Abstract
Plants experience oxidative stress upon exposure to heavy metals that leads to cellular damage. Plants accumulate metal ions that disturb cellular ionic homeostasis. Currently the main interest in metal accumulators lies in the field of phytoremediation when plants are used to “clean up” metal contaminants from the soil. The present study was conducted to evaluate the oxidative stress in chicory plants when subjected to different concentrations of Hg (0, 25, 50 and 75 µM). In order to study oxidative stress and Hg accumulation in chicory various morphological, biochemical and enzymatic parameters at two different stages (23 and 46 days old) were studied. The root and shoot growth, biomass accumulation declined significantly at highest Hg concentration (75 µM) and Hg accumulation was higher in roots than in shoots as indicated by translocation factor < 1. Hydrogen peroxide (H2O2) and thiobarbituric acid reactive substances content increased with increasing concentration of Hg treatments. The change in H2O2 was also revealed by in vivo histochemical detection. The osmolytes and photosynthetic pigment increased significantly up to 50 µMHg, while as decreasing slightly at higher dose (75 µM). The activities of defense enzymes viz. superoxide dismutase, catalase, ascorbate peroxidase, guaiacol peroxidase, glutathione reductase and glutathione S-transferase were found to be positively correlated with Hg-concentration. In conclusion, the chicory plants were found to possess Hg-detoxification capacity which can be recommended for remediating Hg contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution has gained momentum in the current era due to various anthropogenic and geogenic activities which affect quality of soil leading to declining the crop yield (Miransari 2011). Mercury (Hg) is one of the potent toxic elements considered as “priority hazardous substance” due to prolonged residence time and higher mobility (Singh et al. 2016). The accumulation of Hg in plants possess a great threat to agricultural sector because it interact with cellular biomolecules resulting in the oxidative stress by generating reactive oxygen species (ROS), affecting various physiological and biochemical pathways viz. retarted growth, respiration, photosynthesis, cellular development, nitrogen metabolism and water transport (Nagajyoti et al. 2010; Chang et al. 2014). Plants possess innate adaptive mechanisms to combat these oxidative stresses by stimulating antioxidative defense system and biosynthesis of osmoprotectant as shielding agents (Sewelam et al. 2016). The study of dynamic interaction among Hg and the plant systems is an essential approach because Hg has largely been used as seed disinfectant, as an ingredient of various fertilizers and herbicides and besides, the fundamental mechanism of Hg toxicity have not been clearly elucidated (Azevedo and Rodriguez 2012). Therefore, the present study was conducted to investigate the Hg toxicity using Cichorium intybus (chicory) as a model plant. Chicory is an important medicinal plant used in the complementary and alternative system of medicine besides being used as a vegetable. It is well-adapted to contaminated land, tolerant to salt and drought stress, useful for erosion control on slopes and soil building on degraded land; capable of absorbing heavy metals for phytoremediation (Aksoy 2008) and phytomining (Lamb et al. 2001). Thus, the aim of the present study was to better understand the Hg induced oxidative stress in chicory plant and accordingly to develop a convenient approach that can be utilized to measure the degree of toxicity in Hg-polluted soils.
Materials and methods
Experimental design and plant growth
Chicory seeds were procured from Hamdard University, New Delhi, India. Sterilization of healthy seeds was performed for 10 min with 5% NaOCl (w/v) followed by thorough washing with double distilled water for 1 h. Seeds were sown in plastic pots (8 cm diameter) containing ½ kg of acid washed, autoclaved sand. For the growth and development of seedlings, Hoagland nutrient medium (Hoagland and Arnon 1950) with pH 6.5 was used throughout the experiments. The different treatments (0, 25, 50 and 75 µM) of mercury chloride (HgCl2) were prepared in Hoagland nutrient medium and arranged in randomized block design with three replicates. The treatments along with nutrient medium were given at 2 days interval after 7 days of sowing (DAS). The pots along with seedlings were maintained in a growth chamber at 25 ± 2 °C with an 8-h photoperiod, light intensity 300 μmol m−2 s−1 and relative humidity of 60–70%. Primary leaves and roots of 23 and 46-days old seedlings were used for experimental purpose.
Growth analysis
Twenty seedlings (23-days old and 46-days old) were randomly taken to determine root length, shoot length and biomass accumulation for growth analysis.
Biomass accumulation (BA) and relative water content (RWC)
For biomass accumulation, carefully uprooted plants were washed with double distilled water (DDW) for removing adhered sand particles and fresh weight was immediately recorded. For determining the dry weight, the material was dried in a hot air oven at 65 °C till constant weight was achieved. The dried samples were then weighed to record the dry matter (DW) and expressed in percent change over control. The relative water content (RWC) was calculated as described by Chen et al. (2009).
Measurement of Hg content and translocation factor (TF)
Estimation of Hg was done according to the protocol of Cargnelutti et al. (2006). Root and shoot samples were oven dried at 65 °C for 72 h to remove moisture. Dried samples (0.2 g) were ground into fine powder and digested with H2SO4:HClO4 (6:1 v/v) mixtures at 85 °C till white fumes appear. The solution was filtered and diluted to 50 ml and the Hg concentration was determined by atomic absorption spectrophotometry (AAS) with a hollow cathode lamp (Perkin-Elmer Analyst 100, Waltham, MA, USA) equipped with cold vapour chamber and a sodium borohydride (NaBH4) reduction reactor (Perkin-Elmer MHS-20). TF was estimated to determine the relative translocation of metals from roots to shoot according to the Marchiol’s equation. TF < 1 and TF > 1 represent low and high capacity to translocate metals from the roots to the shoots.
Marchiol’s equation (TF = metal concentration in shoots/Metal concentration in roots).
Tolerance index (TI)
TI was calculated according to the Wilkins equation (Wilkins 1957) to measure the ability of plants to grow in the presence of high HgCl2 concentrations in the root zone.
where MLHg and MLC represented the mean length of longest roots in HgCl2 treatments and controls respectively.
