Abstract
Flower opening depends on light–dark periods in diurnal plants, while this role of light in nocturnal plants is elusive. To elucidate this, an experiment with sprays of Oenothera glazioviana was carried out. Sprays were divided into 3 sets. First set of sprays was kept in the laboratory with alternate light and dark period (Control), second set of sprays was kept under continuous light and third set of sprays was kept under continuous darkness held in distilled water or sucrose (0.025 M) along with CoCl2 (0.01 mM). Flowers opened normally in all treatments but the sprays kept under continuous darkness showed maximum flower longevity, floral diameter, % blooms, soluble proteins and sugar fractions as compared to control which instead maintained an increased membrane stability index besides a decrease in lipid peroxidation. The sprays under continuous light showed increased α-amino acid and total phenolic content. The addition of sucrose augmented flower opening even under continuous light or darkness suggesting that light is not an obligatory requirement for flower opening in Oenothera glazioviana.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The process of flower opening is a genetically regulated programme which determines the timing of flower opening and closing between and among the genera (Xu et al. 2007). In case of Oenothera glazioviana flowers open in the afternoon, while as in Oenothera rosea opening occurs in early morning (Wagner et al. 2013). Flower opening is a rapid phenomenon making it an interesting physiological process. In many species, flower opening is accompanied by a high rate of cell expansion rather than simple growth movements. On the basis of mechanics, several categories of flower opening have been distinguished (van Doorn and Meeteren 2003; van Doorn and Kamdee 2014). Depending upon the species, flower opening may be related to the development of tissue adjacent to flower, e.g. during opening in Oenothera species the sepals open in a zipper like fashion. Besides this, the opening process depends on the petal movements, as it involves either abscission of covering parts, growth of pedicel or combination of the both.
In most species, the flower opening shows close relationship with the time of day, while in some species it is hardly influenced by the external regulation i.e. it occurs at any time of the day. Morning opening is often correlated with an increase in temperature and light intensity, besides a decrease in ambient humidity. The reverse is the case with night blooming flowers such as O. lamarkiana. However, many species such as Ipomoea purpurea and Portulaca, are unaffected by relative humidity (Hensel 1905; van Doorn and Meeteren 2003).
Temperature has a well known effect on flower opening. The effect of temperature on flower opening may be due the influence on a rapid growth process (as in spring blooming flowers) or a stimulation of basal growth rate. Besides temperature, light also influences the flower opening in several flowers especially that bloom during the day. Light also regulates the flower opening in some night blooming species. However, inhibition of flower opening is caused by light in O. lamarckiana (Saito and Yamaki 1967; van Doorn and Meeteren 2003; van Doorn and Kamdee 2014).
Sugar status has been found to have an important role in flower opening and closing. Flower opening and closing is determined by the combination of sugar uptake and degradation of reserve carbohydrates. Increasing concentration of soluble sugars in Gladiolus and Freesia florets has been shown to be associated with better opening with increased fresh mass and floral diameter (van Doorn and Kamdee 2014). In most of the species, the flower opening is accompanied with import of sucrose or mobilization of storage carbohydrates (van Doorn and Meeteren 2003). In Medelon roses, the increased concentration of reserve carbohydrates in petals tissue was associated with increased floral diameter and fresh mass (van Doorn et al. 1991; van Doorn and Meeteren 2003; van Doorn and Kamdee 2014). As such flower opening involves complex intervention of many factors along with temperature, light (intensity and duration), darkness and relative humidity (Sigmond 1929; Allard and Garner 1941; Bonde 1955; Bruce 1960; Tukey and Ketellapper 1963; van Doorn 1997; Yamada et al. 2006; van Doorn and Kamdee 2014).
In most species, flower closure responds to external factors in the similar way to opening, while in some species the closure is determined by endogenous rhythm. The presence of endogenous rhythm of opening and closing is tested by keeping the flowers in constant darkness or constant light. The rapid opening and light inhibition of flower opening makes Oenothera an interesting model for the studies related to flower opening and closing. It is in this perspective that the present study was undertaken to assess the possible role of light–dark periodicity along with sugars on flower opening in O. glazioviana.
