Abstract
We investigated the effects of the quality of light used for interrupting the night period, termed the night interruption light (NIL), on morphogenesis, flowering, and the expression of photoperiodic genes in chrysanthemum (Dendranthema grandiflorum) cultivar ‘Gaya Yellow’, a qualitative short-day (SD) plant. Plants were raised in a closed-type plant factory under white (W) light-emitting diodes (LEDs) providing a light intensity of 180 μmol m−2 s−1 photosynthetic photon flux density, under a condition of long-day (LD, 16 h light/8 h dark), short-day (SD, 10 h light/14 h dark), or SD with a 4-h night interruption (NI) provided by 10 μmol m−2 s−1 PPF green (NI-G), blue (NI-B), red (NI-R), far-red (NI-Fr), or W (NI-W) LEDs. Plants grown in the LD condition were the tallest. The SD, NI-B, and NI-Fr conditions induced flowering. Phytochrome A (phyA) and cryptochrome 1 (cry1) were expressed at high levels in plants in NI-B, NI-Fr, and SD conditions. These results suggest that the NIL quality has significant implications on morphogenesis, flowering, and the expression of photoperiodic genes. Flowering was positively affected by the expression of phyA, cry1, and FLOWERING LOCUS T (FT), whereas it was negatively affected by the expression of phyB and anti-florigenic FT/TFL1 (AFT).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Light manipulation is a critical factor to optimizing photosynthesis, and is an important signal for photoperiodic and photomorphogenic responses in plants (Gautam et al. 2015). It is well established that light characteristics affect the photomorphogenesis of plants (Fukuda et al. 2016). The different photosynthetic pigments and photoreceptors of a plant determine its ability to sense and respond to light (Gautam et al. 2015). Phytochromes, the red (R)/far-red (Fr) light receptors, and cryptochromes, the blue (B)/ultraviolet-A light receptors, are the primary photosensory receptors of vascular plants (Kendrick and Kronenberg 1994). Phytochrome A (phyA) promotes flowering under Fr light (Mockler et al. 2003). On the other hand, phytochrome B (phyB), phytochrome D (phyD), and phytochrome E (phyE) act in a partially redundant manner to inhibit flowering under R light (Devlin et al. 1998).
In some plants, the photoperiod may be manipulated to artificially maintain plants in the vegetative stage or induce flowering (Warner and Erwin 2003). Studies on flowering have mainly examined the influence of light duration, intensity, timing, and quality using night interruption (NI, night break) lighting in greenhouses under natural light (Park et al. 2016a, b). Introducing a NI with light during short-day (SD) seasons promotes the flowering of long-day (LD) plants and allows for an accelerated marketing or seed production. Delaying the flowering of SD plants under natural LD conditions can be achieved in a similar manner (Hamamoto et al. 2005; Kang et al. 2008; Park et al. 2017). An intensity of 1–2 μmol m−2 s−1 photosynthetic photon flux density (PPFD) provided by conventional broad-spectrum light sources during the NI is usually adequate to control flowering (Whitman et al. 1998).
Different species display varying flowering responses to the light quality (Higuchi et al. 2012). A NI with R or white (W) light induced flowering of the LD plant petunia (Petunia hybrida) (Park et al. 2016a, b). A NI with Fr light delayed flowering of the day neutral plant geranium (Pelargonium × hortorum) (Park et al. 2017). A NI with a combination of B and R lights (B:R = 1:1) was more effective in promoting flowering of the LD plant cyclamen (Cyclamen persicum) than a NI with B, R, or Fr light alone (Shin et al. 2010). A NI with B and R lights (B:R = 1:1), and R light alone inhibited the flowering of chrysanthemum (Ho et al. 2012). Our previous study (Park et al. 2015) focused on splitting the traditional 4-h NI period into two periods of 2 h each and shifting the night interruption light (NIL) quality to observe its effect on flowering and morphogenesis in chrysanthemum (Dendranthema grandiflorum). The light quality (B, R, Fr, and W) of the first 2 h of the NI exposure affected neither morphogenesis nor flowering, while the light quality (B, R, Fr, and W) of the last 2 h of NI exposure significantly affected both morphogenesis and flowering (Park et al. 2015). Higuchi et al. (2012) also reported the effect of the NIL quality, such as B, R, and Fr, on flowering of chrysanthemum. However, the light intensity of B, R, and Fr they used was quite high: 39.1, 55.3, and 62.5 μmol m−2 s−1 PPF, respectively (Higuchi et al. 2012). This study aimed to investigate the effect of the NIL quality of low intensity on morphogenesis, flowering, and the expression of photoreceptor genes in chrysanthemum cultivar ‘Gaya Yellow’. We hypothesized that the NIL quality, a very low intensity (10 μmol m−2 s−1 PPF) of B, G, R, Fr, or W given for a continuous period of 4 h, would also affect morphogenesis and flowering.
