Abstract
Increasing episodes of high temperature (HT) is becoming a serious threat to rice production throughout the world. Studies on the response of popular rice varieties to HT stress in natural field conditions are very limited. Hence, we tested six popular rice varieties for their response to HT stress imposed by late sowing under field conditions. Effect of heat stress was evaluated based on the following traits: flag leaf chlorophyll content (SPAD), flag leaf temperature, net photosynthetic rate (P N ), stomatal conductance (gs), spikelet fertility and yield per plant (YPP). High temperature stress decreased overall spikelet fertility by 16% and yield per plant by 29%. Based on the heat susceptibility index (HSI) of all the traits, Nagina22 and a restorer line KMR3 were identified as highly tolerant and a high yielding mega variety BPT5204 and a drought tolerant cultivar Vandana were identified as highly susceptible to heat stress. Further, HSI values for SPAD, P N and YPP showed significant correlation indicating that these traits are highly useful in screening genotypes for HT stress in late sown conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is consumed by about 3 billion people in the world and considered as the major source of calories. Increasing rice productivity is essential to keep pace with the rapidly growing world population. The changes in the global climatic conditions in the past few years have become major constraint for rice production. The earth’s climate has warmed by approximately 0.6 °C over the past 100 years and expected to rise up to 4.8 °C by the end of 2100 (IPCC 2013). Furthermore, the present temperatures in most of the rice growing regions are already above optimum for rice growth. Any further increase in episodes of high temperatures during sensitive stages of growth may reduce rice yields drastically. Such yield losses were already experienced by many rice cultivating areas in Asia and parts of western Africa due to frequent heat stress events (Laborte et al. 2012).
Reproductive and grain filing stages are highly susceptible to high temperature in rice. Extremely high temperature, even for a few hours, during flowering can cause complete sterility, while high temperature during grain filling can lead to reduced seed set or grain weight (Yoshida et al. 1981; Matsui et al. 2001; Prasad et al. 2006; Jagadish et al. 2008). A series of phenotypic studies have been conducted to identify rice genotypes tolerant to high temperature at reproductive stage in controlled conditions (Ishimaru et al. 2010; Jagadish et al. 2010; Ye et al. 2011) but most of the genotypes tested are either not grown currently or restricted to narrow geographic limits. However, there are a few reports on the response of popular mega rice varieties to high temperature (Ziska et al. 1996; Prasad et al. 2006; Shi et al. 2014), but these studies were conducted in temperature controlled growth chambers where heat stress was imposed only for few hours or days unlike natural environmental conditions. Hence, it is necessary to test the response of popular rice varieties to high temperature in natural field conditions for development of heat tolerant rice genotypes. Studies performed in natural field conditions are more reliable as compared to simulated growth chambers experiments (Hall 2011). It is possible to evaluate a large number of genotypes in the field for high temperature tolerance by altering sowing dates or growing them in hot regions such as Hyderabad. The objective of the present study was to identify the morphological and physiological response of popular rice varieties to high temperature stress during reproductive stage in natural field conditions.
Materials and methods
Experiments were conducted at Indian Institute of Rice Research (IIRR), Hyderabad, India (Latitude −17.3°N, Longitude 78.40°E and Altitude 524 MSL) during dry season 2012 (December 2011–May 2012). Weather data was obtained from automatic weather station installed at IIRR field.
Plant material and experimental design
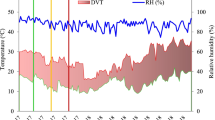
Six popular rice varieties BPT5204, IR64, KMR3, N22 (Nagina 22), Swarna (MTU 7029) and Vandana were evaluated for heat tolerance in this study. The importance of each variety, its parents and year of release is given in Table 1. One set of lines was sown during normal time of sowing 20 December 2012 considered as non-heat stress control. Another set of the same lines was sown after 40 days (30 January 2013) and considered as high temperature stress set. All the lines were transplanted 25 days after sowing with three replications. Plants were transplanted with a spacing of 20 × 15 cm in two rows and each row had 20 plants. Mean daytime/nighttime temperatures and relative humidity throughout the experiment is given in Supplementary Fig. 1.
