Abstract
Autism spectrum disorder (ASD) and schizophrenia spectrum disorders (SZ) are characterized by difficulty with social cognition and atypical reception of facial communication—a key area in the Research Domain Criteria framework. To identify areas of overlap and dissociation between ASD and SZ, we review studies of event-related potentials (ERP) to faces across ASD and SZ populations, focusing on ERPs implicated in social perception: P100, N170, N250, and P300. There were many inconsistent findings across studies; however, replication was strongest for delayed N170 latency in ASD and attenuated N170 amplitude in SZ. These results highlight the challenges of replicating research findings in heterogeneous clinical populations and the need for transdiagnostic research that continuously quantifies behavior and neural activity across neurodevelopmental disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Autism spectrum disorder (ASD) and schizophrenia spectrum disorders (SZ) are highly prevalent neurodevelopmental disorders (ND) with considerable public health impact, costing the USA a combined $423 billion per year (Cloutier et al., 2016). Though these disorders are classified in two separate diagnostic taxonomies, social deficits are diagnostic hallmarks of both, and diagnostic confusion is common, with ASD originally conceptualized as a form of childhood schizophrenia (Wolff, 2004). Social dysfunction is a core symptom of both ASD and SZ, evident in decreased social motivation, reduced social reward, impaired mentalizing, and decreased likelihood of social engagement (Dawson et al., 2005; Dowd & Barch, 2010; Schultz, 2005). Moreover, parallel lines of research indicate commonalities in behavior, genetic pathways, and neural processes, suggesting shared neuropathology (Cristino et al., 2014; Fernandes et al., 2018; Mitchell, 2011; Trevisan et al., 2020; Zheng et al., 2018). These findings are consistent with the hypothesis that common mechanisms contribute to social communication deficits across behaviorally defined categories of ND.
Despite these commonalities, few studies have directly compared distinct diagnostic groups or conceptualized these diagnostic classes as heterogeneous manifestations of shared dysfunction in overlapping neural substrates. For this reason, little is known about whether atypical social perception across NDs reflects common or distinct neural underpinnings. In this review, we focus on literature related to face and emotion processing across ASD and SZ, highlighting what is known, where the disorders converge and diverge, and what work remains to be done to understand social processes related to perception of facial communication across disorders.
Decreased attention to human faces is evident throughout the lifespan in ASD (Dawson et al., 2005; Maestro et al., 2002; Osterling & Dawson, 1994). Though SZ is not usually diagnosed until adolescence or early adulthood, infants later diagnosed with psychosis also show decreased attention to faces (Massie, 1978). Eye-tracking studies of perception of facial stimuli demonstrate atypical face scanning in individuals with ASD and SZ, with both clinical groups spending less time fixating on the eyes and more time fixating on other parts of the face (ASD: Joseph & Tanaka, 2003; Klin et al., 2002; Pelphrey et al., 2002; SZ: Gordon et al., 1992; Phillips & David, 1997). Individuals with ASD and SZ also show deficits in accurately identifying emotions, sorting emotional faces, and matching faces based on emotion (ASD: Baron-Cohen et al., 2001; Hobson, 1986; Ami Klin et al., 1999; Law Smith et al., 2010; SZ: Bellack et al., 1996; Morrison et al., 1988; Mueser et al., 1996). Atypical sensitivity to emotional expressions is a stable feature of multiple NDs and has been shown to associate with chronicity, severity, and type of active symptomatology (Addington & Addington, 1998; Davis & Gibson, 2000; Gaebel & Wolwer, 1992; Kington et al., 2000; Lewis & Garver, 1995). Difficulties in emotion recognition and discrimination in SZ and ASD are associated with specific disruption in social process systems, such as increased social withdrawal and impaired social cognition.
Face and emotion processing rely upon a network of specialized brain regions. Neuroimaging research has clarified the role of specific brain areas, including the fusiform gyrus, superior temporal sulcus, and amygdala, in face perception (Kanwisher, 2001; Kesler-West et al., 2001). Event-related potential (ERP) studies reveal a specific time course for related but distinct stages of face processing in this network (Luo et al., 2009; Wong et al., 2009). This sequence of ERPs is distinguished by scalp topography and chronology and shows sensitivity across perceptual processes, from detecting faces to interpreting differences in facial identity, direction of gaze, and displays of emotion (Bentin et al., 1996; Conty et al., 2007; Eimer & Holmes, 2002; Gosling & Eimer, 2011). These ERPs provide temporally precise indices of function across the regions involved in face perception, or, as conceptualized in the NIMH’s Research Domain Criteria (RDoC) framework, the subdomain reception of facial communication within the broader category of social process systems (Kozak & Cuthbert, 2016). Prior research has demonstrated the relevance of a subset of these components across neurological and psychiatric disorders (Feuerriegel et al., 2015). Here, we focus on their manifestation in ASD and SZ, reviewing a broad, temporally sequential set of ERP components associated with face processing: P100, N170, N250, and P300.
Measured over the occipital cortex as a positive deflection approximately 100 ms after the onset of a visual stimulus, the P100 reflects low-level processing in the visual cortex (V1) that is modulated by age and may be affected by the social nature of stimuli (Key et al., 2005; Mangun, 1995). In typical development (TD), faces elicit a larger (Herrmann et al., 2005; Itier & Taylor, 2004c) and faster (Kuefner et al., 2009; Taylor et al., 2001) P100 than objects, and in a number of studies, inverted faces elicit a larger (Roxane J Itier & Taylor, 2004a, 2004c) but slower (Itier & Taylor, 2004c; Taylor et al., 2001) P100 than upright faces. P100 amplitude can be modulated by emotion (Batty & Taylor, 2003).
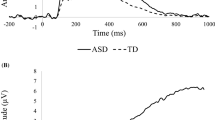
The N170 is a negative-going component, recorded over the occipito-temporal scalp approximately 170 ms after viewing a face, which indexes the earliest stages of face processing (i.e., structural encoding). Neural generators of the N170 have been localized to occipito-temporal sites, including the fusiform gyrus (Itier & Taylor, 2002, 2004b. Rossion et al., 2003; Sadeh et al., 2010), superior temporal sulcus (Itier & Taylor, 2004d; Yovel et al., 2008), lingual gyrus (Shibata et al., 2002), posterior inferotemporal gyrus (Schweinberger et al., 2002; Shibata et al., 2002), and inferior occipital gyrus (Jacques et al., 2018). Like the P100, in TD the N170 exhibits enhanced amplitude (more negative) and faster latency to faces relative to objects (Bentin et al., 1996; Itier & Taylor, 2004c) and larger amplitude but slower latency to inverted than to upright faces (Eimer, 2000; Itier & Taylor, 2004b, 2004c, 2004d; Rossion et al., 2000). For the N170, this “face inversion effect” is not found for upright vs. inverted objects, consistent with the interpretation that the N170 indexes face-selective processing (Eimer, 2000; Itier & Taylor, 2004c). N170 latency tends to be faster (Blau et al., 2007) with greater amplitude (Itier & Taylor, 2004b, 2004c; Rossion et al., 2003) over the right hemisphere than the left. Finally, N170 also may reflect an early indicator of emotion processing. Happy faces elicit faster N170 latencies than faces displaying negative affect (Batty & Taylor, 2003), and fearful faces elicit more negative N170 amplitude than neutral (Batty & Taylor, 2003; Blau et al., 2007) and happy (Brenner et al., 2014) faces.
