Abstract
Heat stress is an abiotic factor that reduces wheat yields. This study assessed the morphological and physiological traits essential for early detection of heat tolerance in wheat and identified suitable genotypes for specific agro-ecologies prone to heat stress in Zambia. Twelve wheat genotypes were evaluated in a heat-stress environment (Chakanka) and a non-stress heat environment (UNZA). Early heat stress indicators like leaf thickness and chlorophyll content had a 92% and 90% relationship with the yields of heat-tolerant genotypes, respectively. An increase in either or both parameters improved the yields of heat-tolerant genotypes relative to the susceptible ones. Among the heat-tolerant genotypes, Entry 48 had the highest yield (5866.5 kg ha−1) at the heat-stress site; this yield was 51.2% higher than that of the most susceptible genotype, Kwale (2864.0 kg ha−1). Entry 48 was more adapted to heat stress and yielded better than others due to early flowering (53.8 days to 50% flowering) and longer grain-filling duration (40.3 days). Entry 48 possessed traits of a climate-smart variety and could be a candidate for breeding future heat-tolerant and high-yielding wheat varieties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heat stress is an abiotic factor that reduces global crop production. It is further exacerbated by global warming. A global atmospheric temperature rise of 2 °C could lead to an increase in yields of adapted crops such as wheat (Triticum aestivum L.), rice (Oryza sativa L.) and maize (Zea mays L.), but each subsequent increase in temperature could reduce yields (Challinor et al., 2014). In wheat, yield declines by 6% for each degree increase in atmospheric temperature above the optimum temperature of 25 °C (Asseng et al., 2015).
Depending on the wheat variety, heat stress can decrease the number of grains, yield and straw mass by up to 28%, 25%, and 25%, respectively (Mahrookashani et al., 2017). In addition, flowering, CO2 assimilation by wheat, seed set, kernel weight, starch and protein content of grains are all lowered by heat stress (Aiqing et al., 2018). Also, grain starch characteristics such as grain starch area, length and volume are reduced (Chaubey et al., 2021). Even the days to anthesis and days to maturity are reduced by 16% and 20%, respectively. The early anthesis causes a further decline in leaf area, above-ground biomass, panicle length, and tillers per plant (Qaseem et al., 2019). Furthermore, heat-stress has been shown to reduce chlorophyll by 19% during anthesis and 25% during grain filling. Similarly, photosynthesis rate declined by 17% and 25% during anthesis and grain filling, respectively (Djanaguiraman et al., 2020). Conversely, fluorescence, stomatal conductance and less-unsaturated lipid species were elevated under heat stress (Djanaguiraman et al., 2020). Despite all these negative effects of heat stress that eventually lower wheat yield, there are concerted efforts to mitigate these, from irrigation to improve transpiration and thus reduce the plant temperature, to growing adapted varieties, priming of seeds with salicylic acid and foliar application (Kousar et al., 2018) and breeding for high-yielding heat-tolerant varieties (Snowdon et al., 2020).
Many breeding strategies have led to development of some heat stress-tolerant wheat varieties across the world; for example, the emmer-derived lines in Australia (Ullah et al., 2018), the thermotolerant SynDT of Korea (Truong et al., 2021), the use of wild species such as Aegilops (Waines, 1994) among others. However, in Zambia, most wheat varieties are susceptible to heat stress, and coupling it with drought lead to higher yield losses, for example, in 2018, there was a 40.9% decline compared to the previous season of 2017 (FAO, 2019). Wheat ranks second after maize with an average annual production of 151,850 tonnes as of 2019 (FAOSTAT, 2021), and an estimated national annual requirement of Zambia is about 414,750 metric tonnes (Mulenga et al., 2019). The average wheat yield in Zambia in 2019 according to FAOSTAT (2021) was 6.7 t ha−1, and production is usually during the rainy season (November–April) or using irrigation during the cool-dry season (May–September). This low yield is largely attributed to increasingly unpredictable rainfall patterns and amounts; diseases and pests; and abiotic stresses such as aluminium toxicity, salinity and heat stress (Tembo, 2019). Furthermore, climate trends from 1960 to 2003 indicate that the mean annual temperature in Zambia has increased by 1.3 °C and will rise to 2.2 °C by 2050 (Ramirez-villegas & Thornton, 2015; World Bank, 2017). This will further be exacerbated by the decline in rainfall amount, especially in the southern region, with as much as 5% during that period (Ramirez-villegas & Thornton, 2015; World Bank, 2017). Currently, the wheat varieties available in Zambia have limited heat stress tolerance, which compromises their yield potential. To fill this gap, high-yielding wheat varieties adapted to increasing global temperatures are required. Although, there have been efforts to breed for heat tolerance in Zambia, precise morphological and physiological markers that inform these decisions are not well studied across Zambian agro-ecological zones. Screening wheat genotypes under different agro-ecological zones whose atmospheric temperatures represent heat stress and non-heat environments could help us to discern traits for early detection of heat stress in wheat and identify site-specific heat-tolerant genotypes. Therefore, this study aimed to assess the morphological and physiological traits essential for early detection of heat tolerance in wheat and identify suitable genotypes for specific agro-ecologies prone to heat stress in Zambia.