Phenotyping and data analysis
Lines were evaluated for heat tolerance based on leaf chlorophyll content (greening index), leaf temperature, net photosynthetic rate, stomatal conductance, spikelet fertility and yield traits. Days to 50% flowering and days to maturity were noted for each variety. Accumulated heat degree days (AHDD) were calculated for each line from sowing to harvesting date in both normal and late sown sets according to d’Alpoim Guedes et al. (2015) and Shi et al. (2015a). The base temperature was considered as 10 °C. Greening index was measured by using soil plant analysis development (SPAD) meter (Minolta chlorophyll meter, SPAD-502) which is an indirect measure of chlorophyll content in the flag leaf. Readings were taken at 3 different positions on the flag leaf and averaged (Ristic et al. 2007). Flag leaf temperature was measured using hand held infra red thermometer with FOV 12:1 (Fischer Scientific, USA) during bright sunny day between 11.00 h and 13.00 h. Net photosynthetic rate and stomatal conductance was recorded on the same leaf during anthesis using portable photosynthesis measuring system (LI6400XT, LI-COR Environmental, USA). Spikelet fertility was calculated as ratio of filled spikelets to total number of spikelets and expressed in percentage. Total panicles from each plant were hand threshed and seed weight was noted for single plant yield data. Heat susceptibility index (HSI) for all the traits was calculated according to Fischer and Maurer (1978) and genotypes were ranked based on the HSI value. Lines with HSI value ≤0.5 are considered as tolerant, ≤1.0 but more than 0.5 are considered as moderately tolerant and lines with >1.0 are considered as susceptible. Two way analysis of variance (ANOVA) was performed using Statistix 8.0 software to determine the significance of variation for all the traits. Statistical significance of the parameter means were determined using Fisher’s LSD test. Correlation analysis was performed for HSI values of all traits.
Results and discussion
The mean day/night temperature from sowing to harvest in the normal sown set was 33.6 ± 0.9/16.1 ± 0.4 °C and mean relative humidity was 71.6 ± 1.3/28.8 ± 1%. In the late sown set, the mean day/night time temperature from sowing to harvest was 36.9 ± 0.8/20.5 ± 0.5 °C and mean relative humidity was 64.3 ± 1.4/25.7 ± 1.1%. Overall, the late sown set was exposed to >3 °C day temperature and >4 °C night temperature compared to normal sown set. Significant differences in SPAD, net photosynthetic rate, spikelet fertility and yield per plant were observed among varieties, between treatments and interaction of variety x treatment. There was a significant difference in leaf temperature only between treatments but not among varieties or interaction of variety x treatment. Stomatal conductance showed significant difference only among the varieties but not between treatment and treatment x variety interaction (Supplementary Table 1).
Effect of high temperature on days to 50% flowering and days to maturity
In general, genotypes delayed their flowering and maturity in late sown condition as compared to normal sown condition (Table 2). Days to flowering (DFF) were delayed from 7 days in Vandana to 23 days in Swarna and days to maturity were delayed from 4 days in IR64 to 22 days in Swarna. The maximum delay in flowering and maturity was in Swarna followed by BPT5204 (15 and 16 days) and KMR3 (14 and 12 days). Among the medium early duration varieties (N22, Vandana and IR64), minimum delay was observed in Vandana (4 and 6 days) followed by N22 (8 and 6 days) and IR64 (11 and 4 days). The mean day length (sun-shine hours) from sowing to maturity in normal sown was 8.8 h and in late sown 9.0 h which is a non-significant difference. Hence the delay in flowering in present experiment could be attributed to HT rather than photoperiod. The accumulated heat degree days (AHDD) was calculated for each variety from sowing to DFF and DFF to maturity in both control and late sown conditions (Table 3) to determine the mean exposure of plants to temperature. Late sown set was exposed to ~648 more AHDD as compared to normal set from sowing to DFF. Swarna (~905), BPT5204 (~757) and KMR3 (~749) were exposed to more AHDD as compared to IR64 (~540), N22 (~506) and Vandana (~429) as their flowering was delayed to June when the temperature gets reduced. Hence, the AHDD from DFF to maturity was comparatively less in BPT5204, Swarna and KMR3. IR64, N22 and Vandana were exposed to more AHDD from anthesis to maturity. There is no significant difference observed in number of days from DFF to maturity in both normal and late sets except in IR64. IR64 matured seven days earlier in late sown conditions compared to normal sown. These results indicate that flowering time is the most adaptive trait and then grain filling and maturity period. Rang et al. 2011 also reported delay in flowering in 5 rice genotypes but under artificial heat stress conditions. The high level of variation in flowering time is one of the key factors in rice breeding which allows it to adapt to different climate conditions (Tanaka et al. 2013).