The N250 is a negative-going component occurring approximately 250 ms post-stimulus (Schweinberger et al., 2002). Though less well characterized than the P100 and N170 components and rarely studied in typically developing populations, the N250 demonstrates increased amplitude in response to emotional expressiveness relative to neutral faces (Balconi & Pozzoli, 2008; Carretié et al., 2001; Labuschagne et al., 2010; Sato et al., 2001; Streit et al., 2001). As such, the N250 is considered a marker of higher-order face processing, such as affect decoding and emotion processing, and is presumed to reflect the modulatory influence of subcortical structures, including the amygdala (Streit et al., 1999). Research on face learning and repetition suggests the N250 is modulated by the identity of a face; familiar or repeated faces evoke a more negative N250 than unfamiliar or novel faces (Gosling & Eimer, 2011; Kaufmann et al., 2009; Tanaka et al., 2006). N250 latency delays to inverted faces have been observed in a face repetition task (Itier & Taylor, 2004b), and, as with the N170, the N250 has been shown to be right lateralized (Kaufmann et al., 2009). There is variation in published studies in terms of scalp electrodes selected to capture the N250 component. For the purposes of the current review, we included all topographies, permitting that the designated component was the N250 and in the context of experimental investigation of facial emotion perception.
The P300 is a large-amplitude positive component measured at central, frontal, and occipital locations approximately 300 ms after stimulus onset. It is associated with detecting novel and significant events (Ferrari et al., 2010; Picton, 1995; Picton, 1992; Polich et al., 1995), as well as with context updating (Donchin, 2008; Donchin & Coles, 1988, 1998). In the context of face perception, the P300 reflects processing of self-relevant stimuli, such as one’s own face (Ninomiya et al., 1998; Onitsuka et al., 2001). Emotional stimuli elicit a greater P300 amplitude than neutral stimuli (Johnston et al., 1986), and salient or attended stimuli elicit a greater P300 response than passively viewed stimuli (Carretié et al., 1997). P300 amplitude is larger to upright than to inverted faces and may be modified by emotional valence (Kestenbaum & Nelson, 1992), though the evidence is mixed (Conroy & Polich, 2007). As with the N250, there has been variance across studies in scalp electrodes selected to capture the P300; as above, we included all studies referencing a P300 in the context of experimental investigation of emotional face perception.
In this review, we highlight findings related to the amplitude and latency of these four ERP components, as elicited in response to face and emotion stimuli in individuals with ASD and SZ. This work expands on prior reviews focused solely on the N170 in ASD (Kang et al., 2018), a review on all four components in SZ (Murashko & Shmukler, 2019), and other disorders (Feuerriegel et al., 2015) by reviewing points of convergence and divergence for additional neural components associated with face processing transdiagnotically.
Methods
Search Criteria
Scopus, Google Scholar, and PubMed were queried using the following search criteria: (ASD OR autis* OR “pervasive developmental disorder” OR PDD-NOS OR Asperger* OR SZ OR schizo*) AND (ERP OR EEG OR electrophys* OR event-related potential OR P100 OR N170 OR N250 OR P300) AND (face OR facial OR emotion*). Reference lists, review articles, and meta-analyses were scanned to identify additional peer-review articles. Searches reflect updated content through December 31, 2020.
Inclusion Criteria: Methods and Components
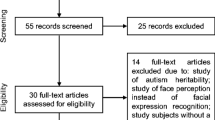
These searches yielded 74 eligible studies (38 ASD, 36 SZ); all included articles were peer-reviewed. Eligible studies were required to investigate at least one of the four key ERP components most commonly investigated in neuroscientific studies of face processing in typical and atypical development: P100, N170, N250, and P300. Studies that did not include at least one of these components were excluded (e.g., McMahon & Henderson, 2015). Included studies were required to use images of human faces as stimuli. Studies in which more than one face or object was simultaneously presented were excluded (e.g., Maher et al., 2016), as were studies in which all visual stimuli were combined with auditory stimuli (Müller et al., 2012). Studies that compared ERPs to faces with two or more emotions were designated as “emotion” studies. “Face” studies were categorized as either (1) studies that included only non-emotionally valenced faces or (2) emotion studies that collapsed across different emotions to examine main effects of diagnostic group. As a result, any particular study could be both a face and an emotion study, just a face study, or just an emotion study. Finally, studies whose results were not possible to interpret in our framework were excluded (e.g., Yang et al., 2017). See Fig. 1 for a flowchart of our inclusion criteria.
Inclusion Criteria: Subjects
Details about population demographics, subject characterization, study methodology, and stimuli are presented in Table 1. Studies were required to examine individuals diagnosed with either ASD or SZ, and those studying only high-risk individuals or relatives were excluded (Wolwer et al., 2012). No studies included both diagnostic groups. Studies were required to report their method for confirming patients’ diagnostic status. In over half of the studies of ASD (21 of 38), diagnosis was confirmed with a gold-standard clinical assessment, the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2012). For the remaining ASD studies, participants entered the study with a prior diagnosis according to DSM-IV or DSM-5 criteria (American Psychiatric Association, 1994; Association, 2013). The DSM-5 diagnosis of ASD removed subtypes, reconfigured the DSM-IV triad of impairments to a dyad, and added atypical sensory responses to the diagnostic criteria; though not identical, both sets of criteria were deemed appropriate for this review in that they both focus on social communication (American Psychiatric Association, 1994). Similarly, the DSM-5 diagnosis of SZ removed subtypes (e.g., paranoid) but was otherwise very similar to the DSM-IV diagnosis (American Psychiatric Association, 1994). In 31 of 36 SZ studies, clinicians confirmed diagnosis based on DSM-IV or ICD-10 criteria. The studies that did not specify these criteria for schizophrenia completed a Schedule for Affective Disorders and Schizophrenia (Spitzer & Endicott, 1979) or the combination of the Structured Clinical Interview and Rating Criteria (SCID) and the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1991). Study samples targeting individuals under three years of age were excluded, given the developmental emergence of some of the ERP components at this point (Itier & Taylor, 2004b). Studies were otherwise included regardless of target age group; however, all SZ studies targeted adults or late adolescents, whereas two-thirds of ASD studies exclusively included children. Studies that included both a child and an adult group analyzed separately were reported on as two separate studies in the “Results” section. Studies without a typically developing (TD) comparison group were excluded. For studies that examined additional clinical groups besides ASD or SZ (Tye et al., 2014; Tye et al., 2013), we focused only on results regarding ASD and SZ.
Results
Details on all study results described below can be found in Table 2. A graphical overview of findings can be found in Fig. 2.
P100 Amplitude
P100 Amplitude in ASD: Face
The majority of ERP literature reported no significant differences in P100 amplitude to faces between individuals with ASD and TD. Twenty-four studies of face processing in ASD reported on P100, 17 of which reported comparable P100 amplitudes in ASD and TD. In these 17 studies, no significant group differences in P100 amplitude to faces were identified across a range of task demands (attending to non-social target images to actively attending to facial stimuli) and in both child and adult samples (Akechi et al., 2010; Apicella et al., 2013; Luyster et al., 2017 [child and adult]; McPartland et al., 2011; Neuhaus et al., 2016; O'Connor et al., 2005 [child and adult]; 2007; Senju et al., 2005; Shen et al., 2017; Sysoeva et al., 2018; Tye et al., 2013; Wagner et al., 2013; Webb et al., 2010; Wong et al., 2008; Yamasaki et al., 2017).