Material and methods
Site description and planting material
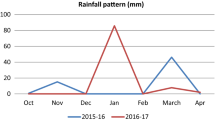
The study was conducted at two sites in Zambia, the University of Zambia (UNZA field station (15° 22′ S and 28° 20′ E)—representing a non-heat stress environment, and at Chakanka farm in Chiawa district (15° 88′ S and 29° 05′ E)—representing heat stress environment. The UNZA field station is 1250 m above sea level (asl) and falls under agro-ecological zone II of Zambia. It receives an average annual rainfall (2009–2019) of between 800 and 1200 mm with an average annual minimum and maximum atmospheric temperatures of 15.6 °C and 28.1 °C respectively (Fig. 1). The soils are classified as acrisols (JAICAF, 2008). While the Chakanka farm in Chiawa district, is at an altitude of 370 m asl in agro-ecological zone I. Its annual average rainfall (2009–2019) is between 600 to 800 mm, and an average annual minimum and maximum atmospheric temperatures of 19.3 °C and 32.2 °C respectively (Fig. 1). Data on atmospheric temperature and rainfall were obtained from the meteorological stations near the trial sites. The soils are classified as cambisols (JAICAF, 2008).
For this study, 12 spring wheat (Triticum aestivum L.) genotypes were obtained from various sources, 10 of these materials were: Entries 22, 32, 39, 48, UNZA WV II, Kwale, Harrier, Pungwa, Chacha and Choza. The remaining two were heat-tolerant checks, Loerie II and Shungu. These materials were acquired from Zambia Seed Company (ZAMSEED) (Kwale, Harrier, Pungwa, Chacha and Choza), Seed-Co (Shungu), University of Zambia (UNZA) (UNZA WV II and Loerie II) and the International Maize and Wheat Improvement Center (CIMMYT) (Entries 22, 32, 39, 48). The checks, Shungu is a short-stature-medium maturing genotype that is tolerant to bacterial leaf streak and Alternaria triticini, it does well in most agro-ecological zones of Zambia, while Loerie II is a medium height genotype that matures early. It is adapted to most agro-ecological zones of Zambia.
Experimental design and agronomic practices
In this study, the site at Chakanka was regarded as a heat-stress environment (see the atmospheric temperature in Fig. 1). The trials were arranged in a randomized complete block design (RCBD) with each treatment (genotype) replicated four times at the two sites. Each plot was 5 m long with five rows spaced at 0.2 m apart. Planting was at a seed rate of 100 kg ha−1 using the hand drilling method on 20th May and 17th June 2019 at UNZA and Chakanka, respectively, and repeated at the same sites on 18th May and 15th June 2020 (Table 1). At the onset of the trial, soil was sampled randomly from Chakanka and UNZA at a depth of 0–30 cm. Five samples were taken in a zigzag method (Maiti, 2013), mixed thoroughly, air-dried, and processed through a 2-mm sieve. The soil was analysed for total N, P, K, Ca, Mg, S, Na, Fe, Mn, B, Cu, Zn, cation-exchange capacity and organic carbon at the University of Zambia, School of Agricultural Sciences, Soil Science Department laboratories. Total N was analysed using the Kjeldahl method (Bremner, 1960), and P, K, Ca, Mg, S, Na, Fe, Mn, B, Cu and Zn using the Mehlich 3 extraction (Mehlich, 1984). Soil pH was measured in a water paste with a 1:2 ratio of soil to water. The texture of the soil after analysis using the hydrometer method was loam at the depth of 0–30 cm and classified as acrisols and cambisols at UNZA and Chakanka, respectively (JAICAF, 2008). The other pre-trial results are presented in Table 2. During sowing, a basal fertilizer with the trade name Compound D (10N:20P:10K) was applied at a rate of 500 kg ha−1. Urea (46% N) was applied in three splits, at early tillering (Zadoks 21), at anthesis (Zadoks 61) and at grain filling (Zadoks 71) (Fowler, 2018) stages at the rates of 300 kg ha−1, 100 kg ha−1 and 200 kg ha−1 respectively. Since these genotypes were planted when the rains had reduced i.e. during winter, supplemental irrigation was done using sprinklers (D-Net™ 8550 from Netafim and supplied by Amiran-Zambia) where a total of 5.50 m3 of water was applied by physiological maturity. Hand weeding was done thrice during the growth period in the 2019 and 2020 seasons (Table 1) and bird scaring began towards physiological maturity.
Growth, yield and heat susceptibility index
Physiological traits and leaf thickness were measured using the MultispeQ device v1.0. This was done by clamping the middle portion of a randomly selected flag leaf using the MultispeQ peg at 50% anthesis (Zadoks 65) (Fowler, 2018) between 10.00 and 13.00 h (Table 1). Despite measuring various fluorescence base parameters, in the results we present relative chlorophyll content, leaf thickness and the difference between ambient and leaf temperatures.