Materials and methods
Experimental set up
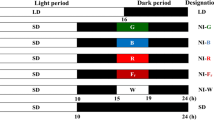
Uniform and healthy sprays of O. glazioviana growing in the Kashmir University Botanic Garden (KUBG) were used for the study. The sprays were harvested at 4 P.M. with their oldest bud at optimal harvest maturity stage (one day before anthesis). The sprays were immediately brought to the laboratory in buckets containing distilled water, defoliated partially and cut to a uniform length of 25 cm. The sprays were divided into 3 sets. One set of sprays was kept as control under laboratory conditions (RT = 23 ± 2 °C). The second set of sprays was kept under continuous light (CL). Set 3rd of sprays was kept under continuous darkness (CD). Each set was further divided into two subsets one held in distilled water and other held in 0.025 M SUC + CoCl2 (0.01 mM). All these replicates were kept in 250 ml flasks containing 80 ml of vase solution. For each test solution, there were 5 replicates, with each flask containing two sprays. The volume of holding solution absorbed per spray was also recorded at periodic intervals. Flower longevity was regarded as terminated when visible signs of senescence like petal inrolling and increased turgidity commenced.
Floral diameter
Floral diameter was recorded at periodic intervals during the experiment and was taken as mean of two perpendicular measurements of the flower.
Fresh mass, dry mass and water content
Fresh and dry mass was recorded on day 2 and 6 after transfer of sprays to holding solutions. For the determination of fresh mass (FM), 10 flowers were taken at fully open stage. These flowers were oven dried at 70 °C for 48 h in paper bags and then put in a desiccator for 48 h before recording their dry mass (DM).
The water content (WC) was measured as difference between fresh and dry mass as
Number of blooms per spray
Number of blooms was recorded regularly by counting the number of buds bloomed on each spray on each day and averaged by dividing the total number of buds bloomed on all the sprays in the treatment on a particular day by the number of sprays receiving the particular treatment. The number of buds that failed to open and aborted was also recorded. Total number of buds on each spray was counted to express the data on percent basis.
Protein estimation
For protein estimation, 1 g petal tissue was macerated in 100 mM (pH 7.2) phosphate buffer containing 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 10% glycerol, 10% polyvinyl pyrrolidone (PVP) and 1 mM Dithiothreitol (DTT). The mixture was centrifuged at 12,000 rpm at 4 °C in a refrigerated centrifuge for 15 min. Soluble proteins were estimated in a suitable aliquot from the supernatant by the method of Lowry et al. (1951).
Membrane stability index (%MSI)
MSI of the petal tissue was recorded at day 2 and 6. 500 mg petal tissue taken from the open flowers was submerged in test tube containing 25 ml deionized water. After incubation for 5 h, the conductivity (C1) of the solution was measured using conductivity meter (Elico). The petals were then boiled for 30 min. and the conductivity (C2) was again measured (Sairam et al. 1997).
Estimation of α-amino acids, Phenols and Sugar fractions
α-amino acids were estimated by the method of Rosen (1957) using glycine as standard. Total phenols were estimated by the method of Swain and Hillis (1959) using gallic acid as standard. Reducing sugars were estimated by the method of Nelson (1944) using d-glucose as standard. Total sugars were estimated as reducing sugars by enzymatic conversion of non-reducing sugars to reducing sugars. Non-reducing sugars were calculated as the difference between total and reducing sugars.
Lipid peroxidation
Lipid peroxidation was determined by the method of Heath and Packer (1968). Petal tissue (250 mg) was macerated in 7.5 ml of 0.1% trichloroacetic acid (TCA) and centrifuged at 15,000×g for 10 min. under refrigeration. One ml of supernatant was taken and mixed with 4 ml of 0.5% TBA diluted in TCA (20%). The reaction was started by incubating the mixture at 95 °C in water bath for 25 min. Reaction was terminated by placing the test tubes in ice. Absorbance was recorded at 532 and 600 nm. Nonspecific absorbance at 600 nm was subtracted from the value at 532 nm. The TBARS (thiobarbituric acid reactive substances) content was calculated using its absorption coefficient (ɛ) of 155 nmol/g fm.
Statistical analysis of the data
Difference between various treatments has been evaluated by simple analysis of variance and least significant difference (LSD) computed at P0.05 using MINITAB (v15.1.2-EQUINOX_Softddl.net) software.