2 Materials and methods
2.1 Plant materials and growth conditions
Cuttings of potted chrysanthemum (Dendranthema grandiflorum ‘Gaya Yellow’, a qualitative SD plant) were stuck in 50-cell plug trays that contained a commercial medium (Tosilee Medium, Shinan Grow Co., Jinju, Korea) and stationed on a glasshouse bench to root. Twelve days after sticking, the rooted cuttings were relocated to a closed walk-in growth chamber. After 12 days of acclimatization in the growth chamber, the plants, approximately 7.9 cm tall, were subjected to the photoperiodic light treatments described below. The growth chamber was maintained at 20 ± 1 °C, 60 ± 10% relative humidity, and 140 ± 20 μmol m−2 s−1 PPF provided by fluorescent lamps (F48T12-CW-VHO, Philips Co Ltd., Eindhoven, the Netherlands). The photoperiodic treatments were provided by an LED system installed 25 cm above the plant canopy. A greenhouse multipurpose nutrient solution (Park et al. 2015, 2016a, b, 2017) was used to fertigate the plants once a day throughout the experiment.
2.2 Photoperiodic light treatments
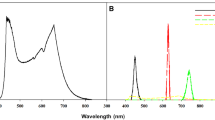
Chrysanthemum is a qualitative SD plant, which means that it will not produce flowers when the daylength is longer than a critical value that is cultivar-dependent. Flowering is inhibited when the required long-night phase is interrupted by a short period of exposure to light. The critical daylength required for flowering in the SD plant used throughout this study was 12 h, and therefore, an uninterrupted dark period longer than 12 h was sufficient to initiate flowering. The plants were grown under W LEDs with a light intensity of 180 μmol m−2 s−1 PPF (MEF50120, More Electronics Co. Ltd., Changwon, Korea). The different photoperiods except the lighted period provided by the NI (mentioned as ‘photoperiod’ hereafter) were as follows: LD (16 h light/8 h dark), SD (10 h light/14 h dark), or SD with a 4-h NI (from 11:00 p.m. to 3:00 a.m.) with a light intensity of 10 μmol m−2 s−1 PPF provided by LEDs. The LD and uninterrupted SD conditions were used as controls. NIL qualities were B (NI-B, 450 nm), G (NI-G, 530 nm), R (NI-R, 660 nm), Fr (NI-Fr, 730 nm), or W (NI-W, 400-700 nm, with 28% B, 37% R, and 15% Fr) (Fig. 1). A spectroradiometer (USB 2000 Fiber Optic Spectrometer, Ocean Optics Inc., Dunedin, FL, USA) scanned the spectral distributions of all photoperiodic treatments at a fixed distance of 25 cm above the bench top at 1-nm intervals. For each photoperiodic treatment, measurements of the spectral distribution and the average maximum absolute irradiance were taken at three locations of the plant growing bench.
A representation of the night interruption light (NIL) quality by light emitting diodes (LEDs) during the 4-h night interruptions (NI) of the 10-h short-day (SD) treatments in Dendranthema grandiflorum ‘Gaya Yellow’: NI-G, green; NI-B, blue; NI-R, red; NI-Fr, far-red; and NI-W, white. LD indicates the 16-h long-day treatment. (Color figure online)
2.3 Data collection and statistical analysis
After 46 days of photoperiodic treatments, the plant height, number of leaves per plant, chlorophyll content, dry mass, fresh and dry weights of the shoot and root, time from treatment initiation to visible flower bud or to visible buds (DVB), number of flowers and flower buds per plant (hereafter referred to as “number of flowers”), and expression of important photoreceptor genes were measured. Ten mg samples of fresh, fully developed young leaves were taken and extracted using 80% ice cold acetone for chlorophyll estimation. Following a 3000-rpm centrifugation, a spectrophotometer (Biochrom Libra S22, Biochrom Co. Ltd., MA, USA) was used to measure the absorbance of the supernatant at 663 and 645 nm. The method of Dere et al. (1998) was used for calculations. To measure dry weights, the shoot and root were dried in an oven (Model FO-450 M, Jeio Technology Co. Ltd., Seoul, Korea) at 75 °C for 3 days and weighed.