Yield and yield parameters: the number of tillers were counted in an area of 0.5 m2, while the number of spikelets and of grains per ear were determined from 10 random plants within the net plot. Harvesting at UNZA was done on 22nd October 2019 in season one and on 20th October 2020 in season two, whereas at Chakanka it was on 29th October 2019 and on 27th October 2020 in season one and two respectively (Table 1). All the five rows were manually harvested. After which, wheat was hand-threshed and winnowed. Weight of grains was obtained for each plot and then converted to kg ha−1 at moisture content of 10–12%. Thereafter, 1000 grains were subsampled from each plot, counted using an automatic seed counter (model SLY-C) and their weights were recorded.
To evaluate heat tolerance among the genotypes, Heat Susceptibility Index (HSI) was calculated using a modification of Fischera and Maurer, (1978) drought susceptibility formula or Mason et al. (2010). Days to 50% flowering (DTF), number of grains per spike (GPS), thousand grain weight (TGW) and yield data of the genotypes were used in the equation below:
where Ys is the mean of yield and yield parameters (DTF, GPS, TWG and yield) of each genotype under heat stress condition; Y is the mean of yield and yield parameters (DTF, GPS, TWG and yield) of each genotype under non-heat stress condition; Xs is the mean of yield and yield parameters (DTF, GPS, TWG and yield) of all genotypes under heat stress condition; X is the mean of yield and yield parameters (DTF, GPS, TWG and yield) of all genotypes under non-heat stress condition.
After calculating the HSI of each genotype, a difference between each genotype’s index and that of the checks (Loerie II and Shungu) was calculated, this was to determine if the measured phenotype traits were better than the checks.
where HSId is the difference between the HSI of a genotype and a check; HSIg is the HSI of a genotype, and HSIc is the HSI of the check.
Data analysis
Analysis of variance was conducted using the Proc Glm statement, and the means compared by Fisher’s Least Significant Difference (LSD) method, at a 95% confidence level using SAS software (SAS, 2002). The site (location), the genotypes and their interactions were fixed factors while the blocks at each site were random factors. Using the HSI, the genotypes were grouped into two: tolerant and susceptible to heat stress (Fig. 2k). This was done by averaging the means of all HSI of the yield and yield parameters (DTF, GPS, TWG and yield). From these groupings, relations between yields and various physiological parameters were done using Proc Reg statement in SAS at 95% confidence level, for example, yield and chlorophyll content, leaf thickness, and leaf-ambient temperature difference.
a HSI of various genotypes calculated using means of yield and yield parameters and categorized into tolerant (HSI < 1) and susceptible (HSI > 1) using Fischera and Maurer, (1978). Any value below zero indicates that the yield parameter is more tolerant to heat than the checks. b–k The difference between heat susceptibility index (HSI) of various genotypes and the checks—Loerie II and Shunga. DTF—days to 50% flowering, GPS—number of grains per spike, TGW—thousand grain weight
Results
Heat susceptibility index, yield traits and yield
Besides the check, Loerie II, Entry 48 was the most tolerant to heat stress, while Kwale was the most susceptible (Fig. 2a). The heat susceptibility index (HSI) difference of various yield and yield parameters among the genotypes and the checks, Loerie II and Shunga, showed that Days to 50% flowering (DTF), number of grains per spike (GPS), thousand grain weight (TGW) and yield of Entries 39, 32 and Kwale were not as tolerant to heat stress as the checks (Fig. 2). However, depending on the parameter, the other seven genotypes, indicated better heat stress tolerance than the checks. For example, the yields of Entry 48 and UNZA WV II only showed more heat stress tolerance than Loerie II, while Harrier’s yield was more tolerant to heat stress than the two checks (Loerie II and Shungu) (Fig. 2).
There was an interaction effect between the sites and the genotypes; yields, 1000 grain weight, number of grains/ear and spikelet/ear, and days to 50% flowering were significant (Table 3). Yields of the evaluated genotypes were significantly different at both heat stress (Chakanka) and non-heat stress (UNZA) sites (Table 3). Under the interaction between site and genotypes, Entry 39 produced the highest yield at UNZA, while Kwale the least at Chakanka. Moreover, this Entry 39’s yield was not significantly different from that of Entry 48 at Chakanka. When focusing on individual sites, the performance of genotypes was different, for example, Entries 39 (8900.0 kg ha−1) and 48 (5866.5 kg ha−1) produced the highest yields at UNZA and Chakanka respectively, while Loerie II (5500.0 kg ha−1) and Kwale (2864.0 kg ha−1) produced the least grain yields at UNZA and Chakanka respectively (Table 3). Generally, each genotype’s yield was higher at UNZA than at Chakanka, for example, the yield of Entry 48 was 21.8% more at UNZA than at Chakanka. The above genotype was categorised as heat tolerant according to HSI (0.7354). Interestingly, Kwale, a heat-susceptible genotype according to HSI (1.3527), had a significantly lower yield at Chaka. In contrast, at UNZA, its yield was higher than Loerie II—one of the checks with the lowest yield (Table 3).