Studies reporting group differences (7 of 24) in P100 amplitude to faces revealed inconsistent directionality of effects. One study of adults found increased P100 amplitude to faces in ASD versus TD (Cygan et al., 2014). One child study and one adult study reported inversion effects (greater P100 amplitude to inverted versus upright faces) in the TD group, but not in the ASD group (Hileman et al., 2011; Webb et al., 2012). Poorer face memory in the study of Webb et al. (2012) was correlated with greater P100 response to inverted versus upright faces in adults with ASD; however, there were no significant differences between groups in processing upright faces or objects. One study of children reported attenuated P100 amplitude to faces in ASD compared to TD, but lacked control stimuli (Batty et al., 2011), and a second study replicated this effect in young adults when using photographs of emotional faces, but reported a slightly larger P100 amplitude in ASD when using realistic drawings of emotional faces (Tseng et al., 2015). An additional study of children reported reduced P100 amplitude in ASD for repeated versus novel faces and objects whereas no difference was found in TD (Key & Corbett, 2014). A final study found reduced P100 lateralization in the ASD group as compared to controls (Luckhardt et al., 2017). Of these seven studies, three found effects across face and objects, and four did not include non-face stimuli. As such, no studies found a face-stimulus specific effect.
P100 Amplitude in ASD: Emotion
There were 13 studies of emotion processing in ASD that reported on P100 amplitude, distributed across childhood, adolescence, and adulthood. Across all studies, P100 was modulated similarly between diagnostic groups in response to emotional faces (Akechi et al., 2010; Apicella et al., 2013; Batty et al., 2011; Hileman et al., 2011; Luckhardt et al., 2017; Luyster et al., 2017 [child and adult]; O'Connor et al., 2005 [child and adult]; 2007; Wagner et al., 2013; Wong et al., 2008; Yamasaki et al., 2017).
P100 Amplitude in SZ: Face
Most studies of face processing (14 of 18) reported comparable P100 amplitudes to face stimuli between SZ and TD groups across a variety of methodologies and sample compositions (Bediou et al., 2007; Brenner et al., 2015; Fukuta et al., 2014; Herrmann et al., 2004; Jetha et al., 2013; P. J. Johnston et al., 2005; Jung et al., 2012; Kim et al., 2015; Lee et al., 2010; Liu et al., 2016; Obayashi et al., 2009; Thoma et al., 2013; Turetsky et al., 2007; Wynn et al., 2008). Among studies reporting P100 amplitude differences, all found attenuated P100 to faces in SZ groups (Caharel et al., 2007; Campanella et al., 2006; Shah et al., 2018; Zhang et al., 2016), though Campanella et al. (2006) found that this effect was specific to a group displaying high SZ symptomatology (compared to TD and low-symptom SZ groups). In all 4 of the studies that found significant differences, participants were actively attending to facial stimuli; 12 of the 14 studies that reported null results also included active attention to stimuli. Importantly, only 3 of 18 studies included control (i.e., non-face) stimuli, and only one of these reported differences between SZ and TD (Shah et al., 2018) while two did not (Obayashi et al., 2009; Wynn et al., 2008).
P100 Amplitude in SZ: Emotion
Out of 16 emotion-processing studies reporting P100 amplitude in SZ, 13 studies reported no significant group differences in P100 amplitude as a function of the emotional content of presented faces (Brenner et al., 2015; Caharel et al., 2007; Campanella et al., 2006; Fukuta et al., 2014; Johnston et al., 2005; Jung et al., 2012; Kim et al., 2015; Lee et al., 2010; Obayashi et al., 2009; Thoma et al., 2013; Turetsky et al., 2007; Wynn et al., 2008; Zhang et al., 2016). One study reported amplitude modulation by emotion in the TD group but not in the SZ group (Bediou et al., 2007). Jetha et al. (2013) reported that moderate shyness in SZ was associated with less emotional reactivity as indicated by P100 amplitude. This finding suggests that differences in P100 amplitude may mark alterations in emotion processing associated with specific symptom dimensions. Finally, Shah et al. (2018) found amplitude responses in the SZ group to be smaller than those of the TD group in sad, angry, and fearful faces occipitally and sad faces parietally. Taken together, these results suggest that P100 amplitude does not specifically index alterations in face or emotion processing.
P100 Latency
P100 Latency in ASD: Face
Almost two-thirds of studies (14 of 22) did not find significant differences between ASD and TD groups regarding P100 latency to faces (Apicella et al., 2013; Cygan et al., 2014; Hileman et al., 2011; Luckhardt et al., 2017; Luyster et al., 2017 [child]; McPartland et al., 2011; O'Connor et al., 2005 [child]; 2007; Senju et al., 2005; Sysoeva et al., 2018; Wagner et al., 2013; Webb et al., 2010; Webb et al., 2012; Wong et al., 2008). Eight studies reported group differences in response to facial stimuli. Two child and two adult studies reported increased latency to faces in ASD groups compared to the TD group (Batty et al., 2011; Luyster et al., 2017 [adult]; Neuhaus et al., 2016; O'Connor et al., 2005 [adult]). Of note, both Luyster et al. (2017) and O'Connor et al.’s (2005) child samples did not show differences between groups despite using the same methods as in their adult samples, where P100 latency delays were identified. A single study found shorter P100 latencies to faces in adults with ASD versus TD (Yamasaki et al., 2017). Two other child studies reported group differences in lateralization, wherein P100 latency was faster in the right versus left hemisphere in TD, but this effect was absent in ASD (Akechi et al., 2010; Tye et al., 2013). A final study showed shorter left hemisphere P100 latencies present only in the ASD group (Shen et al., 2017).
P100 Latency in ASD: Emotion
Twelve of 13 studies of emotion processing in ASD did not report significant group differences between TD and ASD groups in P100 latency sensitivity to emotion (Akechi et al., 2010; Apicella et al., 2013; Hileman et al., 2011; Luckhardt et al., 2017; Luyster et al., 2017 [child and adult]; O'Connor et al., 2005 [child and adult]; 2007; Wagner et al., 2013; Wong et al., 2008; Yamasaki et al., 2017). However, Batty et al. (2011) reported that P100 was modulated by emotion in TD children, but not in ASD.
P100 Latency in SZ: Face
Seven of 11 studies reporting P100 latency to face stimuli reported no significant differences between SZ and TD groups (Campanella et al., 2006; Fukuta et al., 2014; Herrmann et al., 2004; Johnston et al., 2005; Kim et al., 2015; Liu et al., 2016; Thoma et al., 2013). The remaining studies reported longer P100 latency in SZ groups compared to TD groups (Caharel et al., 2007; Lee et al., 2010; Obayashi et al., 2009; Wynn et al., 2008). None of these results are clearly face-specific, however; two studies did not include control stimuli (Caharel et al., 2007; Lee et al., 2010), and two found P100 latency delays in SZ across both face and control stimuli (Obayashi et al., 2009; Wynn et al., 2008).
P100 Latency in SZ: Emotion
Out of nine studies, eight reported no difference between P100 latency in SZ and TD groups in response to emotional faces (Caharel et al., 2007; Campanella et al., 2006; Fukuta et al., 2014; Johnston et al., 2005; Kim et al., 2015; Obayashi et al., 2009; Thoma et al., 2013; Wynn et al., 2008). One study reported sex-specific diagnostic differences in emotion processing: in females, P100 in the left hemisphere was delayed in SZ compared to TD when attending to happy faces (Lee et al., 2010). Moreover, longer latency to happy faces correlated with more negative symptomatology in SZ females. In SZ males, there was no overall delay in P100 compared to the TD group, and longer latency correlated with better performance on a behavioral detection task for happy versus fear faces.