Membrane stability index (MSI)
MSI was determined by estimating the leakage of electrolytes according to the method of Rodriguez-Hernandez et al. (2013).
where EC1 and EC2 are the initial and final electrical conductivity.
Hydrogen peroxide (H2O2) content measurement
H2O2 content was measured according to the method of Velikova et al. (2000). Fresh leaf tissues were homogenized in cold 0.1% (w/v) TCA followed by centrifugation at 12,000×g for 15 min at room temperature. The assay mixture contained supernatant, 10 mM potassium phosphate buffer (pH 7.0) and 1 M potassium iodide (KI). At 390 nm, absorbance of the sample mixture was recorded and the H2O2 level was measured using the extinction coefficient 0.28 µM−1 cm−1 and expressed as nmol g−1 FW.
Histo-chemical detection of H2O2
Leaf H2O2 was visualized using diaminobenzidine (DAB) as substrate according to the method described by Scarpeci et al. (2008). Thoroughly cleaned plant samples were kept in DAB-HCl solution (1 mg/ml, pH 3.8) overnight at 25 °C. The samples were then immersed in the boiling ethanol (70% v/v) for 10 min to remove the green background, which allowed the deep brown polymerization product (by reaction of DAB with H2O2) to be clearly visualized and photographed.
Determination of thiobarbituric acid reactive substances (TBARS)
Lipid peroxidation was measured in terms of 2-thiobarbituric acid (TBA) reactive substances, chiefly malondialdehyde (MDA) as described by Heath and Packer (1968) with slight modifications. The concentration of TBARS was calculated as MDA equivalents using the extinction coefficient of 155 mM−1 cm−1 for MDA, the final decomposition product of lipid peroxidation. The value obtained was used for calculations by the following formula:
“V” represents extraction volume; “W” represents weight of fresh tissue, “ϵ” represent molar extinction coefficient for MDA (ϵ =155 mM−1 cm−1).
Pigment estimation
Fresh leaf tissues (0.2 g) were homogenized in 10 ml of 80% chilled acetone under dark conditions. The chlorophyll content was determined according to the method given by Arnon (1949) followed by an additional step using 100% acetone to guarantee complete extraction process. The formula described by Duxbury and Yentsch (1956) was used to determine carotenoid content and expressed in mg/FW. Anthocyanin pigment was estimated according to Mancinelli et al. (1957).
Measurement of osmolytes
Proline content was determined after reaction with ninhydrin in acidic medium to form the chromophore following the protocol of Bates et al. (1973) with l-proline as standard. The Soluble sugar was estimated following the method of Dey (1990). The total free amino acid content was determined by the ninhydrin test according to the method of Sircelj et al. (2005) with l-leucine as a standard.
Enzyme extraction and activity
Enzyme extraction was done by homogenizing fresh leaf tissues (0.2 g) in pre-chilled mortars with a mixture of 3.0 ml extraction buffer, containing 50 mM phosphate buffer (pH 7.8), 1 mM di-sodium EDTA and 1% PVP. The sample mixture was centrifuged at 4 °C for 30 min at 12,000 rpm and the aliquot was used for the determination of protein content and activities of enzymes. The protocol of Bradford (1976) was used for protein estimation with BSA as standard.
The SOD activity was determined according to the method of Beyer and Fridovich (1987) with slight modifications. The absorbance was measured spectrophotometrically at 560 nm against blank. 50% inhibition of NBT reduction is equal to one unit of SOD activity.
The CAT activity was determined according to the method given by Aebi (1984). The CAT activity was measured using the extinction co-efficient of 0.036 mM−1 cm−1 for H2O2.
Activity of APX enzyme was measured according to the method of Nakano and Asada (1981). The enzyme activity was calculated using the extinction coefficient 2.8 mM−1 cm−1 and expressed in units/mg protein. One unit of enzyme was the amount necessary to decompose 1 μmol of substrate/min at 25 °C.
The enzyme POD (guaiacol specific) was assayed as described by Whitaker and Bernhard (1972). Formation of tetraguaiacol at 470 nm and the extinction coefficient of 26.6 mM−1 cm−1 were used for calculating POD activity. Activity of enzyme was measured as the increase of absorption units per minute per mg protein (U min−1 mg−1 protein).
Glutathione reductase (GR) assay was measured according to the method of Cakmak and Marschner (1992) by monitoring the oxidation of NADPH at 340 nm (extinction coefficient 6.2 mM−1 cm−1). A unit of activity is the amount of enzyme that catalyzes the reduction of 1 μmol of GSSG min−1 mg−1 protein.
Glutathione S-transferase (GST) activity was determined according to the method of Habig and Jacoby (1981). Enzyme activity was measured using the extinction coefficient of the conjugate 9.6 mM−1 cm−1 and expressed as units min−1 mg−1 protein.
Statistical analysis
Results were represented as arithmetic mean ± standard error (SE). All data were subjected to two-way ANOVA using GraphPad Prism 6.0 software. Tukey’s post hoc test was done to identify statistical differences between pairs of means at the 0.05 probability level. All experiments were carried out in triplicates (n = 3) except for growth parameters (root and shoot length, BA, TI and RWC, where n = 10).
Results
Growth response, biomass analysis and RWC
The effects of different Hg treatments on plant growth, biomass accumulation and RWCin C. intybusat 23-days and 46-days are presented in Table 1. The root growth was inhibited with increasing Hg treatments at both 23-days and 46-days reaching the maximum reduction of 46.12% and 15.52% respectively at 75 μM Hg level. The shoot growth showed a concomitant decrease with increase in Hg stress among both stages. However, at 23-day, maximum reduction in shoot growth observed was 29.11% at 25 μM Hg level while as it was 16.59% at 75 μM Hg in 46-days old plants. The reduction in root and shoot length at both stages were non-significant (P ≤ 0.05) among 23 and 46-days old plants. Similarly there was a non-significant (P ≤ 0.05) decrease in biomass accumulation in a dose and time dependent manner. The biomass accumulation was reduced by 20% and 10% in both 23-days and 46-days at 75 μM Hg treatment (Table 1). The RWC under Hg stress increased non-significantly with increase in Hg treatments and the maximum RWC observed was 99.78% at 75 μM Hg in 23-days. However, RWC at 46-days increased with increase in Hg concentration up to 50 μM (91.25%) and thereafter showed a slight decrease to 85.75% at 75 μM Hg dosage (Table 1).
