on jujube fruit.
The titratable acidity is directly related to the concentration of organic acids present in the fruit, which are an important parameter in maintaining the quality of fruits. Hosseini et al. (2021) reported that foliar application of KNO3 had no significant effect on the TSS of barberry fruit. Titratable acidity is directly related to the concentration of organic acids present in the fruit, which are an important parameter in maintaining the quality of fruits (Moradinezhad and Jahani 2019). In apricot, malic acid is the main organic acid. Prasad et al. (2015) reported that foliar application of CaCl2 and KNO3 at a concentration of 2% significantly increased the TA of mango fruit. They stated that calcium and KNO3 being the source of nitrogen might have modified the vegetative growth, which increases metabolism and consequently increases the acidity of fruits. Table 3 shows that the highest TA value was recorded in the KNO3 treatments, while the lowest TA was obtained in control samples. The lower TA may be attributed to a marked increase in malic enzyme and pyruvate decarboxylation reaction during the climacteric period, proportional to the rise in the respiration rate and other metabolic biodegradable response (Mandal et al. 2012). Therefore, it may be concluded that the pre-harvest spray of KNO3 treatment can preserve the sensorial quality of the apricot fruit. Similar results were obtained by Mandal et al. (2012) on guava fruit. A correct balance between sugar and acid contents is crucial for the processing industry. Also, it is essential for the fresh-market production industry because such a ratio determines fruit taste (Caretto et al. 2008). The results also showed that the highest amount of TSS/TA ratio was observed in control followed by calcium chloride 0.5% and the lowest was found in KNO3 treatments. Rabiei et al. (2010) also showed that foliar application of apple trees with KNO3 reduced TSS/TA ratio. They stated that the reduction in TSS/TA ratio might be because the application of potassium slows down the respiration rate and senescence process in the fruit. EL-Seginy (2006) showed that the application of potassium fertilizer did not have a significant effect on the titratable acidity of apricot cv. Canino, which is inconsistent with the results of the current study. These various reports in TSS, TA, and TSS/TA ratio may also be related to the difference between different fruits or apricot cultivars studied in terms of ripening stage and their total soluble solids and titratable acidity of fruit at harvest time.
During growth, many metabolic processes in plants produce reactive oxygen species, but plants have effective antioxidant mechanisms to eliminate reactive oxygen species. One of these mechanisms is the production of phenolic compounds in plants. Phenolic compounds prevent membrane peroxidation by repelling oxygen free radicals and stabilize cell membranes. A close relationship has been reported between K nutritional status and the reduction of free radicals (Leibar et al. 2017). They reported that K plays a very important role in regulating the turgor pressure of protective cells and stomatal movements. The results of the present study showed that the highest phenol content was obtained in 2% KNO3 treatment. Presumably, this increase in total phenol content was related to the role of K in enhancing carbon dioxide fixation, transferring photosynthetic material into other sinks, and preventing the transfer of photosynthetic electrons to oxygen, thus reducing the production of oxygen free radicals and causing accumulation of phenol content. Nguyen et al. (2010) reported that the total phenol content increases with increasing K concentration in basil leaves. They stated that K stimulates the production pathways of secondary metabolites, therefore increasing the total phenol content in potassium-treated samples. Similar to the results of the current study, Ranjbar et al. (2018) reported that Ca increases the amount of total phenol in apple fruit. They suggested that since Ca increases the polysaccharide content in the fruit cell wall and the permeability of the membrane; therefore, the wall strength and cell membrane can be maintained, which leads to preventing the oxidation of phenolic compound. Potassium is an activator for enzymes involved in photosynthesis and the biosynthesis of starch and proteins. When plant growth increases and more photosynthates are produced at higher potassium rates, increased phenolic concentrations may correspondingly occur due to the allocating excess fixed carbon to the Shikimic pathway (Barker and Pilbeam 2015). Medan (2020) reported that foliar application of K and Ca chelate of the apricot trees cv. ‘Royal’ significantly increased leaf area and leaf chlorophyll content. Therefore, the synthesis of secondary metabolites, including phenolic compounds, is also affected.