N170 Amplitude
N170 Amplitude in ASD: Face
Since the initial report of N170 as a marker of atypical social perception in ASD (McPartland et al., 2004), 29 studies have examined N170 amplitude to faces in individuals with ASD, with 13 reporting differences in face processing by diagnosis. Of those 13, 6 studies (2 in children and 4 in adults) reported attenuated amplitude in ASD compared to TD (Churches et al., 2012b; Cygan et al., 2014; Groom et al., 2017; O'Connor et al., 2005 [adult]; Tye et al., 2014; Webb et al., 2012). Seven other studies reported differences in N170 response to faces between ASD and TD groups, but these differences were not always evident in direct statistical comparisons between groups (e.g., absence of lateralization in one group without demonstration of a group by lateralization interaction). One such study of children reported a typical face inversion effect in TD but the opposite effect in ASD (McPartland et al., 2011); conversely, another study found a stronger inversion effect in ASD, though this finding was not face-specific (Sysoeva et al., 2018). Three studies reported right hemisphere lateralization in TD but lack of lateralization in ASD (J. C. McPartland et al., 2011; Tye et al., 2013; Webb et al., 2006). Three studies reported findings of N170 amplitude differentiation between face stimulus types in TD but not ASD (Akechi et al., 2010), active versus passive tasks in TD but not ASD (Churches et al., 2010), and repeated versus novel faces and objects in ASD but not TD (Key & Corbett, 2014).
Most studies reporting correlative analyses demonstrated associations between N170 amplitude and autistic symptomatology or social behavior. More negative N170 amplitude to inverted faces correlated with better face recognition in children with ASD (McPartland et al., 2011) and better social skills in adults with ASD (Webb et al., 2012). Stronger overall N170 amplitude to faces was associated with more typical social behavior in TD children (though not in the ASD group; Hileman et al., 2011), and increased right lateralization was associated with better social communication across both TD and ASD children (Tye et al., 2013).
Sixteen of the 29 studies of N170 amplitude to faces in ASD did not find differences between diagnostic groups (1 of children, 5 distributed across childhood into adolescence, 2 of adolescents, 2 spanning adolescence into early adulthood, and 6 of adults) (Apicella et al., 2013; Batty et al., 2011; Churches et al., 2012a; Faja et al., 2016; Grice et al., 2005; Hileman et al., 2011; Khorrami et al., 2013; Luckhardt et al., 2017; Magnée et al., 2011; McPartland et al., 2004; O'Connor et al., 2005 [child]; 2007; Senju et al., 2005; Wagner et al., 2013; Webb et al., 2010; Wong et al., 2008). Approximately half of these 16 studies did, however, find differences in N170 latency, reported below. Of note, most of these studies did not instruct participants to attend to the faces specifically but included non-face targets to monitor attention. Only 4 of 16 studies with null results required participants to attend to faces. In contrast, all but one study (Webb et al., 2010) that reported differences between ASD and TD groups required participants to actively attend to stimuli. Given evidence that N170 amplitude is enhanced by attention (Churches et al., 2010; Mohamed et al., 2009), this finding suggests that differentiation of N170 amplitude emerges when the task demands are most face-specific.
N170 Amplitude in ASD: Emotion
Eleven of 15 studies reported no significant difference in N170 amplitude between ASD and TD groups during emotional face processing (Apicella et al., 2013; Batty et al., 2011; Hileman et al., 2011; Luckhardt et al., 2017; Luyster et al., 2017 [child and adult]; Magnée et al., 2011; O'Connor et al., 2005 [child and adult]; 2007; Wong et al., 2008). The remaining four studies reported group differences. In two of these studies, N170 amplitude was modulated by emotion in TD groups but not in ASD groups (Akechi et al., 2010; Wagner et al., 2013). One study found the opposite effect, showing N170 amplitude modulation by emotion only in the ASD group (Faja et al., 2016). This last finding is consistent with one other finding in which ASD groups with and without comorbid ADHD showed increased N170 amplitude to neutral relative to fearful faces, whereas amplitude in the TD group was not modulated by emotional valence (Tye et al., 2014). More negative N170 amplitude overall, as well as more negative amplitude to fearful compared to neutral faces, correlated with better social communication skills across both TD and ASD children (Tye et al., 2014). Contrary to patterns detected among face processing findings, only one of these four studies yielding group differences required participants to attend to the emotional valence of the stimuli; the other two required participants to attend to non-social target stimuli. Likewise, four studies with null results tasked participants with identifying the emotional valence of stimuli (O'Connor et al., 2005 [child and adult]; 2007; Wong et al., 2008). Thus, the degree to which explicit demands for attention to emotion may contribute to N170 amplitude alteration in ASD for emotion processing is less clear than for face stimuli.
N170 Amplitude in SZ: Face
Twenty-four out of 32 studies investigating N170 amplitude to faces in adults with SZ reported significant differences in face processing between diagnostic groups. In four of these studies, amplitude to faces was smaller (less negative) in SZ relative to TD, whereas amplitude to objects was not significantly different between groups (Herrmann et al., 2004; Obayashi et al., 2009; Onitsuka et al., 2006; Tsunoda et al., 2012). One study observed N170 attenuation in SZ across inverted face and non-face images (Zheng et al., 2016). Another study showed right lateralization of N170 to low spatial frequency compared to high spatial frequency of fearful faces in SZ but no differentiation in TD groups (Kim et al., 2015). Eighteen additional studies report attenuated N170 amplitude to faces in SZ relative to TD; however, across all these studies, findings were not necessarily face-specific, as several lacked non-face control stimuli (Bediou et al., 2007; Caharel et al., 2007; Campanella et al., 2006; Jetha et al., 2013; Jung et al., 2012; Kirihara et al., 2012; Lee et al., 2010; Onitsuka et al., 2009; Onitsuka et al., 2020; Turetsky et al., 2007; Wang et al., 2020; Zhang et al., 2016) and the remainder showed main effects of group where N170 amplitude attenuation spanned both face and non-face stimuli (Ibanez et al., 2012; Liu et al., 2016; Lynn & Salisbury, 2008; Salisbury et al., 2019; Shah et al., 2018; Wynn et al., 2013). Of note, Salisbury et al. found this effect in both a group of patients presenting with chronic SZ and a group presenting with their first SZ-related hospitalization.
A handful of studies reported correlations between greater N170 amplitude to faces and higher global functioning (Obayashi et al., 2009), less positive SZ symptomatology (Campanella et al., 2006), more positive SZ symptomatology and lower personal and social performance scores (Wang et al., 2020), decreased shyness (Jetha et al., 2013), higher social competency (Tsunoda et al., 2012), and increased extraversion (Kirihara et al., 2012). Together, these studies provide strong evidence for an association between more robust N170 response to faces and better social and psychiatric functioning across both TD and SZ populations. Eight studies reported no significant differences in N170 amplitude to faces between diagnostic groups (Akbarfahimi et al., 2013; Brenner et al., 2015; Komlosi et al., 2013; Lee et al., 2007; Ramos-Loyo et al., 2009; Thoma et al., 2013; Tso et al., 2015; Wynn et al., 2008). However, one found emotion-specific results that are detailed in the following section (Thoma et al., 2013), and the second found decreased N170 differentiation to facial angle as compared to TD controls (Tso et al., 2015). These eight studies have comparable methodologies and group demographics to those studies that do detect group differences.