Mercury content analysis, translocation factor and TI
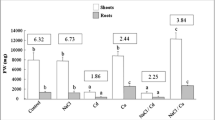
The accumulation of Hg in roots and shoots at 23- and 46-days is illustrated in Fig. 1a, b. The level of Hg accumulation in roots at 23- and 46-days of Hg treatment increased significantly in a dose-dependent manner. The maximum accumulation of Hg content noticed was 25.11 and 28.15 mg g−1 DW at 75 μM Hg in 23- and 46-days plants respectively. Similar trend was observed for accumulation of Hg in shoots and the maximum values measured were 8.11 and 14.92 mg g−1 DW at 75 μM Hg in 23-days and 46-days. Translocation factor values at 75 μM Hg in 23-days and 46-days were 0.33 and 0.53 (Table 2). Tolerance index (TI) values of both shoot and root were significantly different and decreases with increase in Hg stress at both stages. The roots exhibit the highest sensitivity to Hg (TI = 53.87%, at 23 days) at 75 μM (Table 2).
Hg content (mg/g DW) in roots (a) and shoots (b) of Cichorium intybus plants grown in the absence (control) and presence of 25, 50 and 75 µMHg for 23 and 46 days. Data represent the mean ± SE of three different experiments. Alphabetical letters denote significance (P < 0.01) in comparison with control
MSI, H2O2 content and TBARS content
MSI, H2O2 and TBARS content showed different patterns when subjected to Hg treatments. Application of Hg caused a significant increase in MSI at all Hg levels in both 23-and 46-day old plants and the maximum increase was 16.32% and 18.14% at 50 µMHg treatment (Fig. 2a). Hg toxicity resulted in a significant increase of H2O2 content in the leaves at 23-days and 46-days in a dose-dependent manner. The increase was more at 75 μM Hg concentration (89.58% and 78.06%) at 23- and 46-days plants respectively (Fig. 2b). The TBARS content initially decreased at 25 μM Hg level (19.23%) but showed a remarkable increase up to 41.66% at 75 μM Hg level (Fig. 2c). At 46-days, TBARS content increased at all concentrations in a dose-dependent manner with maximum of 93.07% at 75 μM Hg level.
Effect of Hg concentrations (0, 25, 50 and 75 µM) on relative water content (RWC %) (a), membrane stability index (MSI %) (b), hydrogen peroxide content (c) and TBARS content (d) of Cichorium intybus at 23-days and 46-days after sowing: DAS. Data represent the mean ± SE of three different experiments. Alphabetical letters denote significance (P < 0.01) in comparison with control
Hg induced changes in in situ production of H2O2
Leaves from both control (0 µM) and Hg treated samples (25, 50 and 75 µM) were dyed with 3, 3-diaminobenzidine (DAB) solution for H2O2 localization. The results show that control samples were relatively light in color compared to the Hg treated plants. However, the color intensity varied significantly with increase in the Hg concentration, indicating higher accumulation of H2O2 (Fig. 3).
Localization of H2O2 by DAB-mediated tissue printing in leaves of Cichorium intybus treated with distilled water (0 µM) or different concentrations of HgCl2 (25 µM, 50 µM and 75 µM). H2O2 accumulation is indicated by the formation of a red-brown color resulting from polymerization DAB solution in presence of peroxidase enzyme (color figure online)
Photosynthetic pigments
Hg treatment affected the chlorophyll, carotenoid and anthocyanin contents. Total-chl content did not show any significant change in response to Hg stress at both stages except at 25 μM Hg treatment in 23-day old plants where it declined to 50.20% (Fig. 4a). Similarly, carotenoid exhibited a similar trend as it did not show any significant change at both 23 days and 46-days, and the maximum increase was 39.24% and 11.09% respectively at 50 µMHg (Fig. 4b). Anthocyanin content initially at both 23 days and 46-days does not exhibit any significant change in response to Hg treatment except at 75 μM concentration in 23-days old plants where it showed a significant increase of 51.56% (Fig. 4c).
Effect of Hg concentrations on total chlorophyll content (a), carotenoid content (b) and anthocyanin content (c) of Cichorium intybus plants at 23-days and 46-days after sowing; DAS. Data represent the mean ± SE of three different experiments. Alphabetical letters denote significance (P < 0.01) in comparison with control
Effect of Hg on osmolytes and protein content
Proline acts as an osmo-protectant against osmotic disturbance in plant system caused by distinct abiotic stresses. Proline increased by 257.82% and 111.45% at 23- and 46-days respectively subjected to 75 μM Hg stress (Fig. 5a). Soluble sugar content initially showed a reduction of 10.48% at 25 μM Hg level in 23-days but thereafter it increased significantly at all concentrations and the maximum value was 62.23% at 75 μM Hg. However at 46-days, soluble sugar content increased in a concentration-dependent manner and the maximum increase observed at 50 μM Hg level was 34.78% compared to control (Fig. 5b). Free amino acid content increased linearly with Hg treatments at 23-days up to 380.64% at 75 μM Hg but at 46-days, the maximum increase was up to 54.38% at 50 μM (Fig. 5c). Under Hg stress, the total protein content of leaves followed a similar outcome at both 23- and 46-days and the maximum increase observed was 69.09% and 95.42% at 50 μM Hg respectively (Fig. 5d).