Effect of high temperature on greening index and leaf temperature
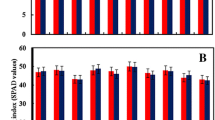
Greening index measured using SPAD meter is an indirect estimator of chlorophyll content in leaves (Netto et al. 2005). A strong relationship between total chlorophyll content and SPAD readings has been reported in several plants (Yadava 1986; Netto et al. 2005). Significant decrease (6–14%) in greening index was observed in BPT5204, Swarna, KMR3 and Vandana. N22 showed 0.2% increase under stress, whereas in IR64 it decreased by 4%, but both were not significant (Fig. 1A). It is well established that high temperature shows negative effect on chlorophyll content (Pradhan et al. 2012; Djanaguiraman et al. 2011). Initially high temperature causes structural alterations in chloroplast protein complexes and reduced activity of enzymes (Ahmad et al. 2010). It also increases the activity of chlorophyllase (Sharkey and Zhang. 2010) and formation of reactive oxygen species (ROS) which results in the loss of photosynthetic pigments (Camejo et al. 2006, Panigrahy et al. 2011). The loss is directly associated with damage of thylakoid membranes (Ristic et al. 2007), lipid peroxidation of chloroplast membranes (Djanaguiraman et al. 2011), and also inhibition of chlorophyll biosynthesis (Sun and Guo 2016).
Effect of early sown and late sown environments on six physiological traits among six popular rice cultivars. A greening index, B leaf temperature (°C), C net photosynthetic rate, D stomatal conductance, E spikelet fertility (%), F yield per plant. Each bar represents cultivar mean ± standard error (n = 3)
A significant increase in leaf temperature was observed in all genotypes. The late sown lines showed an overall increase of 5 °C in leaf temperature compared to early sown lines. The maximum increase in leaf temperature was observed in Vandana and BPT5204 (>6 °C) and minimum in N22, KMR3 and IR64 (<4 °C) (Fig. 1B). KMR3 showed the lowest leaf temperature under HT stress. Genotypes which are able to maintain lower tissue temperature are found to show lesser spikelet sterility in rice under heat stress conditions (Sathishraj et al. 2015). Such genotypes withstand high temperature stress mainly through transpiration cooling mechanism (Amani et al. 1996; Mahan et al. 2011; Julia and Dingkuhn 2013). Lower flag leaf temperature is reported to be a useful trait associated with tolerance to HT stress in rice (Vishnu Kiran et al. 2012; Zhang et al. 2015; Sathishraj et al. 2015).