Results and discussion
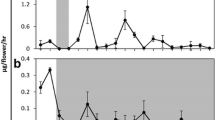
The flowers of O. glazioviana normally open in the late afternoon and senesce rapidly on exposure to sun light in next morning. However, the flower opening time varies from 5:45 PM to 7:00 PM with peak at 6:30 PM. During the present investigation the flowers on the sprays kept under continuous darkness showed hastened flower maturation which was reflected by their opening even during the day time as compared to the flowers from the sprays conditioned at alternate light dark period, where opening occurred only during late afternoon hours. However, the delayed opening was registered in the flowers on the sprays kept under continuous light. The signs of senescence varied in all the treatments. Increased water content in the flowers resulted in flowers stored in the darkness (Fig. 1). Maximum flower longevity, fresh mass, water content, floral diameter and percent blooms were recorded in the samples from flowers kept under continuous darkness as compared to flowers kept as control (alternate light–dark periodicity) (Figs. 2, 3 and 4; Table 1). Our results are in conformity with the earlier studies on Arabidopsis (Redei et al. 1974; Araki and Kameda 1993). Arabidopsis plants have been shown to develop to the differentiation of flowers in darkness under the medium supplemented with sucrose. The flower opening is inhibited by light in O. lamarckiana suggesting the negative role of light in dark blooming flowers (Saito and Yamaki 1967). However, the day opening in case of flowers on the sprays kept under constant conditions (continuous light and darkness) was registered as compared to control. This could be attributed to the fact that constant conditions result in loss of circadian rhythmicity (Hicks et al. 1996; Doyle et al. 2002). Besides extended exposure, light is strongly correlated with increased size of stomata hence increased rate of transpiration and consequently decreased blooming. It has been reported that diurnal rhythm of cut flower opening was abolished when exposed to constant light (Horibe and Yamada 2014). Cobalt chloride was used along with SUC to inhibit vascular blockage, besides maintaining a high water flow rate through stems, leading to significant water uptake (Aslmoshtaghi et al. 2014).
Maximum values of MSI were recorded in the samples from flowers kept as control and transferred to SUC as compared to MSI values of the tissue samples from the sprays kept under CD and CL. The MSI showed a sharp decline with the progression in time from day 2 (D2) to 6 (D6) in all the tissue samples irrespective of treatments (Fig. 5). Decline in MSI values represent a change in membrane permeability leading to solute leakage (Dwivedi, et al. 2016). This can be attributed to the increased membrane stability in the flowers kept as control which resulted in preventing the leakage of proteases from vacuole into the cytoplasm (Fukuchi-Mizutani 2000). Lipid peroxidation (expressed as TBARS content) showed an increase with the progression in time from D2 to D6, irrespective of treatments. However, maximum lipid peroxidation was registered in the tissue samples from the flowers kept under CD and transferred to DW (Fig. 6). The solute leakage from the petal tissue is a result of change in membrane permeability which in turn is the consequence of change in membrane stability. A sharp decline was registered in the sprays kept under CL with the concomitant increase in lipid peroxidation.
During the present investigation, maximum soluble protein content was found in the samples from sprays kept under CD followed by samples from sprays kept as control and least protein content was registered in the sprays stored under CL. However, maximum α-amino acids were recorded in flowers kept under CL. But the content of both proteins and α-amino acids remained more or less constant as flower development progressed from D2 to D6. This can be attributed to elevated activity of proteases involving a cascade of catabolic processes leading to degradation of macromolecules into smaller ones that resulted in elevated levels of α-amino acids in the flowers kept under CL (Trobacher et al. 2006; Xu et al. 2007, Dar et al. 2014).
Maximum phenolic content was registered in the tissue samples from CD treated flowers held in DW followed by CL treated flowers held in DW as compared to flowers kept as control. The total phenolic content increased with the progression in time from D2 to D6 (Table 1). Our results corroborate with the earlier findings on Nerine sarniensis, Rosa hybrid and Tithonia rotundifolia, where increased content of phenolics was associated with improved postharvest life as phenols have been suggested to play an important role in antioxidant defense by scavenging the free radicals and preventing the flowers from oxidative stress (Mwangi et al. 2003; Schmitzer et al. 2010; Ahmad and Tahir 2016). Higher content of total and reducing sugars was registered in the flowers kept under CD irrespective of vase solution. Total and reducing sugar content decreased significantly with the progression in time from D2 to D6 (Table 1). Maximum non-reducing sugar content was registered in the samples from the sprays of the set A. The non-reducing sugar content showed a significant decrease with the progression in time from D2 to D6 (Table 1). Our results suggest that these sugars might have accumulated in the vacuoles of petal tissues due to the stoppage of mobilization in the flowers kept under CL and CD or this might have resulted from reduced respiration rates and limited export. Our results are in contrary to the results obtained for Solanum lycopersicum in which reduction in carbohydrate levels was observed in dark grown seedlings due to induced turnover of sucrose and starch. However, during CL starch degrading enzymes could be inhibited or down regulated resulting in the accumulation of higher sugar fraction in these flowers (Zeeman et al. 2007).