Effect of Hg concentrations on proline content (a), total soluble sugar (b), free amino acids (c) and total protein content (d) of Cichorium intybus plants at 23-days and 46-days after sowing: DAS. Data represent the mean ± SE of three different experiments. Alphabetical letters denote significance (P < 0.01) in comparison with control
Effect of Hg on the activity of enzymatic antioxidants
In the present study, SOD activity showed progressive increase with increasing Hg treatments at both 23- and 46-days. Maximum SOD activity observed was 118.26% and 100.84% at 75 μM Hg level at both stages respectively (Fig. 6a). CAT enzyme also showed linear increase in activity with increase in Hg treatments at both stages and the highest CAT activity measured was 457.14% and 120% at 23- and 46-days respectively at 75 μM Hg (Fig. 6b). With increasing Hg treatment a gradual increase in the activity of POD was observed at all concentrations with maximum POD activity observed was 466.66% and 80% at 23-and 46-days when subjected to 75 μM and 50 μM Hg concentration respectively (Fig. 6c). APX enzyme activity increased significantly with increase in Hg concentration with highest activity of 540.32% at 75 μM Hg in 23-days while as at 46-days it increased up to 838.27% at 50 μM Hg level (Fig. 6d). GR activity showed a concomitant increase with increase in Hg concentration and the maximum increase observed was 540% and 472.72% at 50 μM Hg level at both 23 and 46-days respectively (Fig. 6e). GST activity increased with increase in Hg-concentration and the maximum increase observed was 87.5% and 176.92% at 23- and 46-days respectively at 50 μM Hg level (Fig. 6f).
Effects of Hg stress on SOD (a), CAT (b), POD (c), APX (d), GR (e) and GST (f), antioxidative enzyme activities in leaf tissues of Cichorium intybus plants at 23-days and 46-days after sowing: DAS. Data represent the mean ± SE of three different experiments. Alphabetical letters denote significance (P < 0.01) in comparison with control
Discussion
Effect of Hg on plant growth, biomass accumulation and RWC
Growth inhibition is a common response to heavy metal stress and is one of the most important agricultural indices of heavy metal tolerance. In general, heavy metals inhibit growth by inducing oxidative stress due to the formation of ROS which ultimately damages the plant cell (Chen et al. 2009). In the present study the growth of chicory plants upon Hg stress was significantly affected and inhibition was slightly more in roots than shoots. The shunted growth might be due to Hg restraining aquaporins or by altering the membrane permeability (Malar et al. 2015) or due to the inhibition of mitotic index as previously reported with Cd stress in Elodea canadensis (Vecchia et al. 2005). Similar results were observed in Pterisvittata (Chen et al. 2009) and Nephrolepis exaltata and Jatropha curcas (Gao et al. 2010) grown under Hg stress. Plant biomass is a good indicator for characterizing the growth performance of heavy metal stressed plants. In our study, the biomass accumulation initially increased at low Hg treatment (25 µM) and declined at higher Hg treatment at both 23- and 46-days chicory plants. Almost similar observations were reported under Cd and Hg stress in Triticum aestivum (Sahu et al. 2012); Hg stress in Sesbania drummondii (Venkatachalam et al. 2009); J. curcas (Gao et al. 2010) and Mentha arvensis (Manikandan and Venkatachalam 2011). However, RWC at 23-days old chicory plants was slightly higher than the control plants and our results are in agreement with Malar et al. (2014) who reported same in Eichhornia crassipes under Pb stress condition. Besides, our results also revealed that RWC decreased at higher Hg treatment which might be due to closure of stomata by restrain on stomata. These results are in accordance with Hg stress in Brassica juncea (Shiyab et al. 2009) and in Pteris vittata and Nephrolepis exaltata (Chen et al. 2009).
Mercury content analysis, translocation factor and TI
The present study showed that accumulation of Hg in roots and shoots of chicory plants increased in a dose and time-dependent manner and roots were found to accumulate more Hg compared to shoots. Similar observations were reported earlier in J. curcas (Gao et al. 2010) and in Atriplex codonocarpa (Lomonte et al. 2010). Besides, TF value was found to be < 1 which indicated that large amount of Hg content was stored in the roots. The results also revealed that the tolerance index increased in a dose and time-dependent manner when chicory plants subjected to different concentrations of Hg. The metal sequestration pathway in chicory involves the production of organic acids viz., malic acid and citric acid which causes chelation in the rhizosphere thus prevents its translocation to shoot system (Hajiboland et al. 2006). It has also been reported that chicory also produces amino acids such as; nicotianamine and histidine in response to Cu stress that play an essential role to regulate complexation of Cu inside xylem sap and thus minimizes the potential damage (Welch 1995; Liao et al. 2000).
MSI, TBARS and H2O2 content
Membrane lipid peroxidation is responsible for internal membrane damage caused by several abiotic stresses including heavy metal stress (Ali et al. 2008). The present study revealed that MSI increased upon Hg stress as a result of enhanced formation of ROS which is probably due to the disruption of membrane integrity (Muradoglu et al. 2015). Moreover, MDA content increases in a concentration-dependent manner under Hg stress which induces a higher level of ROS production in chloroplasts and peroxisomes of plants (Hu et al. 2012). Increased lipid peroxidation has also been reported in Vigna radiata (Mondal et al. 2015) under Hg stress. In our study, H2O2 production increased linearly with increase in Hg treatments suggesting a regulatory role of ROS in the cross-talk of stress signaling pathways and redox metabolic signaling (Golldack et al. 2014). Increased H2O2 level in the present study might be due to the inactivation of H2O2 scavenging enzymes.