Effect of high temperature on photosynthesis and stomatal conductance
Photosynthesis is one of the major physiological processes in plants, which is known to be susceptible to stress (Ahmad et al. 2010). Reduction in photosynthetic rate was reported in both vegetative and reproductive stage heat stress in rice (Sailaja et al. 2015). In our study, the mean P N was significantly reduced by 21% in late sown lines compared to early sown lines. The maximum reduction in P N was observed in BPT5204 (39%), Vandana (27%) and Swarna (20%), and minimum in KMR3 (6%), IR64 (11%) and N22 (11%) (Fig. 1C). Increase in leaf temperature leads to increase in transpiration rate which reduces P N . Significant negative relationship was observed between flag leaf temperature and P N in sunflower genotypes also (Kalyar et al. 2013). However, stomatal conductance was not significantly affected by high temperature in the selected genotypes. Mean gs decreased by only 9% under HT stress compared to control. The maximum reduction in gs was observed in BPT5204 (18%) followed by KMR3 (10%) and IR64 (8%). The increase in gs in N22 and Swarna was not significant (Fig. 1D). P N and gs are positively correlated in plants. Genotypes with higher gs show increased transpiration rate which in turn lowers the leaf and canopy temperatures and thus protects the plant from heat induced damage (Egeh et al. 1992). However, in our study P N was significantly reduced in all genotypes in HT stress despite no significant effect on gs. Reduction in rubisco activation or inhibition of ETR can also reduce PN without affecting gs (Salvucci and Crafts-Brandner 2004b).
Effect of high temperature on spikelet fertility and yield
High temperature at reproductive stage caused significant decrease in spikelet fertility and yield per plant in all genotypes. The mean spikelet fertility decreased by 16% and yield per plant by 29% under HT stress as compared to control conditions. The maximum decrease in spikelet fertility was observed in Vandana (25%), followed by BPT5204 (22%), IR64 and Swarna (19%), while N22 and KMR3 showed the least decrease of only 4% and 6% respectively (Fig. 1E). Correspondingly, maximum yield loss was observed in BPT5204 (53%), followed by Vandana (45%), Swarna (35%) and IR64 (24%), and minimum yield loss was in N22 (2%) and KMR3 (6%) (Fig. 1F). Thus, among the six genotypes, N22 and KMR3 showed the minimum reduction in both spikelet fertility and yield per plant under HT stress. N22 is well known for its tolerance to high temperature at seedling (Vishnu Kiran et al. 2012; Sailaja et al. 2014), vegetative (Sailaja et al. 2015) and reproductive stages (Prasad et al. 2006; Jagadish et al. 2011; Poli et al. 2013 and Sailaja et al. 2015) of development and also to both short duration and long duration of HT stress. KMR3 is a restorer line used in the production of the popular rice hybrid KRH2. This is first report of heat tolerance of a restorer line in rice to our knowledge. It is worth testing hybrids developed using KMR3 or introgression lines of KMR3 for heat tolerance. Shi et al. 2014 observed that Swarna, BPT5204 and IR64 are tolerant to short duration (38 °C for 6 h) HT stress in controlled environmental conditions. However, the results were different when the same genotypes were exposed to longer duration (38 °C for 6 consecutive days and 6 h each day) HT stress during day. Sailaja et al. (2015) reported significant reduction in spikelet fertility and grain yield in BPT5204, Swarna and Vandana genotypes when exposed to long duration (reproductive to maturity stage) heat stress in pots kept in polyhouse conditions. In the present study also BPT5204, Swarna and Vandana were highly susceptible to HT stress under late sown field conditions which validates previous studies conducted in pots under controlled conditions (Shi et al. 2014; Sailaja et al. 2015).