The weight of 1000 grains was significantly different among the genotypes only at UNZA (Table 3). At this site, the grains of Entry 48 were significantly compact (54.1 g 1000 grains−1), a similar trend to its grain yield and, its highest number of days of filling the grains. On the other hand, Entry 48’s days to 50% flowering were significantly the lowest (53.8 days) at Chakanka, while Kwale, a susceptible genotype took more days to achieve 50% flowering (62.8 days). Like its lowest yield at Chakanka, Kwale’s 1000-grain weight was the least. Overall, all genotypes took more days to flower (72.2 days) and to fill their grains (55.2 days) at the non-heat stress site (UNZA) than at the heat stress environment (Chakanka), 57.6 and 37.4 days, respectively (Table 3).
There was a significant interaction between the number of spikelets per ear of various genotypes and the sites; across sites, UNZA WV II produced many spikelets per ear (18.9), while Choza had the least, 17 spikelets. The number of spikelets per ear neither followed the trend of any genotype’s yield at Chakanka nor at UNZA. At Chakanka, a heat tolerant genotype, Chacha (19.3) while at UNZA, a susceptible genotype UNZA WV II (20.2), significantly produced the most spikelets per ear. At Chakanka, the number of tillers in 0.5 m2 was conversely proportional to the yield of Entry 48. Indeed, at Chakanka, this genotype significantly produced fewer tillers, while Harrier, a susceptible genotype, produced the most tillers, and Loerie II at Chakanka and UNZA, respectively (Table 3).
Leaf thickness, chlorophyll content, and ambient-leaf temperature difference
Regardless of the genotype, relative chlorophyll was highest at UNZA except for Loerie II. The relative chlorophyll was not significantly different among tolerant and susceptible genotypes at Chakanka. Despite this, the chlorophyll content index value of Loerie II was numerically higher (49.7), while that of Harrier was the least (41.2) (Table 4). At UNZA, Entry 32 had more chlorophyll (60.0), this was not different from other genotypes like Entries 48 and 22, Pungwa, Chacha and Choza. Conversely, Loerie II, whose chlorophyll content index value was highest at Chakanka, had the least at UNZA (48.7) (Table 4).
While leaf thickness was significantly different among the genotypes at Chakanka, it was not at UNZA (Table 4). At Chakanka, the leaves of Entry 39 were thicker (0.36 mm) than other genotypes, although, they were not significantly different from the leaves of Entries 48, 32, and 22, Pungwa, Chacha and Shungu. Even though, there was no significant difference in leaf thickness at UNZA, the leaves of Harrier, a susceptible genotype, were thicker (0.42 mm) than those of Lorrie, a check with the thinnest leaves (0.31 mm) (Table 4).
Under interaction between sites and genotypes, the ambient-leaf temperature difference of the genotypes was not significantly different. However, at Chakanka, it was significant among the genotypes (Table 4). Entries 39 and 48 had the least difference between ambient and leaf temperatures regardless of the location, however, at Chakanka this difference was less than at UNZA, − 8.29 °C and − 10.96 °C, respectively. Kwale, a susceptible genotype which was the exception, had contrasting temperature at both sites. At Chakanka, its difference between ambient and leaf temperature was the lowest (− 4.19 °C), while at UNZA, it was the highest (− 8.12 °C) (Table 4).
Relationship between yield, leaf thickness, chlorophyll content, and ambient-leaf temperature difference
The results from the regression analysis indicated that irrespective of a genotype’s response to heat stress, their chlorophyll content was 89.02% related to yield (R2 = 0.8902) (Fig. 3a). However, this relationship was stronger among the tolerant genotypes, at 89.93% (R2 = 0.8993) (Fig. 3b), than susceptible genotypes, at 89.49% (R2 = 0.8949) (Fig. 3c). Similarly, leaf thickness and yield followed that trend (R2 = 0.9013) (Fig. 3d); they showed a significantly stronger relationship between yield and tolerant genotypes (R2 = 0.9175) (Fig. 3e) in comparison to susceptible genotypes (R2 = 0.8947) (Fig. 3f). The relationships between relative chlorophyll, leaf thickness and yield were positive i.e. an increase in either parameter led to rise in yields—more so of heat-tolerant genotypes than susceptible genotypes. The closer the ambient-leaf temperature difference was to zero, the lower the yields of all the genotypes (Fig. 3g). In addition, any increase in ambient-leaf temperature difference affected yields of the susceptible genotypes more than the tolerant ones.
The relationship between yields of: a all genotypes, b heat tolerant genotypes and c heat susceptible genotypes and relative chlorophyll (SPAD); d all genotypes, e heat tolerant genotypes and f susceptible genotypes and leaf thickness; g all genotypes, h heat tolerant genotypes and i susceptible genotypes and ambient-leaf temperature difference. *p < 0.0
Discussion
Filling the wheat yield gap through breeding is necessary, however, this process must be site-specific to achieve the objective. Most wheat varieties in Sub-Saharan Africa are cultivated in agro-ecologies where they are less adapted, this is in addition to some being outdated and cannot cope with the current vagaries of climate. Certainly, this is the case in Zambia, thus continuous breeding is necessary. Although conventional breeding is a complex and time-consuming process, here we identified traits that can be early measured easily under field conditions. Furthermore, genotypes that are adapted to heat stress and specific agro-ecological zones of Zambia were identified. For example, Entry 48 performed better under the heat stress environment (Chakanka), its yield being more heat tolerant than Loerie II—a check.