Photosynthetic pigments
Photosynthesis is probably the most important metabolic event on earth and is certainly the most important process in understanding maximum crop productivity and minimizes the side-effects of soil contamination. The photosynthetic pigments are considered as one of the most important parameters in evaluating stress and often used as biomarker (Pinheiro et al. 2013). Present study revealed that the total chlorophyll content does not exhibit any significant increase/decrease due to high Hg toxicity during both stages. The reduction in the levels of total chlorophyll content after exposure to Hg and Pb stress has been reported in many plant species viz., in Wolffia arrhiza (Piotrowska et al. 2009) and Najas indica (Singh et al. 2010). Carotenoid and anthocyanin being non-enzymatic antioxidants plays an important in quenching the ROS. Carotenoid content initially showed reduction at low levels of Hg (25 μM) during both stages. Similarly Krupa and Baszynski (1995) reported that Hg > 10 mg−l can inhibit photosynthetic pigment synthesis in plants. The decreased photosynthetic pigment content observed in our study corroborates with the reports of Zhou et al. (2007) in Medicago sativa under Hg stress. It is believed that heavy metal hyper-accumulating plants produce a higher level of photosynthetic pigment contents which might be a potential detoxification mechanism for the efficient removal of heavy metals (Chandra et al. 2009).
Osmolytes and protein content
Proline a known osmolyte accumulates in plants in response to heavy metal stress in order to combat stress conditions and impart tolerance to plants against oxidative stress (Mostofa et al. 2015). Besides, it is also known for its function in the signal transduction pathway and as a source of nitrogen during normal plant metabolism (Tie et al. 2014). Amino acids on the other hand play a pivotal role in plant primary metabolism. Since amino acid biosynthesis is connected to photosynthesis and nitrogen metabolism based on environmental characteristics that effect or changes the free amino acid synthesis (Durzan and Steward 1983). Under Hg stress, proline and free amino acid contents increased considerably at all concentrations among 23-days old chicory plants. Various earlier studies reported that proline contents significantly increased in Phaseolus vulgaris (Khadri et al. 2006), Zea mays (Yoon et al. 2005) under stress conditions. The accumulation of proline and free amino acid was concomitant with increasing stress in chicory plants which is in agreement with the results obtained by John et al. (2008) in Lemna polyrrhiza under Cd and Pb stress and Rahimi et al. (2012) in Foeniculum vulgare under Si stress. Soluble sugar is an important constituent synthesized during photosynthesis and breakdown during respiration by plants. It is believed that under heavy metal stress accumulation of sugar along with other compatible solutes contribute to an osmotic adjustment to allow the plants to minimize sufficient storage reserves to support basal metabolism under stressed environment (Smeekens 2000). In the present study, soluble sugar content showed concomitant increase with increase in Hg treatment at 23-days. However, at 46-days, soluble sugar content underwent a decrease at higher concentration of Hg (75 µM). Our results corroborate with the study in Pisum sativum under salt stress (Ahmad et al. 2008) and Phaseolus vulgaris under Pb stress (Aldoobie and Beltagi 2013). Protein is often considered as a reliable indicator of oxidative heavy metal stress in plants (Plata et al. 2009). Protein content in the present study increased at all levels of Hg treatment at both stages. The increase of protein content under heavy metal stress is possibly as a result of the induction of stress proteins which may comprise various antioxidant enzymes (Lamhamdi et al. 2010). Our results are in accordance with the previous reports of Hg stress in Cucumis sativus (Cargnelutti et al. 2006) and Cu stress in Triticum aestivum (Singh et al. 2007).
Activity of enzymatic antioxidants
HM toxicity has led to the generation of unstable ROS production which in turn leads to oxidative stress in plants and ultimately affects the crop yield (Shahid et al. 2014). To combat stress conditions, plants have developed an innate enzymatic defense pathways (Hassanein et al. 2012) which comprises of SOD, CAT, APX, GPX, GR and GST and inhibit or neutralize toxic effects of free radicals (Chen et al. 2015). SOD plays a key role in cellular defense mechanisms by converting O ·−2 to H2O2 (Alscher and Erturk 2002). In our work the results showed an increased level of SOD activity which might be due to the formation of excess O ·−2 by Hg exposure or over-expression of genes responsible to encode SOD enzyme. Similar results were reported by Pirzadah et al. (2018) in Fagopyrum tataricum under Hg toxicity. CAT plays an important role in maintaining the redox homeostasis of the cell (Das et al. 2015) and catalyses the dismutation of H2O2 into oxygen and water (Gill and Tuteja 2010). In the present study, CAT activity increased linearly in a dose and time-dependent manner during both stages. Similar findings were observed in J. curcas under Pb stress (Shu et al. 2012). POD catalyses H2O2-dependent oxidation of substrate (Hu et al. 2012) and the present study has observed an increase in POD activity with increase in concentration of Hg treatment at 23-days. However at 46-days, the POD activity declined sharply at 75 µMHg treatment. Increased POD activity upon Hg stress has been previously reported in Alfalfa (Plata et al. 2009) and Lycopersiumesculentum (Cargnelutti et al. 2006). Two major enzymes, APX and GR are involved in the ASC/GSH cycle that operates in chloroplasts, cytoplasm, mitochondria as well as in peroxisomes (del Río et al. 2006). Our results indicated that APX activity in leaves increases at 23-days and similar results were also noticed in Phaseolus aureus subjected to Pb stress (Rucinska et al. 1999) and Alfalfa under Hg and Cd stress (Plata et al. 2009). In the present study, GR activity increases in a concentration-dependent manner under Hg stress suggesting operation of ASC/GSH cycle in leaves. A similar observation was reported in Viciafaba plants grown under Cd and Pb stress (Sharifa and Abu-Muriefah 2015). In plants, GST plays a pivotal role in intracellular detoxification of obnoxious chemicals (Reddy et al. 2005). In our study, GST activity showed a positive correlation with Hg stress, indicating that it could catalyse the conjugation of Hg2+ to glutathione (GSH) or act directly as to sequester metal ions (Reddy et al. 2005). The induction of APX, GR and GST provides additional defences against Hg toxicity and keeps the metabolic activities in leaves functional and moreover the oxidative damage imposed by Hg stress is avoided with an altogether increase in activities of antioxidant enzymes.