Heat susceptibility index, correlation analysis and ranking of genotypes
Each cultivar was ranked based on HSI value of SPAD, P N , SF and YPP and mean of all rankings was considered to identify the lines most tolerant or susceptible to high temperature (Table 4). Based on the mean ranking, N22 and KMR3 were in the top two ranks with better performance in all four traits compared to other lines. HSI value less than 1 is considered as criterion to select tolerant lines (Fischer and Maurer (1978). The well-known heat tolerant line, N22 showed HSI value ≤0.5 in all traits. The popular restorer line KMR3 showed HSI ≤0.5 for PN, SF and YPP >0.5 to <1.0 for SPAD. In our previous study, few introgression lines (ILs) of KMR3 x O. rufipogon showed more tolerance to high temperature stress at seedling and vegetative stages (K–13–7, K–50, K–377–13) compared to KMR3 (Prasanth et al. 2012) and later studies confirmed at reproductive stage (K–13–5, K–16–3, K–377–24, and K–458) tolerance of ILs at 7 different locations in India (Prasanth et al. 2016, AICRIP 2017). Thus, KMR3 and its ILs are valuable for the development of high temperature stress tolerant varieties/hybrids in future as these have demonstrated both vegetative and reproductive stage heat tolerance in controlled and field trials in 7 locations. IR64 is a rice mega variety which showed HSI ≤1.0 for the traits SPAD, P N and yield per plant but HSI >1.0 for spikelet fertility. Ye et al. 2011 also reported that IR64 is susceptible at 39 °C and moderately tolerant at 37 °C. Swarna is considered as one of the most adapted rice cultivar in many Asian countries. It showed HSI >1.0 for all the four traits. Vandana, a drought tolerant upland variety and the popular variety BPT5204did not perform well under HT stress and ranked lowest. Vandana showed HSI ≤1.0 for only SPAD and HSI >1.0 for all other traits. BPT5204 showed HSI >1.0 for all the four traits. Overall, N22 showed the lowest HSI with highest rank for 3 traits- flag leaf SPAD, spikelet fertility and yield and KMR3 showed the lowest HSI with highest rank for net photosynthetic rate and second highest rank for spikelet fertility and yield next only to N22. On the other hand, BPT 5204 showed the highest HSI (lowest rank) for flag leaf SPAD, photosynthetic rate and yield and Vandana showed the highest HSI (lowest rank) for spikelet fertility. Thus these lines have distinctly different traits that contribute to their high or low HSI values. Overall, Vandna and BPT5204 were s highly heat susceptible, IR64 and Swarna as moderately susceptible and N22 and KMR3 as highly tolerant based on HSI values.
Correlation analysis was performed for the HSI values of the same four traits to identify the association among them. HSI for SPAD showed significant positive correlation with HSI for P N and HIS for YPP. Significant positive correlations were observed among P N HSI, SFHSI and YPPHSI. As expected, SFHSI was significantly correlated with YPPHSI (Table 5). Pradhan et al. (2015) reported significant positive correlation between SPADHSI and Fv/FmHSI (Chlorophyll fluorescence) under heat stress in wheat chromosomal translocation lines. In our study, it was observed that SPADHSI was significantly correlated with P NHSI. These results confirm that photosynthetic response of plants under high temperature stress is strongly associated with leaf chlorophyll content. The associations indicate strong cause-effect relationships among SPAD, P N and yield per plant which lends support to use of these traits in screening rice genotypes for tolerance to HT stress (Pradhan et al. 2015).
Our results on heat tolerance of six rice varieties under natural field conditions show that high temperature stress in late sown conditions during dry season has adverse effect on yield in BPT5204, Vandana, Swarna and IR64. Mandal et al. (2005) reported yield loss in rice hybrids due to late sowing in dry season. Singh et al. (2016) also reported yield losses in rice in four districts (Jorhat, Kalyani, Ranchi and Bhagalpur) of India because of delayed sowing in dry season. Although the six varieties evaluated in the present study vary in days to flowering and extent of exposure to high temperature, the long duration restorer line KMR3 and the short duration variety N22 are significantly heat tolerant and the long duration variety BPT5204 and short duration variety Vandana are highly susceptible. Field based late sown experiments such as this study conducted to coincide with warmer days/nights present a more realistic assessment of heat tolerance of rice varieties compared to controlled conditions.