N170 Amplitude in SZ: Emotion
A number of studies (12 of 25) suggest altered N170 amplitude during emotion discrimination in SZ compared to TD (Caharel et al., 2007; Campanella et al., 2006; Ibanez et al., 2012; Jung et al., 2012; Kirihara et al., 2012; Lynn & Salisbury, 2008; Onitsuka et al., 2020; Shah et al., 2018; Thoma et al., 2013; Tso et al., 2015; Turetsky et al., 2007; Zhang et al., 2016). Four of these studies reported that TD groups showed modulation of N170 amplitude based on emotional valence, whereas SZ groups did not (Campanella et al., 2006; Ibanez et al., 2012; Kirihara et al., 2012; Lynn & Salisbury, 2008). Four studies reported attenuated N170 amplitude in SZ compared to TD to specific emotions, such as disgust, sadness, and happiness (Caharel et al., 2007; Jung et al., 2012; Onitsuka et al., 2020; Turetsky et al., 2007). One found that N170 amplitude was differentially modulated by emotion in SZ relative to TD: whereas in TD it was larger for happy and fearful faces than for neutral faces, in SZ it was larger for happy faces than for neutral or fearful (Zhang et al., 2016). Another found emotion modulation, with neutral and fearful larger in amplitude than happy and angry faces, in the controls but on the SZ group (Shah et al., 2018). Additionally, reduced left hemisphere N170 amplitude to fearful faces in particular was correlated with severity of blunted affect in patients. Two additional studies found group differences as a function of more nuanced influences on emotion perception, including larger left lateralized N170 amplitude in fearful vs. neutral averted-gaze faces in SZ (Tso et al., 2015), and enhanced N170 amplitude in TD, but not SZ, when the emotion displayed in a stimulus differed from the stimulus emotion proceeding it (Thoma et al., 2013).
Thirteen studies investigating N170 amplitude reported no significant differences between SZ and TD in this ERP component during emotion processing (Akbarfahimi et al., 2013; Bediou et al., 2007; Colleen A Brenner et al., 2015; Jetha et al., 2013; Kim et al., 2015; Komlosi et al., 2013; S. Lee et al., 2007; S.-H. Lee et al., 2010; Obayashi et al., 2009; Ramos-Loyo et al., 2009; Wang et al., 2020; Wynn et al., 2013; Wynn et al., 2008). These studies have comparable population demographics and methodologies to the studies above where differences were detected.
N170 Latency
There were 32 studies of ASD that reported N170 latency. Nineteen of these studies were performed in children, and 13 were in adults.
N170 Latency in ASD: Face
Of the 19 studies investigating N170 latency during face processing in children with ASD, eight reported atypical temporal processing in the ASD group. One study reported face-specific delays in the ASD group compared to the TD group (McPartland et al., 2011); another reported delayed latency to faces compared to objects in the ASD group, contrasted with faster latency to faces than objects in the TD group (Webb et al., 2006); and four studies found slower N170 latency in ASD compared to TD groups across both face and non-face stimuli (M. Batty et al., 2011; Hileman et al., 2011; Khorrami et al., 2013; Luyster et al., 2017 [child]). A study by Gunji et al. (2009) found delayed latency when comparing a child ASD group to adult controls but not to child controls. One study observed shorter N170 latencies in the left hemisphere only in the ASD group across both social and nonsocial stimuli (Shen et al., 2017); this finding is in the opposite direction than others, indicating faster N170 latency in ASD. Eleven studies in children did not find significant between-group differences in N170 latency to faces (Akechi et al., 2010; Apicella et al., 2013; Grice et al., 2005; Luckhardt et al., 2017; O'Connor et al., 2005 [child]; Senju et al., 2005; Sysoeva et al., 2018; Tye et al., 2014; Tye et al., 2013; Wagner et al., 2013; Wong et al., 2008). There were no observable differences in methodology or group characteristics to account for variations in study findings (Table 1).
In adults, seven of 13 studies indicated atypical N170 latency in ASD groups. Across two studies, faces elicited a slower N170 latency in ASD compared to TD, while between-group differences in N170 latency to object images were not significant (McPartland et al., 2004; O'Connor et al., 2007); an additional three studies also found N170 delays to faces, but there were no non-social control stimuli in these experiments (Luyster et al., 2017 [adult]; O'Connor et al., 2005 [adult]; Yamasaki et al., 2017). Two studies suggest a relation between N170 latency and clinical symptoms. McPartland et al. (2004) found that, in ASD but not TD, slower N170 latency to both upright and inverted faces associated with better face memory, suggesting that longer processing times may confer improved behavioral performance. Though Webb et al. (2012) did not find deficits in N170 latency to upright faces relative to either houses or inverted faces or replicate McPartland et al.’s (2004) association between N170 latency with face memory, faster N170 to upright versus inverted faces was related to both better social skills and better face memory. Two studies reported topographic differences in N170 latency between ASD and TD. N170 latency to faces was faster medially than laterally in TD (Webb et al., 2012), but trending toward faster laterally than medially in ASD (Webb et al., 2010). Finally, six studies of adults with ASD found no significant between-group differences or differences in lateralization in N170 latency to face stimuli (Churches et al., 2012a; Churches et al., 2012b; Owen Churches et al., 2010; Cygan et al., 2014; Magnée et al., 2011; Tseng et al., 2015). A recent meta-analysis of face processing studies in ASD indicated that, on average, N170 latency was delayed in ASD relative to TD (Kang et al., 2018).
N170 Latency in ASD: Emotion
Fifteen of sixteen studies of children and adults converge in reporting no emotion-dependent differences in N170 latency between individuals with ASD and TD (Akechi et al., 2010; Apicella et al., 2013; Batty et al., 2011; Hileman et al., 2011; Luckhardt et al., 2017; Luyster et al., 2017 [child and adult]; Magnée et al., 2011; O'Connor et al., 2005 [child and adult]; 2007; Tseng et al., 2015; Wagner et al., 2013; T. K. Wong et al., 2008; Yamasaki et al., 2017). Seven of these studies were of children only, two studies included both child and adult groups, and four studies only included adults. Just one study reported significant differences in emotion perception: when taking ADHD diagnosis into account, Tye et al. (2014) found a group by emotion interaction in which children with ASD showed a longer N170 latency to angry faces than to neutral faces and a shorter latency to happy faces than to fearful faces, whereas children with ASD and comorbid ADHD showed the opposite pattern and TD children did not show any differences in N170 latency across emotions. This finding suggests that a cross-diagnostic approach to identifying neural indices of emotion processing may be more informative than studies restricted to one clinical group.
N170 Latency in SZ: Face
Eleven of 16 studies reported no difference in N170 latency between diagnostic groups in response to face stimuli (Campanella et al., 2006; Herrmann et al., 2004; Johnston et al., 2005; Kim et al., 2015; Lee et al., 2007; Lynn & Salisbury, 2008; Obayashi et al., 2009; Thoma et al., 2013; Tso et al., 2015; Tsunoda et al., 2012; Wynn et al., 2008). The remaining five studies found adults with SZ showed delayed N170 latency to faces compared to TD controls. One of these studies found these results for both upright and inverted faces (Zheng et al., 2016) and showed that N170 latency correlated with both negative and general psychiatric symptoms in SZ patients. However, because the other four studies either had no control stimuli (Caharel et al., 2007; Lee et al., 2010; Wynn et al., 2013) or saw main effects across both face and non-face stimuli (Akbarfahimi et al., 2013; Caharel et al., 2007; Lee et al., 2010), N170 delays in SZ are not necessarily face-specific.