Conclusion
In conclusion, chicory plants subjected to lower concentration of Hg did not experience any oxidative stress and can tolerate the highest concentration of 50 µM of Hg. Tolerance of chicory plants to high levels of Hg may be attributed to the plants capability to sequester the metal and to enhance the antioxidant defense mechanism. The physiological and biochemical responses of chicory plants subjected to different Hg levels can be of great significance in using chicory for remediation of heavy metal polluted sites. Results also confirmed that under high levels of Hg, chicory effectively generated the defense system mediated by enzymatic antioxidants (SOD, CAT, POD, APX, GR and GST) and non-enzymatic antioxidants (osmolytes, photosynthetic pigments) acted in a synergistic manner to scavenge excess of ROS. It was also concluded from the present study that chicory accumulates Hg more in roots than in aerial parts which is a strategy of plants for protecting their more sensitive aerial parts from the deleterious effect of metal stress.
References
Aebi, H. (1984). Catalase in Vitro. Methods in Enzymology, 105, 121–126.
Ahmad, P., Jhon, R., Sarwat, M., & Umar, S. (2008). Responses of proline, lipid peroxidation and antioxidativeenzymes in two varieties of Pisum sativum L. under salt stress. International Journal of Plant Production, 2, 353–360.
Aksoy, A. (2008). Chicory (Cichorium intybus L.): A possible biomonitor of metal pollution. Pakistan Journal of Botany, 40, 791–797.
Aldoobie, N. F., & Beltagi, M. S. (2013). Physiological, biochemical and molecular responses of common bean (Phaseolus vulgaris L.) plants to heavy metals stress. African Journal of Biotechnology, 12, 4614–4622.
Ali, B., Hayat, S., Fariduddin, Q., & Ahmad, A. (2008). 24-Epibrassinolide protects against the stress generated by salinity and nickel in Brassica juncea. Chemosphere, 72, 1387–1392.
Alscher, R. G., & Erturk, N. L. (2002). Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Environmental and Experinmental Botany, 53, 1331–1341.
Arnon, D. (1949). Copper enzymes in isolated chloroplasts, polyphenoloxidase in beta vulgaris. Plant Physiology, 24(1), 1–15.
Azevedo, R., & Rodriguez, E. (2012). Phytotoxicity of mercury in plants: A review. Journal of Botany, 848, 614–616.
Bates, L. S., Walderen, R. D., & Taere, I. D. (1973). Rapid determination of free proline for water stress studies. Plant and Soil, 39, 205–207.
Beyer, W. F., & Fridovich, I. (1987). Assaying for superoxide disniutase activity: Some large consequences of minor changes in conditions. Analytical Biochemistry, 161, 559–566.
Bradford, M. M. A. (1976). Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dyes binding. Analytical Biochemistry, 72, 248–254.
Cakmak, I., & Marschner, H. (1992). Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiology, 98, 1222–1227.
Cargnelutti, D., Tabaldi, L. A., Spanevello, R. M., Jucoski, G. O., Battisti, V., Redin, M., et al. (2006). Mercury toxicity induces oxidative stress in growing cucumber seedlings. Chemosphere, 65, 999–1006.
Chandra, R., Bharagava, R. N., Yadav, S., & Mohan, D. (2009). Accumulation and distribution of toxic metals in wheat (Triticum aestivum L.) and Indian mustard (Brassica campestris L.) irrigated with distillery and tannery effluents. Journal of Hazardeous Materials, 162, 1514–1521.
Chang, C. Y., Yu, H. Y., Chen, J. J., Li, F. B., Zhang, H. H., & Liu, C. P. (2014). Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the Pearl River Delta, South China. Environmental Monitoring and Assessment, 186, 1547–1560.
Chen, J., Shafi, M., Li, S., Wang, Y., Wu, J., Ye, Z., et al. (2015). Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Science Reporter, 5, 13554.
Chen, J., Shiyab, S., Han, F. X., Monts, D. L., Waggoner, A. W., & Su, Z. Y. (2009). Bioaccumulation and physiological effects of mercury in Pteris vittata and Nephrolepis exaltata. Ecotoxicology, 18, 110–121.
Das, P., Nutan, K. K., Singla-Pareek, S. L., & Pareek, A. (2015). Oxidative environment and redox homeostasis in plants: Dissecting out significant contribution of major cellular organelles. Frontiers of Environmental Science and Engineering, 2, 70.
del Río, L. A., Scandalio, L. M., Corpas, F. J., Palma, J. M., & Barroso, J. M. (2006). Reactive oxygen species and reactive nitrogen species in peroxisomes, production, scavenging, and role in cell signaling. Plant Physiology, 141, 330–335.
Dey, P. M. (1990). Oligosaccharides. In P. M. Dey & J. B. Harborne (Eds.), Methods in plant biochemistry carbohydrates (Vol. 2, pp. 189–218). London: Academic Press.
Durzan, D. J., & Steward, F. C. (1983). Nitrogen metabolism. In P. P. Preeti, A. K. Tripathi, D. S. Vivek, & R. G. S. Bidwell (Eds.), Plant physiology: A treatise, 1 (Vol. VIII, pp. 55–65). New York: Academic Press.
Duxbury, A. C., & Yentsch, C. S. (1956). Plankton pigment monograph. Journal of Marine Research, 15, 93–101.
Gao, S., Yang, C., Tang, L., Zhu, J., Xu, Y., Wang, S., et al. (2010). Growth and antioxidant responses in Jatropha curcas seedling exposed to mercury toxicity. Journal of Hazardeous Materials, 182, 591–597.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48, 909–930.
Golldack, D., Li, C., Mohan, H., & Probst, N. (2014). Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Frontiers in Plant Science, 5, 151.
Habig, W. H., & Jacoby, W. B. (1981). Glutathione S-transferases (rats and humans). Methods of Enzymology, 77, 218–231.
Hajiboland, R., Niknam, V., Ebrahim-Zadeh, H., & Mozafari, A. (2006). Uptake, transport and chelation of Cu and Zn at toxic levels in tolerant and sensitive species from North West of Iran. Journal of Sciences, Islamic Republic of Iran, 17(3), 203–214.