Our study suggests that higher content of sugars registered in the tissue samples from the sprays kept under CD and CL might play a pivotal role as potential ROS scavenger to avert the effect of continuous light on the sprays kept under CL and mimic the effect of continuous darkness on the sprays kept under CD. Couee et al. (2006) reported that glucose feeding of the Oxidative Pentose Phosphate (OPP) pathway can enhance NADPH production, which is a major cofactor of ROS scavenging pathways. Thus, the blocking of photoperiodic control could shift the flowering in O. glazioviana to an unknown autonomous mechanism.
Conclusions
Flower opening in many flowers is regulated by either light/length of the dark period or temperature. However, the effect of rise in temperature has been shown to be higher in the morning than in the evening in some day blooming flowers and a similar time of day effect was found in the response to light of night blooming flowers held in darkness, making the process of flower opening a complex physiological process. Although some interplay between circadian rhythm and environmental factors is now known but the molecular mechanisms governing the process of flower opening are still elusive. During the present study increased blooming, fresh mass and water content in the sprays kept under CD clearly indicate the photo-inhibition of opening by light. Moreover, increase in the solute leakage with the concomitant increase in lipid peroxidation was registered in flowers kept under CL. We conclude that sugars have an important role by either acting as respiratory substrates or mimicking the effect of light in the sprays of O. glazioviana kept under CD.
References
Ahmad, S. S., & Tahir, I. (2016). Storage protocol for improving the postharvest performance in cut scapes of Iris versicolor. Acta Horticulturae, 1060, 71–79.
Allard, H. A., & Garner, W. W. (1941). Responses of some plants to equal & unequal ratios of light and darkness in cycles ranging from 1 hour to 72 hours. Journal of Agricultural Research, 63, 305–330.
Araki, T., & Komeda, Y. (1993). Flowering in darkness in Arabidopsis thaliana. The Plant Journal, 4(5), 801–811.
Aslmoshtaghi, E., Jafari, M., & Rahemi, M. (2014). Effects of Daffodil flowers and cobalt chloride on vase life of cut Rose. Journal of Chemical Health Risks, 4(2), 1–6.
Bonde, E. K. (1955). The effect of various cycles of light & darkness on the growth of tomato & cocklebur plants. Physiologia Plantarum, 8, 913–923.
Bruce, V. G. (1960). Environmental entrainment of circadian rhythms. Cold Spring Harbor Symposium, 25, 29–48.
Couee, I., Cecile, S., Gwenola, G., & Abdelhak, A. (2006). Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. Journal of Experimental Botany, 57(3), 449–459.
Dar, R. A., Tahir, I., & Ahmad, S. S. (2014). Sugars & sugar alcohol have their say in the regulation of flower senescence in Dianthus chinensis L. Scientia Horticulturae, 174, 24–28.
Doyle, M. R., Davis, S. J., Bastow, R. M., McWatters, H. G., Kozma-Bognar, L., Nagy, F., et al. (2002). The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature, 419, 74–77.
Dwivedi, S. K., Ajay, A., Virendra, P. S., Sairama, R. R., & Bhattacharya, C. (2016). Effect of sodium nitroprusside on differential activity of antioxidants and expression of SAGs in relation to vase life of gladiolus cut flowers. Scientia Horticulturae, (pp. 158–165).
Fukuchi-Mizutani, M., Ishiguro, K., Nakayama, T., Utsunomiya, Y., Tanaka, Y., Kusumi, T., et al. (2000). Molecular and functional characterization of a rose lipoxygenase cDNA related to flower senescence. Plant Science, 160, 129–137.
Heath, R. L., & Packer, L. (1968). Photo peroxidation in isolated chloroplasts-Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125, 189–198.
Hensel, E. P. (1905). On the movements of petals. Nebraska University Studies, 5, 191–228.
Hicks, K. A., Millar, A. J., Carre, I. A., Somers, D. E., Straume, M., Meeks-Wagner, D. R., et al. (1996). Conditional circadian dysfunction of the Arabidopsis early-flowering 3 mutant. Science, 274, 790–792.