A randomized complete block design was employed in this experiment using 3 replications with 2 plants in each. To minimize the effects of changing treatment locations, the treatment locations were randomly mixed between replications in a controlled environment. The statistical significance of the collected data was analyzed using SAS (Statistical Analysis System, V. 9.1, Cary, NC, USA). Tukey’s studentized range test at p < 0.05 was used to assess the differences among the treatment means. Sigma Plot 10.0 (Systat Software, Inc., San Jose, CA, USA) was used for graphing.
2.4 Total RNA isolation, cDNA synthesis, and quantitative polymerase chain reaction (PCR) of selected genes
Independent PCRs with equal amounts of cDNA were performed using primers of phytochrome A (phyA), phytochrome B (phyB), cryptochrome 1 (cry1), Anti-florigenic FT/TFL1 family protein (AFT), and FLOWERING LOCUS T (FT) genes of the sequence from Arabidopsis thaliana (Table 1). The methods described in Park et al. (2015) were used for total RNA isolation and semi-quantitative reverse transcription PCR analysis of selected genes.
3 Results
3.1 Morphogenesis
Plants grown under LD and all NI treatments were taller than those grown in the SD condition (Fig. 2a). The greatest plant height was observed in the NI-R treatment (18.2 cm), followed by the NI-Fr treatment (17.7 cm) for the NI treatments. Dry mass was the greatest for plants in the LD treatment (Fig. 2b). Among the NI treatments, the dry mass was the greatest for plants in the NI-W treatment (1.938 g) due to the relatively higher growth rate of both the shoot and root. The dry mass of plants in the NI-G (1.535 g), NI-B (1.679 g), and SD (1.239 g) treatments were similar. The weights of the shoot and root, both dry and fresh, showed a similar trend with the relative growth rate (Park 2014). The number of leaves per plant was 93% higher for plants grown in the LD treatment compared to that for plants grown in the SD treatment (Fig. 2c), and was the lowest for plants grown in the NI-Fr (82.6) and SD (80.3) treatments. The leaf area showed a similar tendency to the number of leaves per plant (Fig. 2d). Plants grown in the NI-W treatment (370.0 cm2) showed the greatest leaf area, followed by plants in the NI-G treatment (355.6 cm2), and then by plants in the NI-R treatment (353.5 cm2). The chlorophyll content was the greatest for plants grown in the SD control (Fig. 2e) and was lower for plants in all NI treatments, especially NI-Fr. The NI-R treatment was an exception; plants displayed similar chlorophyll levels as plants in the SD control. The chlorophyll content was 60% lower for plants grown in the NI-Fr treatment compared to those grown in the SD treatment.
The effects of the night interruption light (NIL) quality on the a plant height, b dry mass, c number of leaves per plant, d leaf area, and e chlorophyll content in Dendranthema grandiflorum ‘Gaya Yellow’ (see Fig. 1 for details of the light quality of the NI). Vertical bars indicate ± S.E. of the means for n = 3. Means accompanied by different letters are significantly different (p < 0.05) according to the Tukey’s studentized range test
3.2 Flowering
The NI-B, NI-Fr, and SD treatments induced complete flowering (Fig. 3 and Table 2). The DVB was higher for plants grown in the NI-B and NI-Fr treatments than that of plants in the SD control (Table 2). The number of flowers per plant was 31% higher for plants in the NI-Fr treatment than that of plants in the SD control (Table 2). The difference in the number of flowers per plant was insignificant between plants in the NI-B treatment and in the SD control (Table 2).