Conclusion
Popular rice varieties revealed contrasting response to high temperature under late sown field conditions. Out of the six varieties tested, a known heat tolerant variety N22 and a restorer line KMR3 were found to be highly tolerant. The mega variety IR64 was identified as moderately tolerant to high temperature based on all the six traits. BPT5204 and Vandana were the most heat susceptible varieties. High temperature tolerant restorer line may be deployed for the development of heat tolerant rice hybrids in future. Even though N22 is highly tolerant to high temperature, it has low absolute yield and grain quality (Jagadish et al. 2008) and its mutants with higher yield and heat tolerance may be valuable. Identification and introgression of useful genes from N22 to heat susceptible mega varieties like BPT5204 and Swarna using marker assisted selection may facilitate improvement of heat tolerance in these varieties.
References
Ahmad, A., Diwan, H., & Abrol, Y. P. (2010). Global climate change, stress and plant productivity. In A. Pareek, S. K. Sopory, H. J. Bohnert, & Govindjee (Eds.), Abiotic stress adaptation in plants: Physiological, molecular and genome foundation (pp. 503–521). Berlin: Springer.
AICRIP. (2017). All India coordinated rice improvement project (AICRIP). Annual Progress Reports 2017. Hyderabad, Indian institute of rice research.
Amani, I., Fischer, R. A., & Reynolds, M. P. (1996). Canopy temperature depression association with yield of irrigated spring wheat cultivars in a hot climate. Journal of Agronomy Crop Science, 7, 119–129.
Camejo, D., Jiménez, A., Alarcón, J. J., Torres, W., Gómez, J. M., & Sevilla, F. (2006). Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Functional Plant Biology, 33, 177–187.
d’Alpoim Guedes, J., Jin, G., & Bocinsky, R. K. (2015). The impact of climate on the spread of rice to North-Eastern China: A new look at the data from Shandong Province. PLoS ONE, 10(6), e0130430.
Djanaguiraman, M., Prasad, P. V. V., & Al-Khatib, K. (2011). Ethylene perception inhibitor 1-MCP decreases oxidative damage of leaves through enhanced antioxidant defense mechanisms in soybean plants grown under high temperature stress. Environmental and Experimental Botany, 71, 215–223.
Egeh, A. O., Ingram, K. T., & Zamora, O. B. (1992). High temperature effects on leaf gas exchange of four rice cultivars. Philippines Journal Crop Science, 17, 21–26.
Fischer, R. A., & Maurer, R. (1978). Drought resistance in spring wheat cultivars. I. Grain yield responses in spring wheat. Australian Journal of Agricultural Sciences, 29, 892–912.
Hall, A. E. (2011). Breeding cowpea for future climates. In S. S. Yadav, R. Redden, J. L. Hatfield, H. Lotze Campen, & A. J. W. Hall (Eds.), Crop adaptation to climate change. Oxford: Wiley.
IPCC (2013). Working group I contribution to the IPCC fifth assessment report climate change (2013). The physical science basis, summary for policymakers. Available online at: http://www.climatechange2013.org/images/%20report/WG1AR5_SPM_FINAL.pdf.
Ishimaru, T., Hirabayashi, H., Ida, M., Takai, T., San-Oh, Y. A., Yoshinaga, S., et al. (2010). A genetic resource for early-morning flowering trait of wild rice Oryza officinalis to mitigate high temperature-induced spikelet sterility at anthesis. Annals of Botany, 106, 515–520.
Jagadish, S. V. K., Cairns, J., Lafitte, R., Wheeler, T. R., Price, A. H., & Craufurd, P. Q. (2010). Genetic analysis of heat tolerance at anthesis in rice (Oryza sativa L.). Crop Science, 50, 1–9.
Jagadish, S. V. K., Craufurd, P. Q., & Wheeler, T. R. (2008). Phenotyping rice mapping population parents for heat tolerance during anthesis. Crop Science, 48, 1140–1146.
Jagadish, S. V. K., Muthurajan, R., Rang, Z. W., Malo, R., Heuer, S., Bennett, J., et al. (2011). Spikelet proteomic response to combined water deficit and heat stress in rice (Oryza sativa cv. N22). Rice, 4, 1–11.