In general, the yield of each genotype was higher at UNZA than at Chakanka. This could be attributed to the favourable weather conditions. The rainfall amounts at UNZA were higher than at Chakanka (Fig. 1), thus the cumulative residual soil moisture from the rainy season and the irrigation was advantageous to the genotypes at UNZA. Also, the atmospheric average temperature during the growth period at UNZA (23.2 °C) was within the optimal range for higher yields than at Chakanka (26.3 °C) (Fig. 1). Acevedo et al. (2002) stated that the optimal atmospheric temperature is 20–25 °C, the temperature at UNZA was within this range. Furthermore, the initial soil analysis at UNZA indicated more macronutrients, (except for N), micronutrients, organic carbon and higher pH than at Chakanka (Table 2). The presence of these nutrients probably boosted wheat nutrition at UNZA, despite basal application of N, P and K.
Balla et al., (2019), reported that heat stress at any stage of wheat development leads to a reduction in grain yield. In our study, the yields of all genotypes evaluated under heat stress were lower than those under optimal environment. However, focusing on the heat stress environment (Chakanka) revealed that Entry 48’s yield was the highest. This could be related to the high weight of grains and their number per ear. Moreover, it flowered earlier than the other genotypes, a trait observed as a drought escape strategy (Shavrukov et al., 2017), and yet important as a coping mechanism under heat stress (Kazan & Lyons, 2016). It also took more days to fill its grains, this was contrary to other studies that observed shortening of the grain filling period under heat stress (Dias & Lidon, 2009). Combined, the above traits thus contributed to Entry 48’s higher number of full grains. These responses of Entry 48 were an indication of heat tolerance, a calculation of the heat susceptibility index (HSI) further confirmed the superiority of Entry 48’s yield against Loerie II. Cementing Entry 48’s superiority was a genotype like Kwale whose HSI indicated susceptibility to heat stress, and produced low grain yields which corresponded to the lowest number of grains per ear. This strengthened the assumption that early flowering and longer duration of grain filling leading to production of large grains is a survival strategy to heat stress. This is contrary to other studies which observed decline in the sizes and the number of grains in an ear (Akter & Islam, 2017). However, these traits are only measured at harvesting; for early indication of heat stress, physiological traits would suffice. For example, ambient-leaf temperature differences, chlorophyll content, leaf thickness etc. Therefore, their inclusion is essential as early detection tools in conventional breeding strategies.
Since leaf temperature is highly dependent on the ambient temperature (Yu et al., 2015), the internal temperature of a heat tolerant genotype nears the ambient temperature than a heat susceptible genotype, as observed in Entry 48. This could be due to several reasons, one being regulation of the opening of the stomatal pores to control cooling of the plant, which is possibly associated with the thickness of the leaves. In fact, Deva et al., (2020) noted that heat-tolerant genotypes are cooler than heat susceptible genotypes because of leaf thickness. Moreover, thicker leaves, as was with Entry 48, a heat tolerant genotype, avoid thermal damage than thinner leaves, they have more thermal stability (Leigh et al., 2012). These plants with thicker leaves are more tolerant to heat stress (Nautiyal et al., 2008). The controlling of stomatal conductivity in thicker leaves regulates leaf temperature through evaporative cooling driven by transpiration (Tricker et al., 2018), which contradicts most expectations. Reason being that they could loss more water through this process leading to wilting or even permanent wilting. Yet, they adapt to this because the thicker leaves have higher leaf water content i.e. the succulent leaves. This succulency and high reflectance help regulate leaf temperature (Leigh et al., 2012). The other reason for the almost comparable temperatures, is that thicker leaves offer a buffer between inner leaf temperature and the atmospheric temperature (Liu et al., 2020).
Thicker leaves positively correlate with chlorophyll content (Jumrani et al., 2017; Marenco & Nascimento, 2009). Unlike heat susceptible genotypes, leaf thickness in heat tolerant genotypes corresponded to their chlorophyll content. Feng et al. (2014) also observed low chlorophyll in heat susceptible genotypes. These traits of the heat tolerant genotypes, especially in Entry 48, combined with increased stomatal opening under elevated temperatures (Reynolds-henne et al., 2010) probably led to enhanced gaseous exchange and stomatal conductance, and thus improved photosynthesis (Marks & Lechowicz, 2007). In summary, Mcmillen and Mcclendon (1983) too observed an important relationship between leaf thickness and photosynthesis, making leaf thickness a trait worth measuring in heat stress studies.