Horibe, T., & Yamada, K. (2014). Petals of cut rose flower show diurnal rhythmic growth. Journal of the Japanese Society for Horticultural Science, 83(4), 302–307.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193(1), 265–275.
Mwangi, M., Chatterjee, S. R., & Bhattacharjee, S. K. (2003). Changes in the biochemical constituents of ‘‘Golden gate’’ cut rose petals as affected by precooling with ice cold water spray, pulsing and packaging. Journal of Plant Biology, 30, 95–97.
Nelson, N. A. (1944). Photometric adaptation of the Somogyi method for the determination of glucose. Journal of Biological Chemistry, 153, 375–380.
Redei, G. P., Acedo, G., & Gavazzi, G. (1974). Flower differentiation in Arabidopsis. Stadler Symposium, 6, 135–168.
Rosen, H. A. (1957). Modified ninhydrin colorimetric analysis for amino acids. Archives of Biochemistry and Biophysics, 67(1), 10–15.
Sairam, R. K., Deshmukh, D. S., & Shukla, D. S. (1997). Tolerance to drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. Journal of Agronomy and Crop Science, 178, 171–177.
Saito, M., & Yamaki, T. (1967). Retardation of flower opening in Oenothera lamarckiana caused by blue and green light. Nature, 214, 1027.
Schmitzer, V., Veberic, R., Osterc, G., & Stampar, F. (2010). Color and phenolic content changes during flower development in ground cover rose. Journal of American Society of Horticultural Sciences, 135(3), 195–202.
Sigmond, H. (1929). About the blossoming of Hedera helix L. and the influence of this process by the light. Booklets of the Botanisches Zentralblatt, 46, 68–92.
Swain, T., & Hillis, W. E. (1959). The phenolic constituents of Prunus domestica I. The quantitative analysis of phenolic constituents. Journal of the Science of Food and Agriculture, 10, 63–68.
Trobacher, C. P., Senatore, A., & Greenwood, J. S. (2006). Masterminds or minions? Cysteine proteinases in plant programmed cell death. Canadian Journal of Botany, 84, 651–667.
Tukey, H. B., & Ketellapper, H. J., Jr. (1963). Length of the light-dark cycle and plant growth. American Journal of Botany, 50, 110–115.
van Doorn, W. G. (1997). Effects of pollination on floral attraction and longevity. Review article. Journal of Experimental Botany, 48, 1615–1622.
van Doorn, W. G., Groenewegen, G., van de Pol, P. A., & Berkholst, E. M. (1991). Effects of carbohydrate and water status on flower opening of cut Madelon roses. Postharvest Biology and Technology, 1, 47–57.
van Doorn, W. G., & Kamdee, C. (2014). Flower opening and closure: An update. Journal of Experimental Botany, 65(20), 5749–5757.
van Doorn, W. G., & van Meeteren, U. (2003). Flower opening and closure: A review. Journal of Experimental Botany, 54, 1801–1812.
Wagner, W.L., Kyra, N., Krakos, P., & Hoch, C. (2013). Taxonomic changes in Oenothera sections Gaura and Calylophus (Onagraceae). PhytoKeys, (pp. 61–72).
Xu, X., Gookin, T., Jiang, C. Z., & Reid, M. (2007). Genes associated with opening and senescence of Mirabilis jalapa flowers. Journal of Experimental Botany, 58(8), 2193–2201.
Yamada, T., Ichimura, K., & van Doorn, W. G. (2006). DNA degradation and nuclear degeneration during programmed cell death in petals of Antirrhinum, Argyranthemum, and Petunia. Journal of Experimental Botany, 57, 3543–3552.
Zeeman, S. C., Delatte, T., Messerli, G., Umhang, M., Stettler, M., & Mettler, T. (2007). Starch breakdown: Recent discoveries suggest distinct pathways and novel mechanisms. Functional Plant Biology, 34, 465–473.
Acknowledgement
The authors thank Head Department of Botany, University of Kashmir for providing the necessary facilities during the course of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Dar, R.A., Tahir, I., Nisar, S. et al. Is light dark periodicity an obligate requirement for flower opening in Oenothera glazioviana Micheli: a nocturnal flower. Ind J Plant Physiol. 23, 467–473 (2018). https://doi.org/10.1007/s40502-018-0382-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-018-0382-8