Julia, C., & Dingkuhn, M. (2013). Predicting temperature induced sterility of rice spikelets requires simulation of crop-generated microclimate. European Journal of Agronomy, 49, 50–60.
Kalyar, T., Rauf, S., Teixeira da silva, J. A., Haidar, S., & Iqbal, Z. (2013). Utilization of leaf temperature for the selection of leaf gas-exchange traits to induce heat resistance in sunflower (Helianthus annuus L.). Photosynthetica, 51(3), 419–428.
Laborte, A., Nelson, A., Jagadish, K., Aunario, J., Sparks, A., Ye, C.(2012). Rice feels the heat. In Redoña (Ed.), Rice Today. July–Sep, 30–31.
Mahan, J. R., Young, A. W., & Payton, P. (2011). Deficit irrigation in a production setting: Canopy temperature as an adjunct to ET estimates. Irriation Science, 30, 127–137.
Mandal, N., Nag, K., & Ghosh, M. (2005). Planting date effects on phenological development, yield, and quality of hybrid rice. Tropical Agriculture, 82, 34–39.
Matsui, T., Omasa, K., & Horie, T. (2001). The difference in sterility due to high temperature during the flowering period among japonica-rice varieties. Plant Production Science, 4, 90–93.
Netto, A. T., Campostrini, E., de Oliveira, J. G., & Bressan-Smith, R. E. (2005). Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Scientia Horticulturae, 104, 199–209.
Ogiso-Tanaka, E., Matsubara, K., Yamamoto, S-i, Nonoue, Y., Wu, J., Fujisawa, H., et al. (2013). Natural variation of the RICE FLOWERING LOCUS T 1 contributes to flowering time divergence in rice. PLoS ONE, 8(10), e75959.
Panigrahy, M., Neelamraju, S., Nageswara Rao, D., & Ramanan, R. (2011). Heat tolerance in rice mutants is associated with reduced accumulation of reactive oxygen species. Biologia Plantarum, 55, 721.
Poli, Y., Ramana Kumari, B., Panigrahy, M., Vinukonda, V. P., Nageswara Rao, D., Voleti, S. R., et al. (2013). Characterization of a Nagina22 rice mutant for heat tolerance and mapping of yield traits. Rice, 6, 36.
Pradhan, G. P., & Prasad, P. V. V. (2015). Evaluation of wheat chromosome translocation lines for high temperature stress tolerance at grain filling stage. PLoS ONE, 10(2), e0116620.
Pradhan, G. P., Prasad, P. V. V., Fritz, A. K., Kirkham, M. B., & Gill, B. S. (2012). High temperature tolerance in Aegilops species and its potential transfer to wheat. Crop Science, 52, 292–304.
Prasad, P., Boote, K., Allen, L., Sheehy, J., & Thomas, J. (2006). Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Research, 95, 398–411.
Prasanth, V. V., Chakravarthi, D. V. N., VishnuKiran, T., Venkateswara Rao, Y., Panigrahy, M., Mangrauthia, S. K., et al. (2012). Evaluation of rice germplasm and introgression lines for heat tolerance. Annals of Biological Research, 3(11), 5060–5068.
Ristic, Z., Bukovnik, U., & Prasad, P. V. V. (2007). Correlation between heat stability of thylakoid membranes and loss of chlorophyll in winter wheat under heat stress. Crop Science, 47, 2067–2073.
Sailaja, B., Anjum, Nazreen, Vishnu Prasanth, V., Sarla, N., Subrahmanyam, D., Voleti, S. R., et al. (2014). Comparative study of susceptible and tolerant genotype reveals efficient recovery and root system contributes to heat stress tolerance in rice. Plant Molecular Biology Reporter, 32, 1228–1240.
Sailaja, B., Subrahmanyam, D., Neelamraju, S., Vishnukiran, T., Rao, Y. V., Vijayalakshmi, P., et al. (2015). Integrated physiological, biochemical, and molecular analysis identifies important traits and mechanisms associated with differential response of rice genotypes to elevated temperature. Frontiers of Plant Science, 6, 1044.