N170 Latency in SZ: Emotion
Eleven of 13 studies of emotion processing reported no significant differences between SZ and TD in N170 latency modulation to emotional faces (Caharel et al., 2007; Campanella et al., 2006; Johnston et al., 2005; Kim et al., 2015; Lee et al., 2007; Lynn & Salisbury, 2008; Obayashi et al., 2009; Thoma et al., 2013; Tso et al., 2015; Wynn et al., 2013; Wynn et al., 2008). Two studies reported a differential effect of emotional faces on N170 latency in SZ versus TD. In a passive viewing study, adults with SZ showed a delayed N170 to fearful faces relative to other emotional faces, whereas controls displayed the shortest latencies to fearful faces (Akbarfahimi et al., 2013). Investigating gender differences, Lee et al. (2010) reported emotion-specific differences between females, but not males, with SZ and TD: in the right hemisphere, happy and fearful faces elicited a delayed N170 in females with SZ compared to those with TD. Slower N170 latency to happy faces in females with SZ was also correlated with increased expression of negative SZ symptomatology. An earlier study from the same group reported faster N170 latency to happy and neutral faces was associated with increased positive and negative SZ symptomatology, but found no significant differences between groups (Lee et al., 2007); thus, examining gender-specific effects may clarify the nature of processing deficits and relations with clinical symptoms.
N250 Amplitude
N250 Amplitude in ASD: Face
Two studies in adults with ASD reported on N250 amplitude, and both found significant, yet conflicting, group differences. When viewing a series of neutral faces and instructed to attend to a target face, relative to TD adults, adults with ASD showed reduced N250 amplitude to targets (Churches et al., 2012a). Groups did not differ in their N250 response to non-target faces. In contrast, Webb et al. (2010) reported enhanced N250 amplitude to both familiar and unfamiliar face stimuli in the ASD group relative to TD adults. In the same study, N250 amplitude difference between familiar versus unfamiliar faces was right lateralized in ASD, while it was uniform across hemispheres in the TD group. Differing methodologies across these two studies may have elicited different main effects. The first used unfamiliar target faces in an active viewing task demanding attention and facial memory; the second used familiar faces in a passive viewing task. Of note, Webb et al. (2010) also reported that increased right lateralization of N250 to familiar versus unfamiliar faces in the ASD group correlated with lower ADOS scores in communication and social domains.
N250 Amplitude in ASD: Emotion
There were no studies of emotion processing in ASD that reported N250 amplitude.
N250 Amplitude in SZ: Face
Three of eight studies of SZ measuring N250 amplitude to faces reported group differences. Two of these studies reported attenuated N250 in SZ groups relative to TD groups to both face and non-face stimuli (Wynn et al., 2013; Wynn et al., 2008). These studies used the same methodology, and the authors noted that while their TD groups were different across the two studies, their SZ groups were highly overlapping. A third investigated sex differences and reported larger N250 amplitudes in males versus females with SZ when attending to faces; this sex difference was absent in TD (Jung et al., 2012). Overall, however, no between-group (SZ versus TD) differences were significant. The remaining five studies did not find significant differences in N250 amplitude between groups (Brenner et al., 2015; Kim et al., 2015; Lee et al., 2010; Liu et al., 2016; Turetsky et al., 2007).
N250 Amplitude in SZ: Emotion
Seven of the eight studies reported above used emotional stimuli, but none found group differences in N250 amplitude as a function of the emotional valence of faces.
N250 Latency
N250 Latency in ASD
There were no studies of face or emotion processing in individuals with ASD that reported N250 latency.
N250 Latency in SZ
There were five studies of face and emotion processing in SZ that reported N250 latency; however, none found significant group differences, either to faces overall or to emotional faces specifically (Kim et al., 2015; Lee et al., 2010; Liu et al., 2016; Wynn et al., 2013; Wynn et al., 2008).
P300 Amplitude
P300 Amplitude in ASD: Face
Three of four studies of ASD reporting on P300 amplitude detected differences between diagnostic groups, albeit in conflicting directions. In two studies, familiar faces elicited greater P300 than unfamiliar faces in TD children, whereas this effect was absent in children with ASD (Gunji et al., 2013; Gunji et al., 2009). In contrast, Key and Corbett (2014) found enhanced P300 amplitude to repeated faces in children with ASD relative to the TD group. P300 amplitude was also greater to repeated faces than to neutral stimuli in ASD but not TD. However, in this study, the same pattern was seen for repeated objects, suggesting the enhanced P300 effect during stimulus repetition was not necessarily face-specific. When measuring neural response to averted and direct eye gaze, there were no significant differences in P300 amplitude between children with ASD and TD to either stimulus type (Senju et al., 2005).
P300 Amplitude in ASD: Emotion
There were no studies of emotion processing in ASD that reported P300 amplitude.
P300 Amplitude in SZ: Face
Seven of 11 studies of SZ reporting on P300 amplitude detected diagnostic differences during reception of facial stimuli. In five of these studies, P300 amplitude was attenuated in SZ relative to TD, although these results were either found across both faces and control stimuli (Shah et al., 2018; Ueno et al., 2004) or had no control stimuli (Onitsuka et al., 2020; Ramos-Loyo et al., 2009; Turetsky et al., 2007). However, greater P300 amplitude was correlated with lower negative SZ symptomatology (Ueno et al., 2004) and better emotion identification (Turetsky et al., 2007), suggesting its relevance for social symptomatology specifically. In a study of sex differences, while P300 amplitude was equivalent in TD males and females, females with SZ showed a larger P300 amplitude to faces than males with SZ; overall, there were no between-group differences with regard to P300 amplitude (Lee et al., 2010). Finally, a study investigating effects of antipsychotic medication reported that, relative to TD controls, the SZ group showed attenuated P300 amplitude to faces, but only after exposure to 12 months of medication (Mori et al., 2012). Four studies reported no group differences in P300 amplitude to faces (Bediou et al., 2007; Johnston et al., 2005; Jung et al., 2012; Kim et al., 2015). There were no clear patterns among results to indicate methodological or sample differences accounting for this variability.
P300 Amplitude in SZ: Emotion
Three of 11 studies of emotion processing reported significant group differences in P300 amplitude to emotional faces in SZ. When observing images of infant faces and objects, TD adults showed the largest P300 amplitude to a crying infant face and the smallest amplitude to a happy face (Ueno et al., 2004), suggesting that an infant’s distress is most salient to TD adults. In the SZ group as a whole, P300 amplitude was not modulated by the emotional valence of the infant face; however, when the SZ group was divided by subtype, adults with paranoid SZ showed the same pattern as the TD group, while those with non-paranoid SZ showed an inverted effect, with larger P300 amplitude to happy than to crying faces. In a second study, fearful adult faces elicited greater P300 amplitude than neutral faces in TD adults tasked with attending to emotional valence. There was no modulation of the P300 response by emotion in the SZ group (Bediou et al., 2007). A third study found that TD adults had higher P300 amplitudes to fearful and neutral faces than the SZ group, but the groups responded similarly to happy and sad faces (Onitsuka et al., 2020). The remaining eight studies found no significant group differences in emotion processing as measured by P300 amplitude (Johnston et al., 2005; Jung et al., 2012; Kim et al., 2015; Lee et al., 2010; Mori et al., 2012; Ramos-Loyo et al., 2009; Shah et al., 2018; Turetsky et al., 2007).
P300 Latency
P300 Latency in ASD
Two face processing studies in ASD reported on P300 latency; neither found group differences (Gunji et al., 2013; Senju et al., 2005). No emotion processing studies in ASD reported on P300 latency.
P300 Latency in SZ
Three of five studies of face and emotion processing that reported on P300 latency in SZ found group differences. Two studies found delayed P300 in SZ compared to TD in response to all stimuli, regardless of whether stimuli were faces or objects, or of the emotional valence of face stimuli (Lee et al., 2010; Ueno et al., 2004). Latency differences were, however, modulated by paranoia. Specifically, adults with non-paranoid SZ showed longer P300 latency to faces than TD adults, whereas individuals with paranoid SZ did not differ from the TD group (Ueno et al., 2004). This pattern parallels the P300 amplitude finding from the same study. In a third study, adults with SZ who had been medicated for 12 months showed delayed P300 latency to happy—but not neutral or sad—faces relative to TD adults (Mori et al., 2012). In the same study, a slowing effect of medication on P300 latency in SZ was apparent: adults with SZ showed slower P300 latency after 12 months of medication compared to their responses while medication-naïve and medicated for a shorter, 3-month interval. Two studies did not report group differences in P300 latency (Johnston et al., 2005; Kim et al., 2015).