Vitamin C (ascorbic acid) has important antioxidant and metabolic functions in both plants and animals, but humans and a few other animal species have lost the capacity to synthesize it. Plant-derived ascorbic acid is thus the major source of vitamin C in the human diet (Alkadi 2020). Ascorbate usually acts as an antioxidant. It typically reacts with oxidants of active oxygen species (ROS), such as the hydroxyl radical formed from hydrogen peroxide. Ascorbate can terminate chain radical reactions by electron transfer. On the other hand, it has been proven that one of the reasons for the increase of ROS is the production of ethylene in plants. Moreover, an increase in the hormone ethylene has been shown to cause the synthesis of ascorbic acid hydrolyzing enzymes (such as ascorbate oxidase and ascorbate peroxidase) (Alkadi 2020). Our results showed that the application of CaCl2 and KNO3 maintained the level of vitamin C in apricot fruit compared to the control. Foliar application of these chemicals preserved vitamin C likely due to delayed ripening and/or reduced ethylene production. Kazemi (2013) reported that the treatment of strawberry fruits with KNO3 significantly increased ascorbic acid content, which is consistent with the results of the current study. They stated that the increase in vitamin C content was due to the synthesis of enzymes and proteins by potassium application. In addition, Ramezanian et al. (2009) found that foliar CaCl2 spraying on pomegranate fruits at the full bloom stage increased the ascorbic acid content of fruits at harvest. Because Ca delays the synthesis of ethylene, and ROS may be reduced, vitamin C levels in the fruit maintained. Higher vitamin C content in CaCl2-treated fruits could be related to inhibiting the action of calcium on the activity of ascorbic acid oxidase that uses ascorbate as a substrate (Singh et al. 2005), which is in line with the results of the current study. Similar results have been reported by Yousefi et al. (2015) on apricot fruit cv. Jahangiri. They showed that foliar application of 0.5% CaCl2 (46 days after full bloom) increased the amount of vitamin C by 3-fold compared to the control at harvest.
Many physiological disorders of fruits are associated with low Ca levels, and because of its phloem immobility, foliage-applied Ca is not redistributed from sprayed leaves to the fruit (Atkinson et al. 2015). Generally accepted is that multiple sprays as well as the concurrent application of Ca to leaves, stems, and fruit are required and early-season sprays appear to be more effective than late-season sprays (Fernández et al. 2013). It is well-known that Ca is an essential substance in maintaining the structure of the cell wall, which also reduces ethylene and delays the ripening process of fresh products (Mohebbi et al. 2020). Ngamchuachit et al. (2014) reported that with increasing applied Ca concentrations (CaCl2 and Ca lactate), the Ca content of the tissue also increased. They showed that Ca treatment reduces ion leakage, which is why maintaining cell wall integrity in calcium-treated fruits. Our results showed that foliar application with CaCl2 1% increased the calcium content of fruit tissue by about 40% compared to control. This increase in tissue Ca content may be due to Ca treatment that contains more available Ca ions to interact with the binding sites in the cell wall and plasma membrane. In agreement with our results, Tzoutzoukou and Bouranis (1997) reported that pre-harvest multiple foliar application of apricot trees with CaCl2 at 0.5% concentration, resulting in a 50% increase in the calcium content of fruit pulp in apricot cv. Bebekou. Also, the firming effect of Ca-treated apricot results primarily from the interaction with the cell wall and middle lamella, which is consistent with the results obtained from the evaluation of tissue firmness in the present study. Madani et al. (2016) also reported similar results on papaya fruit. Similar to our results, Yousefi et al. (2015) reported that foliar application of CaCl2 (0.5%) significantly increased the calcium content of apricot fruit cv. Jahangiri.
5 Conclusions
Higher yield and fruit size are important for the producer, while nutritional value and firmness are more important qualitative factors from consumers and postharvest viewpoint because apricot is a highly perishable fruit. Interestingly, obtained results of the present study revealed that pre-harvest foliar spray of potassium nitrate (KNO3) at a concentration of 1% has a great potential for fertilization of apricot and is recommendable for practical application on apricot trees as fruit fresh weight doubled, firmness increased, and the nutritional value of apricot fruit improved by increasing the total phenol, calcium content, and vitamin C compared to the control. Moreover, calcium chloride (CaCl2) 1% significantly increased the strength and calcium content of treated fruit more than KNO3 treatment. It is well-known that increased calcium content also effectively extends the quality of fresh apricot and reduces postharvest losses during long-term storage and marketing. So, it can be concluded that likely a combination of KNO3 and CaCl2 1% multiple application at pre-harvest stage has more beneficial and economic effects on quantitative and qualitative traits of fresh apricot. Therefore, further study is required to investigate the influence of the pre-harvest multiple foliar application of these chemicals and their combined effect on the yield and quality of fresh apricot fruit at harvest.