Altogether, the yield of Entry 48 was higher under heat stress conditions and more tolerant than all other genotypes, except Loerie II, one of the checks. Reynolds et al. (2007) observed that the drivers of yield under heat stress environments were soluble carbohydrates content, light interception, and canopy temperature. Canopy temperature is influenced by many factors, including leaf thickness and size (Konrad et al., 2021), a parameter measured in this study. Overall, the physiological factors measured indicated that some hidden processes which were not measured (stomatal conductivity or net photosynthesis) in this study might have influenced the yields too. However, working under certain conditions with limited resources, the traits measured here are still effective tools for screening of heat tolerance, and for the first time Entry 48 has been identified as a heat tolerant genotype, yielding better under heat stress environment. The traits of Entry 48 identify it as a climate-smart variety, and a candidate in breeding for future heat tolerant and high yielding wheat varieties. Furthermore, identification of genes that initiate heat tolerance in Entry 48 in future could improve breeding not only of wheat but other crops too.
Conclusion
Sustainable improvement of crop yields per acreage in the changing climate will combat Sub-Saharan Africa’s hunger, breeding of heat-tolerant crops is just one of the strategies, particularly of wheat, a crop consumed by many people. In as much as breeding has advanced in other parts of the world e.g. through genetic markers and gene editing, most of these tools are lagging behind in Sub-Saharan Africa, mostly due to minimum resources. Therefore, effective and efficient tools for conventional breeding and easily measurable parameters that can detect heat stress early are important. This study has shown: (1) that even though yield and yield parameters are the ultimate indication of the best adapted genotypes to heat stress, early indicators such as chlorophyll content, leaf thickness, ambient-leaf temperature differences are equally important in screening for heat-tolerant genotypes; (2) an increase in either of or both these parameters improves the yields of heat-tolerant genotypes than susceptible ones; (3) Entry 48 is more tolerant to heat stress, and only dwarfed by Loerie II’s HSI; and (4) among the measured parameters, Entry 48 derived its tolerance through early flowering and longer duration of grain filling, and hence improving the weight of grains in the ear and the number of grains.
Availability of data and materials
Datasets are available from the corresponding author(s) on reasonable request.
References
Acevedo, E., Silva, P., & Silva, H. (2002). Wheat growth and physiology. In B. C. Curtis, S. Rajaram, & G. H. Macpherson (Eds.), Bread wheat, improvement and production (Issue 30, p. 554). Food and Agriculture Organization of the United Nations. https://eur-lex.europa.eu/legal-content/PT/TXT/PDF/?uri=CELEX:32016R0679&from=PT%0Ahttp://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:52012PC0011:pt:NOT
Aiqing, S., Somayanda, I., Sebastian, S. V., Singh, K., Gill, K., Prasad, P. V. V., & Jagadish, S. V. K. (2018). Heat stress during flowering affects time of day of flowering, seed set, and grain quality in spring wheat. Crop Science, 58, 380–392. https://doi.org/10.2135/cropsci2017.04.0221
Akter, N., & Islam, M. R. (2017). Heat stress effects and management in wheat. A review. Agronomy for Sustainable Development, 37, 1–17. https://doi.org/10.1007/s13593-017-0443-9
Asseng, S., Ewert, F., Martre, P., Rötter, R. P., Lobell, D. B., Cammarano, D., Kimball, B. A., Ottman, M. J., Wall, G. W., White, J. W., Reynolds, M. P., Alderman, P. D., Prasad, P. V. V., Aggarwal, P. K., Anothai, J., Basso, B., Biernath, C., Challinor, A. J., De Sanctis, G., … Zhu, Y. (2015). Rising temperatures reduce global wheat production. Nature Climate Change, 5(2), 143–147. https://doi.org/10.1038/nclimate2470
Balla, K., Karsai, L., Bonis, P., Kiss, T., Berki, Z., Horvath, A., Mayer, M., Bencze, S., & Veisz, O. (2019). Heat stress responses in a large set of winter wheat cultivars (Triticum aestivum L.) depend on the timing and duration of stress. PLoS ONE, 14(9), e0222639. https://doi.org/10.1371/journal.pone.0222639
Bremner, J. M. (1960). Determination of nitrogen in soil by the Kjeldahl method. The Journal of Agricultural Science, 55(1), 11–33. https://doi.org/10.1017/S0021859600021572
Challinor, A. J., Watson, J., Lobell, D. B., Howden, S. M., Smith, D. R., & Chhetri, N. (2014). A meta-analysis of crop yield under climate change and adaptation. Nature Climate Change, 4(4), 287–291. https://doi.org/10.1038/nclimate2153