Hassanein, R. A., Hashem, H. A., & Khalil, R. R. (2012). Stigmasterol treatment increases salt stress tolerance of faba bean plants by enhancing antioxidant systems. Plant Osmics Journal, 5, 476–485.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125, 189–198.
Hoagland, D. R., & Arnon, D. I. (1950). The water-culture for growing plants without soil. California Agricultural Experiment Station Circular, 347(32) (Rev).
Hu, R., Sunc, K., Suc, X., Pana, Y., Zhanga, Y., & Wanga, X. (2012). Physiological responses and tolerance mechanisms to Pb in two xerophils: Salsolapasserina Bunge and Chenopodium album L. Journal of Hazardeous Materials, 205–206, 131–138.
John, R., Ahmad, P., Gadgil, K., & Sharma, S. (2008). Effect of cadmium and lead on growth, biochemical parameters and uptake in Lemna polyrrhiza L. Plant Soil and Environment, 54, 262–270.
Khadri, M., Tejera, N. A., & Lluch, C. (2006). Alleviation of salt stress in common bean (Phaseolus vulgaris) by exogenous abscisic acid supply. Journal of Plant Growth and Regulation, 25, 110–119.
Krupa, Z., & Baszyński, T. (1995). Some aspects of heavy metals toxicity towards photosynthetic apparatus direct and indirect effects on light and dark reactions. Acta Physiology of Plant, 17, 177–190.
Lamb, A. E., Anderson, C. W. N., & Haverkamp, R. G. (2001). The induced accumulation of gold in the plants Brassica juncea, Berkheyacoddii and chicory. Chemistry in New Zeeland, 1, 34–36.
Lamhamdi, M., Bakrim, A., Aarab, A., Lafont, R., & Sayah, F. (2010). A comparison of lead toxicity using physiological and enzymatic parameters on spinach (Spinaciaoleracea) and wheat (Triticumaestivum) growth. Moroccan Journal of Biology, 6–7, 64–73.
Liao, M. T., Hedley, M. J., Wooly, D. J., Brooks, R. R., & Nichols, M. A. (2000). Copper uptake and translocation in chicory (Cichorium intybus L. cv. Grasslands Puna) and tomato (Lycopersicon esculentum Mill. cv. Rondy) plants grown in NFT system II. The role of nicotianamine and histidine in xylem sap copper transport. Plant and Soil, 223, 243–252.
Lomonte, C., Sgherri, C., Baker, A. J. M., Kolev, S. D., & Navari-Izzo, F. (2010). Antioxidative response of Atriplex codonocarpa to mercury. Environmental and Experinmental Botany, 69, 9–16.
Malar, S., Sahi, S. V., Favas, P. J. C., & Venkatachalam, P. (2015). Assessment of mercury heavy metal toxicity-induced physiochemical and molecular changes in Sesbania grandiflora L. International Journal of Environmental Science and Technology, 12, 3273–3282.
Malar, S., Vikram, S. S., Favas, P. J. C., & Perumal, V. (2014). Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths (Eichhorniacrassipes (Mart.). Botanical Studies, 55, 54.
Mancinelli, A. L., Huang-Yang, C. P., Lindquist, P., Anderson, O., & Rabino, I. (1957). Photo-control of anthocyanin synthesis, III. The action of streptomycin on the synthesis of chlorophyll and anthocyanin. Plant Physiology, 55, 251–257.
Manikandan, R., & Venkatachalam, P. (2011). Risk assessment of mercury ion heavy metal exposure on physiological and biochemical changes and DNA damage using RAPD analysis in Mentha arvensis seedlings. Plant Cell Biotechnology and Molecular Biology, 12, 41–50.
Miransari, M. (2011). Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metal. Biotechnology Advances, 29, 645–653.
Mondal, N. K., Das, C., & Datta, J. K. (2015). Effect of mercury on seedling growth, nodulation and ultra-structural deformation of Vigna radiate (L) Wilczek. Environmental Monitoring and Assessment, 187, 241.
Mostofa, M. G., Hossain, M. A., Fujita, M., & Tran, L. S. P. (2015). Physiological and biochemical mechanisms associated with trehalose-induced stress tolerance in rice. Science Reporter, 5, 11433.
Muradoglu, F., Gundogdu, M., Ercisli, S., Encu, T., Balta, F., Jaafar, H. Z. E., et al. (2015). Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biology Research, 48, 1–11.
Nagajyoti, P. C., Lee, K. D., & Sreekanth, T. M. V. (2010). Heavy metals, occurrence and toxicity for plants: A review. Environmental Chemistry Letters, 8, 199–216.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiology, 22, 867–880.
Pinheiro, J. N., Marques, C., Pinto, G., Mestiri, A., Mendo, S., Gomes, N. C., et al. (2013). The performance of Fraxinus angustifolia as a helper for metal phytoremediation programs and its relation to the endophytic bacterial communities. Geoderma, 202–203, 171–182.
Piotrowska, A., Bajguz, A., Godlewska, B., Czerpak, R., & Kaminska, M., (2009). Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lamnaceae). Environmental and Experinmental Botany, 66, 507–513.
Pirzadah, T. B., Malik, B., Tahir, T., Irfan, Q. M., & Rehman, R. U. (2018). Characterization of mercury-induced stress biomarkers in Fagopyrum tataricum plants. International Journal of Phytoremediation, 20(3), 225–236.
Plata, J. S., Villasante, C. O., Flores-Caceres, M. L., Escobar, C., del Campo, F. F., & Hernandez, L. E. (2009). Differential alterations of antioxidant defenses as bio-indicators of mercury and cadmium toxicity in Alfalfa. Chemosphere, 77, 946–954.
Rahimi, R., Mohammakhani, A., Roohi, V., & Armand, N. (2012). Effects of salt stress and silicon nutrition on cholorophyll content, yield and yield components in fennel (Foeniculumvulgar Mill.). International Journal of Agriculture Crop Science, 4, 1591–1595.