References
Aksoy U, Kara S, Misirli A, Can HZ, Seferoglu G (1993) Effect of potassium nitrate and hydrogen cyanamide on apricot. In X International symposium on apricot culture, 384 pp 431-440. https://doi.org/10.17660/actahortic.1995.384.68
Alexa E, Lalescu D, Berbecea A, Camen D, Poiana MA, Moigradean D, Bala M (2018) Chemical composition and antioxidant activity of some apricot varieties at different ripening stages. Chil J Agric Res 78:266–275. https://doi.org/10.4067/S0718-58392018000200266
Alkadi H (2020) A review on free radicals and antioxidants. Infectious disorders-drug targets (Formerly Current Drug Targets-Infectious Disorders) 20:16–26. https://doi.org/10.2174/1871526518666180628124323
Aslantürk B, Altuntaş E, Ozturk B (2021) Effects of modified atmosphere packaging and methyl jasmonate treatments on fruit quality and bioactive compounds of apricot fruit during cold storage. J Agric Sci (in press)
Atkinson D, Jackson JE, Sharples RO (2015) Mineral nutrition of fruit trees. Studies in the agricultural and food sciences. Elsevier, London
Barker AV, Pilbeam DJ (2015) Handbook of plant nutrition. CRC press, London
Ben-Oliel G, Kant S, Naim M, Rabinowitch HD, Takeoka GR, Buttery RG, Kafkafi U (2005) Effects of ammonium to nitrate ratio and salinity on yield and fruit quality of large and small tomato fruit hybrids. J Plant Nutr 27:1795–1812. https://doi.org/10.1081/PLN-200026430
Caretto S, Parente A, Serio F, Santamaria P (2008) Influence of potassium and genotype on vitamin E content and reducing sugar of tomato fruits. Hortic Sci 43:2048–2051. https://doi.org/10.21273/hortsci.43.7.2048
Chuah AM, Lee YC, Amaguchi Y, Takamura H, Yin LJ, Matoba T (2008) Effect of cooking on the antioxidant properties of coloured peppers. J Food Chem 111:20–28. https://doi.org/10.1016/j.foodchem.2008.03.022
Cronje RB, Sivakumar D, Mostert PG, Korsten L (2009) Effect of different preharvest treatment regimes on fruit quality of litchi cultivar ‘Maritius’. J Plant Nutr 32:19–29. https://doi.org/10.1080/01904160802530987
Ekinci N, Özdüven F, Gür E (2016) Effects of preharvest foliar calcium applications on the storage quality of ‘0900 Ziraat’ sweet cherry cultivar. Erwerbs-Obstbau 58:227–231. https://doi.org/10.1007/s10341-016-0281-y
EL-Seginy A (2006) Response of "Canino" apricot trees to different irrigation and potassium treatments. Alexandria Science Exchange 27:64–76
Emmons CL, Peterson DM, Paul GL (1999) Antioxidant capacity of oat (avena sativa L.) extracts. 2. In vitro antioxidant activity and contents of phenolic and tocol antioxidants. J Agric Food Chem 47:4894–4898. https://doi.org/10.1021/jf990530i
Fernandes AM, Gazola B, Nunes JGDS, Garcia EL, Leonel M (2017) Yield and nutritional requirements of cassava in response to potassium fertilizer in the second cycle. J Plant Nutr 40:2785–2796. https://doi.org/10.1080/01904167.2017.1382520
Fernández V, Sotiropoulos T, Brown PH (2013) Foliar fertilization: scientific principles and field practices. International fertilizer industry association, France
Ghorbani E, Bakhshi D, Fallahi E, Rabiei B (2017) Evaluation of pre-harvest foliar calcium applications on 'Fuji' apple fruit quality during cold storage. Aust J Crop Sci 11:228–233. https://doi.org/10.21475/ajcs.17.11.02.p5853
Hegazi ES, Mohamed SM, El-Sonbaty MR, Abd El-Naby SKM, El-Sharony TF (2011) Effect of potassium nitrate on vegetative growth, nutritional status, yield and fruit quality of olive cv. Picual. J Hort Sci Ornament Plants 3:252–258. https://doi.org/10.21608/ajs.2018.34126
Hernández-Muñoz P, Almenar E, Ocio MJ, Gavara R (2006) Effect of calcium dips and chitosan coatings on postharvest life of strawberries (fragaria × ananassa). Postharvest Biol Technol 39:247–253. https://doi.org/10.1016/j.postharvbio.2005.11.006