The effects of the night interruption light (NIL) quality provided at 10 μmol m−2 s−1 PPF on the flowering of Dendranthema grandiflorum ‘Gaya Yellow’ measured 46 days after treatment: a side view and b top view (see Fig. 1 for the details of the light quality)
3.3 Photoreceptor gene expression analysis
The expression of phyA was lower for plants in the LD, NI-G, and NI-W treatments than for plants in the SD control (Fig. 4a). phyA expression was more pronounced for plants in the NI-B, NI-R, and NI-Fr treatments compared to that for plants in the SD control (Fig. 4a). The expression of phyB was the greatest for plants in the NI-R treatment (Fig. 4b). Cry1 expression was the highest for plants in the NI-B treatment followed by the SD control (Fig. 4c). The expression of FT and AFT was the highest for plants in the NI-Fr treatment (Fig. 4d, e).
The effects of the night interruption light (NIL) quality provided at 10 μmol m−2 s−1 PPF on the relative gene expression of aphyA, bphyB, ccry1, dFT, and eAFT of Dendranthema grandiflorum ‘Gaya Yellow’ (see Fig. 1 for details of the light quality of the NI). Vertical bars indicate ± S.E. of the means for n = 3. Means accompanied by different letters are significantly different (p < 0.05) according to the Tukey’s studentized range test
4 Discussion
4.1 Plant height
For all of the NI treatments, the NI-R treatment produced the tallest plants (Fig. 2a). These results agree with results reported by Kim et al. (2004), where the lengths of the stem and the internode were the greatest when chrysanthemum was exposed to R and R + Fr lights during the photoperiod. However, the effects of R light on stem elongation were inconsistent. Heo et al. (2002) found that R light during the photoperiod stunted stem elongation in marigold (Tagetes sp.); this difference may be attributed to different synergistic interactions of the phytochromes acting to inhibit stem elongation. A high level of phytochrome photo-equilibrium (Φ = PFr/P total ratio: ratio of PFr to total phytochrome) during the photoperiod hindered chrysanthemum growth. The results of the current study suggest that plant growth responses differ by the quality of light given during the photoperiod and by the quality of light given during the NI.
4.2 Leaf growth
The NI-W treatment promoted overall vegetative growth, indicated by increases in the dry mass, number of leaves per plant, and leaf area over the other NI treatments (Fig. 2b–d), even at low intensities. The W LEDs may be more efficient for photosynthesis than LEDs with just one or two narrow bandwidth wavelengths (e.g. G, B, R, or a combination of B and R), since W LEDs have the advantage of having a broad spectrum more similar to the sunlight (Park et al. 2012). The chlorophyll content was lower for plants in the NI-Fr treatment than for plants in the SD control (Fig. 2e). Similar results were reported by Li and Kubota (2009) who described that a “dilution” effect by increased biomass led to a decline in phytochemicals, such as chlorophyll and carotenoid, accompanying supplemental Fr during the photoperiod, since an increase in the dry weight was shown for plants grown under a combination of W and Fr lights.
4.3 Flowering and photoreceptor gene expression
In petunia (a LD plant), flowering was observed in the LD, NI-G, NI-Fr, and NI-W treatments (Park et al. 2016a, b). Petunia plants under a 6-h day extension and 4-h NI generally flowered earlier than under 2-h NI (Oh and Runkle 2016). In geranium (a day neutral plant), flowering was not affected by the quality of the NIL, and all plants flowered in the SD, LD, NI-B, NI-G, NI-Fr, and NI-W treatments (Park et al. 2017). In chrysanthemum (a SD plant), previous studies have reported that flowering was induced by NI-B, NI-Fr, and SD treatments (Stack et al. 1998; Higuchi et al. 2012; Jeong et al. 2012), which is similar to our findings in this study. The mutant hy4-101 alleles of Arabidopsis in the Columbia ecotype background caused delayed flowering in both SD and LD conditions with either day extensions or NI, and a NI-B treatment had a stronger effect than NI-W or NI-R treatments (Bagnall et al. 1996). In our study, the NI-B treatment induced flowering with high-level expressions of phyA, cry1, and FT (which are thought to be flowering promoter genes), possibly due to lower levels of the flowering inhibitor genes phyB and AFT (Fig. 4). phyA promotes flowering by Fr light (Johnson et al. 1994; Mockler et al. 2003). phyB mediates the NI-induced inhibition of flowering (Higuchi et al. 2013). Cryptochromes induce flowering, and the function of cryptochromes in floral induction has been observed using mutations in cry1 and cry2 (Mockler et al. 1999). In addition, FT and TFL-like genes are crucial to the integration of both exogenous and endogenous signals that control flowering (Ferrier et al. 2011). AFT systemically inhibits flowering and is central to the obligate photoperiodic response (Higuchi et al. 2013). The flowering that was observed in the NI-Fr treatment despite high levels of phyB and AFT expression may be due to the high expression level of FT observed in that treatment (Fig. 4b, d, e). The high level of phyB expression observed in the NI-G, NI-W, and NI-R treatments explains the lack of flowering in those environments (Fig. 4b). The DVB observed for plants in the NI-Fr and NI-B treatments in this study was prolonged by 1 day and 4 days, respectively. The number of flowers per plant was higher for plants in the NI-Fr treatment than for plants in the NI-B and SD treatments. Similarly, Park et al. (2015) reported that a shifting of light from B to Fr during the NI resulted in a higher number of flowers per plant, which may be due to a high light energy induction and shade avoidance response, a behavior marked by darkness evasion by shoots via lengthening of the internodes. The early flowering of phyB mutants of Arabidopsis in SD conditions seems to reflect the constitutive shade avoidance phenotype (Franklin 2008; Franklin and Quail 2010). The overall expression patterns of photomorphogenic genes observed in this study differed from those described in previous studies (Higuchi et al. 2012, 2013). Possible explanations for this disparity are differences in the chrysanthemum genotypes used, the experimental environment, the sample collection time, the plant part from which the samples were collected, and the specific wavelengths of LEDs used in both studies.
In summary, the NI-G, NI-B, NI-R, and NI-W treatments promoted plant growth and leaf expansion. The NI-Fr treatment promoted plant growth, decreased the chlorophyll content, and suppressed leaf expansion. In conclusion, morphogenesis, flowering, and the expression of photoperiodic genes were highly affected by the NIL quality. Flowering was positively affected by the expression of phyA, cry1, and FT, while it was negatively affected by the expression of phyB and AFT. One possible practical application of this technique in the production of floricultural crops, either in plant factories or greenhouses, would be the inhibition of flowering of SD plants by using the R and W lights as the NIL. Further studies on the effect of the intensity and positioning of B light are needed to understand its effect on flowering control of SD plants.
References
Bagnall DJ, King RW, Hangarter RP (1996) Blue-light promotion of flowering is absent in hy4 mutants of Arabidopsis. Planta 200:278–280
Dere S, Gunes T, Sivaci R (1998) Spectrophotometric determination of chlorophyll-a, b and total carotenoid contents of some algae species using different solvents. Turk J Bot 22:13–17
Devlin PF, Patel SR, Whitelam GC (1998) Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell 10:1479–1487
Ferrier T, Matus JT, Jin J, Riechmann JL (2011) Arabidopsis paves the way: genomic and network analyses in crops. Curr Opin Biotechnol 22:260–270
Franklin KA (2008) Shade avoidance. New Phytol 179:930–944
Franklin KA, Quail PH (2010) Phytochrome functions in Arabidopsis development. J Exp Bot 61:11–24
Fukuda N, Ajima C, Yukawa T, Olsen JE (2016) Antagonistic action of blue and red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environ Exp Bot 121:102–111
Gautam P, Terfa MT, Olsen JE, Torre S (2015) Red and blue light effects on morphology and flowering of Petunia × hybrida. Sci Hortic 184:171–178
Hamamoto H, Hideo S, Tadahisa H (2005) Budding response of horticultural crops to night break with red light on alternate days. Environ Control Biol 43:21–27
Heo JW, Lee CW, Chakrabarty D, Paek KY (2002) Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a light emitting diode (LED). Plant Growth Regul 38:225–230
Higuchi YK, Sumitomo K, Oda A, Shimizu H, Hisamatsu T (2012) Days light quality affects the night-break response in the short-day plant chrysanthemum, suggesting differential phytochrome-mediated regulation of flowering. J Plant Physiol 169:1789–1796