Reddy, A. M., Kumar, S. G., Jyothsnakumari, G., Thimmanaik, S., & Sudhakar, C. (2005). Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bangal gram (Cicer arietinum L.). Chemosphere, 60, 97–104.
Rodriguez-Hernandez, M. D., Moreno, D. A., Carvajal, M., & Ballesta, M. D. M. (2013). Interactive effects of boron and NaCl stress on water and nutrient transport in two broccoli cultivars. Functional Plant Biology, 40, 739–748.
Rucinska, R., Waplak, S., & Gwozoz, E. A. (1999). Free radical formation and activity of antioxidant enzymes in lupin roots exposed to lead. Plant Physiology and Biochemistry, 37, 187–194.
Sahu, G. K., Upadhyay, S., & Sahoo, B. B. (2012). Mercury induced phytotoxicity and oxidative stress in wheat (Triticum aestivum L.) plants. Physiology and Molecular Biology of Plants, 18, 21–31.
Scarpeci, T. E., Zanor, M. I., Crrillo, N., Mueller-Roeber, B., & Valle, E. M. (2008). Generation of superoxide anion in chloroplast of Arabidopsis thaliana during active photosynthesis: A focus on rapidly induced. Plant and Molecular Biology, 66, 361–378.
Shahid, M., Pourrut, B., Dumat, C., Nadeem, M., Aslam, M., & Pinelli, E. (2014). Heavy-metal induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Reviews of Environmental Contamination and Toxicology, 232, 1–44.
Sharifa, S., & Abu-Muriefah. (2015). Effects of Silicon on membrane characteristics, photosynthetic pigments, antioxidative ability, and mineral element contents of faba bean (Vicia faba L.) plants grown under Cd and Pb stress. International Journal of Advanced Research in Biological Sciences, 2, 1–17.
Shiyab, J., Chen, J., Han, F. X., Monts, D. L., Matta, F. B., Gu, M., et al. (2009). Phytotoxicity of mercury in Indian mustard (Brassica juncea L.). Ecotoxicology and Environmental Safety, 72, 619–625.
Shu, X., Yin, L. Y., Zhang, Q. F., & Wang, W. B. (2012). Effect of Pb toxicity on leaf growth, antioxidant enzyme activities, and photosynthesis in cuttings and seedlings of Jatropha curcas L. Environmental Science and Pollution Research, 19, 893–902.
Singh, D., Nath, K., & Sharma, Y. K. (2007). Response of wheat seed germination and seedling growth under copper stress. Journal of Environmental Biology, 28, 409–414.
Singh, S., Parihar, P., Singh, R., Singh, V. P., & Prasad, S. M. (2016). Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Frontier Plant Science, 6, 1143.
Singh, R., Tripathi, R. D., Dwivedi, S., Kumar, A., Trivedi, P. K., & Chakrabarty, D. (2010). Lead bioaccumulation potential of an aquatic macropyte Najas indica are related to antioxidant system. Bioresources Technology, 101, 3025–3032.
Sircelj, H., Tausz, M., Grill, D., & Batic, F. (2005). Biochemical responses in leaves of two apple tree cultivars subjected to progressing drought. Journal of Plant Physiology, 162, 1308–1318.
Smeekens, S. (2000). Sugar-induced signal transduction in plants. Annual Review of Plant Biology, 51, 49–81.
Sewelam, N., Kazan, K., & Schenk, P.M. (2016) Global plant stress signalling: Reactive oxygen species at the cross-road. Frontiers in Plant Science, 7, 187. https://doi.org/10.3389/fpls.2016.00187.
Tie, S. G., Tang, Z. J., Zhao, Y. M., & Li, W. (2014). Oxidative damage and antioxidant response caused by excess copper in leaves of maize. African Journal of Biotechnology, 11, 4378–4384.
Vecchia, F. D., Larocca, N., Moro, I., Defaveri, S., Andreoli, C., & Rascio, N. (2005). Morphogenetic, ultra structural and physiological damages suffered by submerged leaves of Elodea canadensis exposed to cadmium. Plant Science, 168, 329–338.
Velikova, V., Yordanov, I., & Edreva, A. (2000). Oxidative stress and some antioxidant system in acid rain treated bean plants: Protective role of exogenous polyamines. Plant Science, 51, 59–66.
Venkatachalam, P., Srivastava, A. K., Raghothama, K. G., & Sahi, S. V. (2009). Genes induced in response to mercury-ion-exposure in heavy metal hyper accumulator Sesbania drummondii. Environmental Science and Technology, 43, 843–850.
Welch, R. M. (1995). Micronutrient nutrition of plants. Critical Reviews in Plant Science, 14, 49–82.
Whitaker, J. R., & Bernhard, R. A. (1972). Experiments for an introduction to enzymology. Davis, CA: The Whiber Press.
Wilkins, D. A. A. (1957). Technique for the measurement of Pb tolerance in plants. Nature, 180, 37–38.
Yoon, B. S., Jin, C. J., Un, P. S., & Cho, D. H. (2005). Change in photosynthesis, proline content, and osmotic potential of Corn seedling under high saline condition. Korean Journal of Crop Science, 50, 28–31.
Zhou, Z. S., Huang, S. Q., Guo, K., Mehta, S. K., Zhang, P. C., & Yang, Z. M. (2007). Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. Journal of Inorganic Biochemistry, 101, 1–9.
Acknowledgements
We are thankful to Prof. A. Hemantaranjan for going through the manuscript, providing valuable inputs and suggestions.
Funding
RUR is thankful to the University of Kashmir for seed grant money.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict on interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malik, B., Pirzadah, T.B., Tahir, I. et al. Growth and physiological responses in chicory towards mercury induced in vitro oxidative stress. Plant Physiol. Rep. 24, 236–248 (2019). https://doi.org/10.1007/s40502-019-00442-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-019-00442-2