Discussion
Here we have reviewed the results of studies reporting on P100, N170, N250, and P300 ERP components in response to neutral and emotional face stimuli in individuals with autism spectrum disorders and schizophrenia. Our review sheds light on patterns of replicability in the literature and highlights multiple areas marked by inconsistent findings and in need of further research.
For the P100, which reflects early stages of visual processing, there is little evidence of amplitude or latency alterations in ASD across face or emotion processing. Scattered findings suggest that P100 amplitude may index neural processes relevant to ASD, but primary P100 deficits are unlikely. As with face processing, P100 latency does not seem to be a reliable indicator of emotion processing differences between ASD and TD groups. While P100 latency may index variability in speed of low-level visual processing across groups, it does not capture behavioral differences in face or emotion processing between ASD and TD groups. In schizophrenia, there is similarly little evidence for P100 amplitude or latency alterations being effective indices of face or emotion processing deficits.
The N170, a well-studied index of face processing, was the component most consistently demonstrating atypical neural response across the two neurodevelopmental disorders. In ASD studies, approximately 40% of all findings reported diminished amplitude and delayed latency to faces in ASD compared to TD; however, several of these studies did not include non-face control stimuli or found effects that were not specific to faces. Two-thirds of the studies that found significant differences required active attention to stimuli, whereas three-quarters of the studies that found no significant differences were passive viewing tasks. This distinction suggests that N170 deficits in ASD are particularly prominent when attentional demands are involved. There was little evidence of N170 amplitude or latency reliably indexing deficits in emotion processing in ASD. In studies of SZ, evidence for N170 latency delays to faces and differential N170 latency by emotion was mixed. However, there was strong evidence of diminished N170 amplitude to both face and non-face stimuli, indicating differences by diagnosis in early visual—but not necessarily face-specific—processing. There was also strong evidence of diminished N170 amplitude in SZ from studies of emotion processing. In general, N170 amplitude was differentially responsive to neutral versus emotionally valenced faces in TD groups, whereas SZ groups did not tend to show this specialization. This pattern may suggest that processing the emotional content of faces, rather than just faces themselves, may be most specifically atypical in SZ. Moreover, a recent systematic review on the N170 in SZ noted that correlations with clinical features were strongest in older individuals and that abnormalities in electrophysiological findings are more associated with more severe symptomatology (Murashko & Shmukler, 2019). In contrast, early structural processing of faces in general, more so than differentiation of particular emotions, may be more consistently impacted in ASD. However, the utility and reliability of the N170 as a biomarker in ASD remain debated and experiment features such as sample size and effect size must be considered closely (Kang et al., 2019; Vettori et al., 2019).
Across the N250 and P300 components, there are too few studies in ASD to draw conclusions despite promising group differences. Further research comparing N250 and P300 amplitude in ASD and TD, along with re-analysis of previously published studies that did not include these components in their original analysis, may help elucidate the neural underpinnings of atypical face and emotion perception. Many studies that measured N250 and P300 amplitude found group differences in face perception, indicating that both components may be understudied but promising leads for indexing social cognition deficits.
In SZ, there is a slightly larger literature, but evidence for diminished N250 and P300 amplitude to face stimuli is mixed. N250 amplitude was attenuated to face, but not emotion, stimuli in approximately 40% of SZ studies, but N250 latency appears to be unaffected. P300 amplitude to face stimuli appear to be more reliably affected, and P300 amplitude may both mark symptom levels and be affected by medication treatments. Though findings from a small sample of studies suggest P300 latency may reliably index cognitive processing deficits for both face and emotion stimuli in SZ, the relative paucity of literature measuring P300 latency makes it difficult to identify this component as a sensitive neural marker of social cognition differences.
To expand both ASD and SZ literatures, given that ongoing, continuous EEG is recorded before segmentation for ERP analyses, it is likely that pre-existing data from studies reporting on earlier components (e.g., P100 and/or N170) could provide an opportunity to analyze N250 and P300 components to test for group differences during face and emotion processing. Such re-analysis of existing data may provide a quick and efficient mechanism to address outstanding questions of whether later, attentional neural responses are impacted in the context of reception of facial communication, particularly in ASD where existing data is more limited.
Accounting for Variability Across Studies
Variation in methodologies—such as stimulus design and subject factors—may account for some of the inconsistencies among studies. Differences in low-level visual characteristics, such as luminance, contrast, and color, can produce significantly different waveforms (Rossion & Caharel, 2011; Rousselet & Pernet, 2011), yet most studies did not provide specific details on the low-level visual aspects of their stimulus set. Likewise, there were many different types of stimuli across studies. For example, while all studies included face stimuli, the nature of these faces varied (i.e., upright vs. inverted, familiar vs. unfamiliar, neutral vs. emotional, child vs. adult). Moreover, faces were sometimes the sole stimuli within a study paradigm, whereas other studies included non-face conditions. Study design, such as the use of blocking by stimulus type (e.g., face vs. non-face; neutral vs. emotional faces) vs. randomly interspersing all stimuli, can affect ERPs due to repetition and habituation effects (Bentin & Peled, 1990; Ravden & Polich, 1999), which few studies examined. Importantly, many studies claiming group differences in face processing did not include non-face control stimuli, preventing conclusions regarding the specificity of reported findings to faces.
Participant tasks varied widely across studies, from passive viewing, to providing button-press responses to occasional attentional control trials not included in later analyses, to providing task-relevant verbal responses to each trial. Variability in task design yields inconsistencies in the nature and burden of the cognitive load placed on the participant and may manipulate how intensely and/or consistently the participant is attending to the stimuli. Unfortunately, there were very few instances in which clear patterns of replicable vs. less consistent findings were revealed as a function of particular aspects of stimulus type, task design, or demands for participant attention and task engagement. Finally, no studies in this review monitored participant gaze with eye tracking; thus, while participants were ostensibly looking toward the screen (based on experimenter observations or attention monitoring tasks), gaze was not directly measured.
Subject factors also may have contributed to variability across studies. For example, not all studies matched clinical and control groups by IQ, making it difficult to ascertain which observed differences were due to diagnosis versus may have been driven by differences in cognitive functioning. Moreover, most studies did not account for sex when matching controls, and this variable may drive certain effects or correlations in ASD and SZ groups, as highlighted by several studies (Jung et al., 2012; Lee et al., 2010). ERP components indexing face perception change in amplitude and latency over the lifespan (Batty et al., 2011; Itier & Taylor, 2004b), yet, when comparing studies of ASD and SZ, we are limited by the fact that most studies of ASD are in children, while all studies of SZ are in adults. Participants in clinical groups, especially SZ, were often medicated with psychoactive agents. In their longitudinal investigation of the effects of antipsychotic use on ERP waveforms, Mori and colleagues (Mori et al., 2012) reported changes in patients’ P300 amplitude and latency between drug-naïve and medicated time points, and another study reported a positive correlation between medication dosage and P300 amplitude to emotional compared to neutral faces (Komlosi et al., 2013). Many studies did not report on the medication status of patients, and still others reported medication status but did not discuss testing for correlations between ERP waveforms and medication dose or controlling for medication status. Thus, effects of medication differences among participants and between clinical and control groups could also contribute to variability in findings across studies.