Higuchi Y, Narumi T, Oda A, Nakano Y, Sumitomo K, Fukai S, Hisamatsu T (2013) The gated induction system of a systemic floral inhibitor, antiflorigen, determines obligate short-day flowering in chrysanthemums. Proc Natl Acad Sci USA 110:17137–17142
Ho CH, Yang CM, Hsiao CL (2012) Effects of nighttime lighting with specific wavebands on flowering and flower quality of chrysanthemum. Crop Environ Bioinform 9:265–277
Jeong SW, Park S, Jin JS, Seo O, Kim GS, Kim YH, Bae H, Lee G, Kim ST, Lee WS, Shin SC (2012) Influences of four different light-emitting diode lights on flowering and polyphenol variations in the leaves of chrysanthemum. J Agric Food Chem 60:9793–9800
Johnson E, Bradley M, Harberd NP, Whitelam GC (1994) Photoresponses of light-grown phyA mutants of Arabidopsis (phytochrome A is required for the perception of day length extensions). Plant Physiol 105:141–149
Kang KJ, Oh W, Shin JH, Kim KS (2008) Night interruption and cyclic lighting promote flowering of Cyclamen persicum under low temperature regime. Hortic Environ Biotechnol 49:72–77
Kendrick RE, Kronenberg GHM (1994) Photomorphogenesis in plants, 2nd edn. Kluwer Academic Publishers, Dordrecht
Kim HH, Goins GD, Wheeler RM, Sager JC (2004) Green-light supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes. Hortic Sci 39:1617–1622
Li Q, Kubota C (2009) Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ Exp Bot 67:59–64
Mockler TC, Guo H, Yang H, Duong H, Lin C (1999) Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development 126:2073–2082
Mockler T, Yang H, Yu W, Parikh D, Cheng Y, Dolan S, Lin C (2003) Regulation of photoperiodic flowering by Arabidopsis photoreceptors. Proc Natl Acad Sci USA 100:2140–2145
Oh W, Runkle ES (2016) Flowering and morphological responses of petunia and pansy as influenced by lamp type and lighting period to provide long days. Korean J Hortic Sci Technol 34:207–219
Park YG (2014) Flowering and morphogenesis of photoperiodic floricultural plants in response to quality and lighting position of night interruption light. Ph.D. Thesis. Gyeongsang National University. http://academic.naver.com/article.naver?doc_id=80088464. Accessed 25 August 2014
Park YG, Park JE, Hwang SJ, Jeong BR (2012) Light source and CO2 concentration affect growth and anthocyanin content of lettuce under controlled environment. Hortic Environ Biotechnol 53:460–466
Park YG, Muneer S, Jeong BR (2015) Morphogenesis, flowering, and gene expression of Dendranthema grandiflorum in response to shift in light quality of night interruption. Int J Mol Sci 16:16497–16513
Park IS, Cho KJ, Kim J, Cho JY, Lim TJ, Oh W (2016a) Growth and flowering responses of petunia to various artificial light sources with different light qualities. Korean J Hortic Sci Technol 34:55–66
Park YG, Muneer S, Soundararajan P, Manivannan A, Jeong BR (2016b) Light quality during night interruption affects morphogenesis and flowering in Petunia hybrida, a qualitative long-day plant. Hortic Environ Biotechnol 57:371–377
Park YG, Muneer S, Soundararajan P, Manivannan A, Jeong BR (2017) Light quality during night interruption affects morphogenesis and flowering in geranium. Hortic Environ Biotechnol 58:212–217
Shin JH, Jung HH, Kim KS (2010) Night interruption using light emitting diodes (LEDs) promotes flowering of Cyclamen persicum in winter cultivation. Hortic Environ Biotechnol 51:391–395
Stack PA, Drummond FA, Stack LB (1998) Chrysanthemum flowering in a blue light-supplemented long day maintained for biocontrol of thrips. HortScience 33:710–715
Warner RM, Erwin JE (2003) Effect of photoperiod and daily light integral on flowering of five Hibiscus sp. Sci Hortic 97:341–351
Whitman CM, Heins RD, Cameron AC, Carlson WH (1998) Lamp type and irradiance level for daylength extensions influence flowering of Campanula carpatica ‘Blue Clips’, Coreopsis grandiflora ‘Early Sunrise’, and Coreopsis verticillata ‘Moonbeam’. J Am Soc Hortic Sci 123:802–807
Acknowledgements
This work was supported by the BK21 Plus program, at Gyeongsang National University, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, Y.G., Jeong, B.R. Night interruption light quality changes morphogenesis, flowering, and gene expression in Dendranthema grandiflorum. Hortic. Environ. Biotechnol. 60, 167–173 (2019). https://doi.org/10.1007/s13580-018-0114-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-018-0114-z