Chaubey, R. K., Bhutia, D. D., Navathe, S., Mishra, V. K., Singh, A. K., & Chand, R. (2021). Interrelationships among different grain characteristics of wheat grown under optimum and late sowing date conditions in the Eastern Indo-Gangetic plains of India. Cereal Research Communications, 49(1), 10. https://doi.org/10.1007/s42976-020-00123-z
Deva, C. R., Urban, M. O., Challinor, A. J., Falloon, P., & Svitákova, L. (2020). Enhanced leaf cooling is a pathway to heat holerance in common bean. Frontiers in Plant Science, 11, 1–17. https://doi.org/10.3389/fpls.2020.00019
Dias, A. S., & Lidon, F. C. (2009). Evaluation of grain filling rate and duration in bread and durum wheat, under heat stress after anthesis. Journal of Agronomy and Crop Science, 195, 137–147. https://doi.org/10.1111/j.1439-037X.2008.00347.x
Djanaguiraman, M., Narayanan, S., Erdayani, E., & Prasad, P. V. V. (2020). Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biology, 20(268), 1–12. https://doi.org/10.1186/s12870-020-02479-0
FAO. (2019). GIEWS Country Brief Zambia. https://www.fao.org/giews/countrybrief/country.jsp?code=ZMB&lang=AR
FAOSTAT. (2021). FAOSTAT. FAOSTAT. http://www.fao.org/faostat/en/#data/QC
Feng, B., Liu, P., Li, G., Dong, S. T., Wang, F. H., Kong, L. A., & Zhang, J. W. (2014). Effect of heat stress on the photosynthetic characteristics in flag Leaves at the grain-filling stage of different heat-resistant winter wheat varieties. Journal of Agronomy and Crop Science, 200, 143–155. https://doi.org/10.1111/jac.12045
Fischera, R. A., & Maurer, R. (1978). Drought resistance in spring wheat cultivars. I. Grain yield responses. Australian Journal of Agricultural Research, 29, 897–912. https://doi.org/10.1071/AR9780897
Fowler, D. B. (2018). Growth stages of wheat. In A practical guide to successful winter wheat production (Issue September, pp. 1001–1012). Ducks Unlimited Canada and Conservation Production Systems Limited.
JAICAF. (2008). Agriculture and forestry in Zambia: Present situation and issues for development.
Jumrani, K., Singh, V., Govind, B., & Pandey, P. (2017). Impact of elevated temperatures on specific leaf weight, stomatal density, photosynthesis and chlorophyll fluorescence in soybean. Photosynthesis Research, 131(3), 333–350. https://doi.org/10.1007/s11120-016-0326-y
Kazan, K., & Lyons, R. (2016). The link between flowering time and stress tolerance. Journal of Experimental Botany, 67(1), 47–60. https://doi.org/10.1093/jxb/erv441
Konrad, W., Katul, G., & Roth-nebelsick, A. (2021). Leaf temperature and its dependence on atmospheric CO2 and leaf size. Geological Journal, 56, 866–885. https://doi.org/10.1002/gj.3757
Kousar, R., Qureshi, R., Din, J., Munir, M., & Shabbir, G. (2018). Salicylic acid mediated heat stress tolerance in selected bread wheat genotypes of Pakistan. Pakistan Journal of Botany, 50(6), 2141–2146.
Leigh, A., Sevanto, S., Ball, M. C., Close, J. D., Ellsworth, D. S., Knight, C. A., Nicotra, A. B., & Vogel, S. (2012). Do thick leaves avoid thermal damage in critically low wind speeds? New Phytologist, 194(2), 477–487. https://doi.org/10.1111/j.1469-8137.2012.04058.x
Liu, W., Zheng, L., & Qi, D. (2020). Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecology and Evolution, 10(15), 8166–8175. https://doi.org/10.1002/ece3.6519
Mahrookashani, A., Siebert, S., Huging, H., & Ewert, F. (2017). Independent and combined effects of high temperature and drought stress around anthesis on wheat. Journal of Agronomy and Crop Science, 203(6), 453–463. https://doi.org/10.1111/jac.12218
Maiti, S. K. (2013). Soil sampling techniques. In Ecorestoration of the coalmine degraded lands (pp. 259–264). Springer India. https://doi.org/10.1007/978-81-322-0851-8
Marenco, R. A., & Nascimento, H. C. S. (2009). Relationship between specific leaf area, leaf thickness, leaf water content and SPAD-502 readings in six Amazonian tree species. Photosynthetica, 47(2), 184–190. https://doi.org/10.1007/s11099-009-0031-6
Marks, C. O., & Lechowicz, M. J. (2007). The ecological and functional correlates of nocturnal transpiration. Tree Physiology, 27, 577–584. https://doi.org/10.1093/treephys/27.4.577
Mason, R. E., Mondal, S., Beecher, F. W., & Hays, D. B. (2010). QTL associated with heat susceptibility index in wheat (Triticum aestivum L.) under short-term reproductive stage heat stress. Euphytica, 174, 423–436. https://doi.org/10.1007/s10681-010-0151-x
Mcmillen, G. G., & Mcclendon, J. H. (1983). Dependence of photosynthetic rates on leaf density thickness in deciduous woody plants grown in sun and shade. Plant Physiology, 72, 674–678. https://doi.org/10.1104/pp.72.3.674
Mehlich, A. (1984). Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Communications in Soil Science and Plant Analysis, 15(12), 1409–1416. https://doi.org/10.1080/00103628409367568
Mulenga, B. P., Kabisa, M., & Chapoto, A. (2019). Zambia agriculture status report, 2019.