Salvucci, M. E., & Crafts-Brandner, S. J. (2004). Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiology, 134, 1460–1470.
Sathishraj, R., Bheemanahalli, R., Ramachandran, M., Dingkuhn, M., Muthurajan, R., & Jagadish, S. V. K. (2015). Capturing heat stress induced variability in spikelet sterility using panicle, leaf and air temperature under field conditions. Field Crops Research, 190, 10–17.
Sharkey, T. D., & Zhang, R. (2010). High temperature effects on electron and proton circuits of photosynthesis. Journal of Integrative Plant Biology, 52, 712–722.
Shi, W., Ishimaru, T., Gannaban, R. B., Oane, W., & Jagadish, S. V. K. (2014). Popular rice (Oryza sativa L.) cultivars show contrasting responses to heat stress at gametogenesis and anthesis. Crop Science, 55, 589–596.
Shi, P., Tang, L., Wang, L., Sun, T., Liu, L., Cao, W., et al. (2015). Post-heading heat stress in rice of South China during 1981–2010. PLoS ONE, 10(6), e0130642.
Singh, P. K., Singh, K. K., Rathore, L. S., Baxla, A. K., Bhan, S. C., Akhilesh Gupta, G. B., et al. (2016). Rice (Oryza sativa L.) yield gap using the CERES-rice model of climate variability for different agroclimatic zones of India. Current Science, 110, 405–413.
Sun, A. Z., & Guo, F. Q. (2016). Chloroplast retrograde regulation of heat stress responses in plants. Frontiers in Plant Science, 7, 398.
Vishnu Kiran, T., Sravan Raju, N., Senguttuvel, P., Vijayalakshmi, P., Venkateswara Rao, Y., Surekha, K., Neeraja, C. N., & Voleti, S. R. (2012). Screening of hybrids and parental lines for association of physiological traits to identify heat-tolerant and nitrogen-use efficient rice genotypes. 6th IHRS (pp. 169–177).
Vishnu Prasanth, V., Basava, Kumari Ramana, Suchandranath Babu, M., Venkata Tripura, V. G. N., Rama Devi, S. J. S., Mangrauthia, S. K., et al. (2016). Field level evaluation of rice introgression lines for heat tolerance and validation of markers linked to spikelet fertility. Physiology and Molecular Biology of Plants, 22(2), 179–192.
Yadava, U. L. (1986). A rapid and nondestructive method to determine chlorophyll in intact leaves. Horticultural Science, 21(6), 1148–1150.
Ye, C., May, A., Argayoso, A., Edilberto, D., Redon, A., Sierra, S. N., et al. (2011). Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breeding, 131(1), 33–41.
Yoshida, S., Satake, T., & Mackill, D. (1981). High temperature stress. IRRI Research Paper, 67, 1–15.
Zhang, C. X., Fu, G. F., Yang, X. Q., Yang, Y. J., Zhao, X., Chen, T. T., et al. (2016). Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity. Journal of Agronomy Crop Science, 202(5), 394–408.
Ziska, L. H., Manalo, P. A., & Ordonez, R. A. (1996). Intraspecific variation in the response of rice (Oryza sativa L.) to increased CO2 and temperature: Growth and yield response of 17 cultivars. Journal of Experimental Botany, 47, 1353–1359.
Acknowledegments
This work was supported by the National Innovations on Climate Resilient Agriculture (NICRA), Indian Council of Agricultural Research (ICAR), Ministry of Agriculture, Govt. of India [F. No. Phy/NICRA/2011–2012].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vishnu Prasanth, V., Suchandranath Babu, M., VGN, T.V. et al. Response of popular rice varieties to late sown high temperature conditions in field. Ind J Plant Physiol. 22, 156–163 (2017). https://doi.org/10.1007/s40502-017-0287-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-017-0287-y