Hosseini A, Moradinezhad F, Khayyat M, Aminifard MH (2021) Influence of foliar application of calcium nitrate and potassium nitrate on qualitative and quantitative traits of seedless barberry (berberis vulgaris L.). Erwerbs-Obstbau 63 (in Press). https://doi.org/10.1007/s10341-021-00553-x
Kanai S, Moghaieb RE, El-Shemy HA, Panigrahi R, Mohapatra PK, Ito J, Fujita K (2011) Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Sci 180:368–374. https://doi.org/10.1016/j.plantsci.2010.10.011
Kazemi M (2013) Influence of foliar application of 5-sulfosalicylic acid, malic acid, putrescine and potassium nitrate on vegetative growth and reproductive characteristics of strawberry cv. ‘Selva’. J Biol Environ Sci 7:93–101. https://doi.org/10.36632/mejas/2019.9.4.16
Kirmani SN, Wani GM, Wani MS, Ghani MY, Abid M, Muzamil S, Malik AR (2013) Effect of preharvest application of calcium chloride (CaCl2), gibberlic acid (GA3) and napthelenic acetic acid (NAA) on storage of plum (prunus salicina L.) cv. Santa Rosa under ambient storage conditions. J Sci Food Agric 8:812–818. https://doi.org/10.5897/AJAR12.1708
Lal S, Kumar D, Singh DB, Ahmed N, Kumar R, Dar GA (2011) Effect of pre-harvest application of calcium chloride and gibberellic acid on shelf-life and post-harvest quality of apricot (prunus armeniaca L.) cv. Harcot. J Hort Sci 6:46–51. https://doi.org/10.3923/pjn.2009.861.865
Langer SE, Marina M, Burgos JL, Martínez GA, Civello PM, Villarreal NM (2019) Calcium chloride treatment modifies cell wall metabolism and activates defense responses in strawberry fruit (fragaria× ananassa, Duch). J Sci Food Agric 99:4003–4010. https://doi.org/10.1002/jsfa.9626
Leibar U, Pascual I, Aizpurua A, Morales F, Unamunzaga O (2017) Grapevine nutritional status and K concentration of must under future expected climatic conditions texturally different soils. J Soil Sci Plant Nutr 17:385–397
Li W, Liu L, Wang Y, Zhang Q, Fan G, Zhang S, Liao K (2020) Genetic diversity, population structure, and relationships of apricot (prunus) based on restriction site-associated DNA sequencing. Hort Res 7:1–13. https://doi.org/10.1038/s41438-020-0284-6
Madani B, Mirshekari A, Yahia E (2016) Effect of calcium chloride treatments on calcium content, anthracnose severity and antioxidant activity in papaya fruit during ambient storage. J Sci Food Agric 96:2963–2968. https://doi.org/10.1002/jsfa.7462
Mandal G, Dhaliwal HS, Mahajan BVC (2012) Effect of pre-harvest application of NAA and potassium nitrate on storage quality of winter guava (psidium guajava). Indian J Agric Sci 82:985–989. https://doi.org/10.1007/s13197-010-0085-2
Manganaris GA, Vasilakakis M, Diamantidis G, Mignani I (2007) The effect of postharvest calcium application on tissue calcium concentration, quality attributes, incidence of flesh browning and cell wall physicochemical aspects of peach fruits. Food Chem 100:1385–1392. https://doi.org/10.1016/j.foodchem.2005.11.036
Martins V, Garcia A, Alhinho AT, Costa P, Lanceros-Méndez S, Costa MMR, Gerós H (2020) Vineyard calcium sprays induce changes in grape berry skin, firmness, cell wall composition and expression of cell wall-related genes. Plant Physiol Biochem 150:49–55. https://doi.org/10.1016/j.plaphy.2020.02.033
Medan RA (2020) Effect of foliar application of potassium and calcium on vegetative growth, yield and fruit quality of "royal" apricot trees. Plant Cell Biotechnol Mol Biol 21:106–112
Mohebbi S, Babalar M, Zamani Z, Askari MA (2020) Influence of early season boron spraying and postharvest calcium dip treatment on cell-wall degrading enzymes and fruit firmness in ‘starking delicious’ apple during storage. Sci Hortic 259:108822. https://doi.org/10.1016/j.scienta.2019.108822