Nautiyal, P. C., Zala, P. V., Pujari, S. D., Basu, M., Dhadhal, A. B., & Nandre, M. B. (2008). Evaluation of wild Arachis species for abiotic stress tolerance: I. Thermal stress and leaf water relations. Euphytica, 159, 43–57. https://doi.org/10.1007/s10681-007-9455-x
Qaseem, M. F., Qureshi, R., & Shaheen, H. (2019). Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse wheat (Triticum aestivum L.) genotypes varying in sensitivity to heat and drought stress. Scientific Reports, 9, 6955. https://doi.org/10.1038/s41598-019-43477-z
Ramirez-villegas, J., & Thornton, P. K. (2015). Climate change impacts on African crop production (119, Issue 119). www.ccafs.cgiar.org
Reynolds, M. P., Pierre, S. C., Saad, S. I. A., Vargas, M., & Condon, G. A. (2007). Evaluating potential genetic gains in wheat associated with stress-adaptive trait expression in elite genetic resources under drought and heat stress. Crop Science, 47(3), 172–189. https://doi.org/10.2135/cropsci2007.10.0022IPBS
Reynolds-henne, C. E., Langenegger, A., Mani, J., Schenk, N., Zumsteg, A., & Feller, U. (2010). Interactions between temperature, drought and stomatal opening in legumes. Environmental and Experimental Botany, 68, 37–43. https://doi.org/10.1016/j.envexpbot.2009.11.002
SAS, I. I. (2002). SAS/STAT. In SAS/STAT 9.2 Cary, NC, USA (9.2). SAS Institute Inc.
Shavrukov, Y., Kurishbayev, A., Jatayev, S., Shvidchenko, V., Zotova, L., Koekemoer, F., Groot, S. D., Soole, K., & Langridge, P. (2017). Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Frontiers in Physiology, 8(1950), 1–8. https://doi.org/10.3389/fpls.2017.01950
Snowdon, R. J., Wittkop, B., Chen, T. W., & Stahl, A. (2020). Crop adaptation to climate change as a consequence of long-term breeding. Theoretical and Applied Genetics, 133(11), 1–12. https://doi.org/10.1007/s00122-020-03729-3
Tembo, B. (2019). A review of rain-fed wheat production constraints in Zambia. Journal of Agriculture and Crops, 5(9), 158–161. https://doi.org/10.32861/jac.59.158.161
Tricker, P. J., Elhabti, A., Schmidt, J., & Fleury, D. (2018). The physiological and genetic basis of combined drought and heat tolerance in wheat. Journal of Experimental Botany, 69(13), 3195–3210. https://doi.org/10.1093/jxb/ery081
Truong, H. A., Lee, H., Kishii, M., Hong, S. W., & Lee, H. (2021). Development and characterization of synthetic hexaploid wheat for improving the resistance of common wheat to leaf rust and heat stress. Agronomy, 11(18), 1–17. https://doi.org/10.3390/agronomy11010018
Ullah, S., Bramley, H., Daetwyler, H., He, S., Mahmood, T., Thistlethwaite, R., & Trethowan, R. (2018). Genetic contribution of Emmer wheat (Triticum dicoccon Schrank) to heat tolerance of bread wheat. Frontiers in Plant Science, 9, 01529. https://doi.org/10.3389/fpls.2018.01529
Waines, J. G. (1994). High temperature stress in wild wheats and spring wheats. Australian Journal of Plant Physiology, 21, 705–715. https://doi.org/10.1071/pp9940705
World Bank. (2017). Climate-smart agriculture in Zambia. CSA country profiles for Africa series. World Bank.
Yu, M. H., Ding, G. D., Gao, G. L., Sun, B. P., Zhao, Y. Y., Wan, L., Wang, D. Y., & Gui, Z. Y. (2015). How the plant temperature links to the air temperature in the desert plant Artemisia ordosica. PLoS ONE, 10(8), 1–14. https://doi.org/10.1371/journal.pone.0135452
Acknowledgements
The authors are deeply grateful to the University of Zambia (UNZA) and the Regional Universities Forum for Capacity Building in Agriculture (RUFORUM) for funding this work. Their gratitude also goes to colleagues at Ministry of Higher Education—Zambia (MOHE) Zambia Research Institute (ZARI) and International Institute of Tropical Agriculture (IITA) for their research and technical support.
Funding
This study was funded by University of Zambia (UNZA) and the Regional Universities Forum for Capacity Building in Agriculture (RUFORUM).
Author information
Authors and Affiliations
Contributions
PC, SC, DL, KK, and JOO conceived and designed the experiment. PC conducted the experiment, collected, and entered the data. JOO analysed and presented the data. PC and JOO drafted the manuscript, it was reviewed by SC, DL and KK. JOO edited and revised the manuscript’s final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chileshe, P., Chikuta, S., Lungu, D. et al. Evaluation of wheat genotypes for heat stress tolerance and identification of early stress indicators. Plant Physiol. Rep. 28, 405–417 (2023). https://doi.org/10.1007/s40502-023-00735-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-023-00735-7