Mohit ML, Thakur J (2017) Effect of different nitrogenous fertilizers on fruit quality and yield of apricot (prunus armeniaca L.). Journal of Pharmacognosy and Phytochemistry 6:217–220
Moradinezhad F, Jahani M (2019) Effect of potassium permanganate, 1-Methylcyclopropene and modified atmosphere packaging on postharvest losses and quality of fresh apricot cv. Shahroudi. J Hortic Postharvest Res 2:39–48. https://doi.org/10.22077/jhpr.2018.1207.1007
Moradinezhad F, Ghesmati M, Khayyat M (2019) Postharvest calcium salt treatment of fresh jujube fruit and its effects on biochemical characteristics and quality after cold storage. J Hortic Res 27:39–46. https://doi.org/10.2478/johr-2019-0009
Mosa W, Abd El-Megeed NA, Paszt LS (2015) The effect of the foliar application of potassium, calcium, boron and humic acid on vegetative growth, fruit set, leaf mineral, yield and fruit quality of 'Anna' apple trees. J Exp Agric Int 8:224–234. https://doi.org/10.9734/ajea/2015/16716
Muzzaffar S, Bhat MM, Wani TA, Wani IA, Masoodi FA (2018) Postharvest biology and technology of apricot. In: Mir S, Shah M, Mir M (eds) Postharvest biology and technology of temperate fruits. Springer, Cham, pp 201–222. https://doi.org/10.1007/978-3-319-76843-4_8
Ngamchuachit P, Sivertsen HK, Mitcham EJ, Barrett DM (2014) Effectiveness of calcium chloride and calcium lactate on maintenance of textural and sensory qualities of fresh-cut mangos. J Food Sci Technol 79:786–794. https://doi.org/10.1111/1750-3841.12446
Nguyen PM, Kwee EM, Niemeyer ED (2010) Potassium rate alters the antioxidant capacity and phenolic concentration of basil (ocimum basilicum L.) leaves. Food Chem 123:1235–1241. https://doi.org/10.1016/j.foodchem.2010.05.092
Nielsen SS (2010) Food analysis. Springer, New York, p 550. https://doi.org/10.1007/978-1-4419-1478-1
Niu J, Liu C, Huang M, Liu K, Yan D (2021) Effects of foliar fertilization: a review of current status and future perspectives. J Soil Sci Plant Nutr 21:104–118. https://doi.org/10.1007/s42729-020-00346-3
Ortiz A, Graell J, Lara I (2011) Cell wall-modifying enzymes and firmness loss in ripening ‘golden reinders’ apples: a comparison between calcium dips and ULO storage. Food Chem 128:1072–1079. https://doi.org/10.1016/j.foodchem.2011.04.016
Ozturk K, Ölmez HA, Colak S, Celik B (2001) Effects of potassium nitrate on cold resistance of Cataloglu apricot variety. In: XII international symposium on apricot culture and decline, vol 701, pp 713–718. https://doi.org/10.17660/actahortic.2006.701.127
Öztürk B, Ağlar E, Karakaya O, Saracoğlu O, Sefa GÜN (2019) Effects of preharvest GA3, CaCl2 and modified atmosphere packaging treatments on specific phenolic compounds of sweet cherry. Turk J Food Agric Sci 1:44–56
Prasad B, Dimri DC, Bora L (2015) Effect of pre-harvest foliar spray of calcium and potassium on fruit quality of pear cv. Pathernakh. Sci Res Essays 10:376–380. https://doi.org/10.5897/sre2015.6246
Pretel MT, Souty M, Romojaro F (2000) Use of passive and active modified atmosphere packaging to prolong the postharvest life of three varieties of apricot (prunus armeniaca, L.). Eur Food Res Technol 211:191–198. https://doi.org/10.1007/s002170050022
Rabiei V, Shirzadeh E, Sharafi Y, Mortazavi N (2010) Effects of postharvest applications of calcium nitrate and acetate on quality and shelf-life improvement of Jonagold apple fruit. J Med Plant Res 5:4912–4917. https://doi.org/10.17660/actahortic.2011.903.109
Ramezani S, Shekafandeh A (2011) Influence of Zn and K sprays on fruit and pulp growth in olive. Iran Agric Res 30:1–10
Ramezanian A, Rahemi M, Vazifehshenas MR (2009) Effects of foliar application of calcium chloride and urea on quantitative and qualitative characteristics of pomegranate fruits. Sci Hortic 121:171–175. https://doi.org/10.1016/j.scienta.2009.01.039
Ranjbar S, Rahemi M, Ramezanian A (2018) Comparison of nano-calcium and calcium chloride spray on postharvest quality and cell wall enzymes activity in apple cv. red delicious. Sci Hortic 240:57–64. https://doi.org/10.1016/j.scienta.2018.05.035
Singh S, Singh AK, Joshi HK (2005) Prolonging storability of Indian gooseberry (emblica officinalis) under semi-arid ecosystem of Gujarat. Indian J Agric Sci 75:647–650. https://doi.org/10.2212/spr.2012.1.5
Sinha A, Jawandha SK, Gill PPS, Singh H (2019) Influence of pre-harvest sprays of calcium nitrate on storability and quality attributes of plum fruits. J Food Sci Technol 56:1427–1437. https://doi.org/10.1007/s13197-019-03621-z
Solhjoo S, Gharaghani A, Fallahi E (2017) Calcium and potassium foliar sprays affect fruit skin color, quality attributes, and mineral nutrient concentrations of ‘red delicious’ apples. Int J Fruit Sci 17:358–373. https://doi.org/10.1080/15538362.2017.1318734
Soliman SS, Alebidi AI, Al-Obeed RS, Al-Saif AM (2018) Effect of potassium fertilizer on fruit quality and mineral composition of fig (ficus carica L. cv. brown Turkey). Pak J Bot 50:1753–1758
Sud G, Bhutani VP (1988) Effects of soil and foliar applications of urea on productivity and the tree nitrogen status of apricot. J Hortic Sci 63:583–585. https://doi.org/10.1080/14620316.1988.11515895
Sušin J, Kmecl V, Gregorčič A (2006) A survey of nitrate and nitrite content of fruit and vegetables grown in Slovenia during 1996–2002. Food Addit Contam 23:385–390. https://doi.org/10.1080/02652030600573715
Tzoutzoukou CG, Bouranis DL (1997) Effect of preharvest application of calcium on the postharvest physiology of apricot fruit. J Plant Nutr 20:295–309. https://doi.org/10.1080/01904169709365251
Yousefi S, Amiri ME, Mirabdulbaghi M (2015) Biochemical properties and fruit quality of "Jahangiri" (prunus armeniaca L.) apricot fruit under calcium chloride treatment. Agron Res Mold 48:81–94. https://doi.org/10.1515/cerce-2015-0055
Zeraatgar H, Davarynejad GH, Moradinezhad F, Abedi B (2018) Effect of salicylic acid and calcium nitrate spraying on qualitative properties and storability of fresh jujube fruit (ziziphus jujube mill.). Notulae Botanicae Horti Agrobotanici Cluj-Napoca 46:138–147. https://doi.org/10.15835/nbha46110743
Zhang J, LVJ, Dawuda MM, Xie J, Yu J, Li J, Gan Y (2019) Appropriate ammonium-nitrate ratio improves nutrient accumulation and fruit quality in pepper (capsicum annuum L.). Int J Agron 9:683. https://doi.org/10.3390/agronomy9110683
Availability of Data and Material
All data presented in the manuscript.
Code Availability
Not applicable.
Funding
The authors would like to acknowledge the financial support of the University of Birjand for this research under contract number of 26135/D on 12/11/1398.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The authors will follow the Ethical Responsibilities of Authors and COPE rules.
Consent to Participate
On behalf of all co-authors, I believe the participants are giving informed consent to participate in this study.
Consent for Publication
I, Farid Moradinezhad, give my consent for a submitted manuscript to be published in the Journal of Soil Science and Plant Nutrition free of charge.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moradinezhad, F., Dorostkar, M. Pre-harvest Foliar Application of Calcium Chloride and Potassium Nitrate Influences Growth and Quality of Apricot (Prunus armeniaca L.) Fruit cv. ‘Shahroudi’. J Soil Sci Plant Nutr 21, 1642–1652 (2021). https://doi.org/10.1007/s42729-021-00468-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-021-00468-2