Variability in clinical profiles within participant samples could also have affected results. With respect to subject characterization, many studies did not report confirming ASD or SZ diagnoses in the context of the study with an ADOS (for ASD; Lord et al., 2012) or SCID (SCID, for SZ; First, 1995). Lack of rigorous characterization of participants may mean that some clinical groups were not composed entirely of participants with gold-standard ASD or SZ diagnoses and/or may have included individuals falling short of diagnostic threshold. Many studies did not report level or severity or particular profiles of symptomatology within patient samples, despite the fact that ASD and SZ samples tend to be phenotypically heterogeneous. With disorders that are behaviorally defined (and which likely lump individuals with multiple different etiological origins based on their symptoms; Happé et al., 2006; Insel, 2010), re-grouping participants by subtypes or symptom profile can be a helpful way of finding patterns. Conducting correlations and regressions accounting for symptom levels on well-validated measures like the ADOS (for ASD; Lord et al., 2012) and Positive and Negative Syndrome Scale (PANSS, for SZ; Kay et al., 1987), as well as broader measures of symptoms that cut across diagnoses, may help further clarify the association between clinical profiles and neural processing abnormalities. For example, when dividing their SZ group into paranoid and non-paranoid subgroups, Ueno et al. (2004) found that the paranoid subgroup showed similar P300 amplitude and latency to the TD group, whereas the non-paranoid subgroup differed in P300 amplitude to emotional faces and in latency to all stimuli. When looking at the SZ group as a whole, however, these differences were obscured.
Related to the need to carefully characterize and account for variation in symptom profiles among participants is the need to recruit large study samples in order to capture heterogeneity and enable statistical analyses that are adequately powered to detect effects using covariates and regressions. Among the studies highlighted in this review, 50% of studies in ASD (18 of 38) and approximately 25% of those in schizophrenia (8 of 36) had fewer than 15 participants per group. Moreover, whereas nearly 25% of SZ studies (8 of 36) included 30 or more participants per group, only approximately 10% of ASD studies (4 of 38) had sample sizes this large. Small samples are particularly vulnerable to spurious or non-generalizable findings (Button et al., 2013). Thus, it may be the case that some of the inconsistencies across studies relate to the relatively small sample sizes, particularly in the ASD literature. Moving forward, recruiting large samples to enable examination of more homogenous subsets of participants and to allow for evaluation of the association between ERP components and clinical traits measured in a continuous fashion might help to explain differences among studies where samples can be quite variable. Performing meta-analyses will also help to disentangle type two error due to small sample size from biologically real effects (e.g., Fernandes et al., 2018; Kang et al., 2018; Zheng et al., 2018).
Considerations for Future Research
This review of electrophysiological studies of face perception in ASD and SZ indicates that the N170, and possibly the N250 and P300, hold promise as biomarkers indexing the integrity of social perceptual systems across neurodevelopmental disorders and for providing clues about underlying neural dysfunction within and across disorders. Several caveats for future research emerge from these findings.
First, this review makes clear that alterations in the highlighted ERP indices are not diagnostically specific. Similar patterns of atypical processing at identical components were observed in both ASD and SZ. This suggests that, consistent with the RDoC framework, it may be most useful to evaluate the underlying neural bases of neurodevelopmental disorders in terms of specific, dimensionally measured processes related to function and dysfunction transdiagnostically, rather than designing studies around a particular diagnostic category. Although the reviewed evidence does not make a reliable case that any one particular ERP component may be a consistent diagnostic biomarker, correlations with symptomatology and key associated features (e.g., emotion recognition) indicate promise with respect to other areas of biomarker development. These include classifying individuals into subgroups relevant to treatment selection (stratification biomarkers), demonstrating the influence of an intervention on a targeted neural system (target engagement biomarkers), or offering short-term indication of intervention effects on symptoms or underlying neural systems (early efficacy biomarkers; McPartland, 2016). Our review focused specifically on ASD and schizophrenia; however, social cognition and these ERP components are germane to multiple other psychiatric diagnoses (Feuerriegel et al., 2015). Increased focus on transdiagnostic samples can provide greater clarity regarding the information these ERP indices provide about the neural processes underlying neurodevelopmental and psychiatric disorders.
As the variability in findings across existing studies makes clear, it is necessary to carefully consider individual differences in participant characteristics, such as age, both within and across diagnostic groups. Differences in findings reported within ASD between child and adult samples and cross-diagnostically between studies of ASD and SZ may reflect differences in age-related characteristics of particular samples. For example, some alterations in neural responses may be more prominent in adults, reflecting later-onset difficulties or premature developmental plateaus, whereas some may be most notable in young children and resolve over time. Future research on markers of social perception must either constrain age ranges to reduce variability, conduct studies with sufficient statistical power to co-analyze age effects, or examine participant samples longitudinally to study the divergence of particular neural responses as they relate to emergence or amelioration of symptoms over time. Though age effects are less germane to schizophrenia, a disorder most commonly occurring in adulthood, whether alterations in ERP response to face stimuli are present in prodromal phases is an important question. Moreover, as other factors such as cognitive ability are also demonstrated to influence ERP indices, these characteristics must be considered and controlled, either statistically or through reduction of sample heterogeneity. Finally, prioritization of exploring hypotheses in large participant samples, either within individual studies or by harnessing “big data” sources, such as the National Institute of Mental Health’s RDoC or NDAR data repositories (data-archive.nimh.nih.gov), will maximize the probability of detecting robust, clinically meaningful, and replicable findings.
Studies will benefit from increased consistency and methodological rigor in both experimental design and equipment. Based on existing data, it cannot be determined to what degree differences in EEG data recording and processing methods (e.g., high versus low impedance EEG systems, electrode density, choice of scalp topography for component extraction) contributed to the diversity of results. Similarly, research on social perception must include appropriate control stimuli. In the research reviewed here, in some cases, inference was limited by omission of non-face stimuli, stimuli matched for low-level visual features, and, in studies of emotion, both neutral/natural and emotional faces. Because many studies focused exclusively on social stimuli, it was often impossible to determine whether reported anomalies reflected specific difficulties in social brain circuitry or more general problems with object perception.
Many of these principles have been recognized by researchers and funding agencies, and within-disorder studies incorporating these features are in progress. Though not in the context of electrophysiological markers, the schizophrenia field recognized more than a decade ago with the MATRICS initiative the importance of identifying reliable markers and developing standardized batteries of cognitive tests for use in diagnosis and assessing treatment outcomes (Marder & Fenton, 2004). The autism field has more recently begun promoting a similar agenda, with increased focus on the potential utility of electrophysiological biomarkers for capturing heterogeneity and predicting treatment. To this end, the Autism Biomarkers Consortium for Clinical Trials (www.asdbiomarkers.org) is evaluating a battery of EEG and eye-tracking social-communicative biomarkers in a large, rigorously characterized sample of children with ASD, with careful attention to participant characteristics, meticulous control of stimulus, task, and EEG recording parameters across sites, and a longitudinal component to provide information about developmental effects. Likewise, the European Autism Interventions — A Multicentre Study for Developing New Medications (EU-AIMS; Murphy & Spooren, 2012) is investigating similar markers, as well as those from additional biomarker domains, such as functional magnetic resonance imaging (fMRI). Although these initiatives are focused on single disorders, their scope and thoroughness are likely to advance understanding of the practical utility of ERP indices of face perception for developing novel interventions and guiding clinical trials.
Finally, the scarcity of studies including multiple diagnostic categories within the same project highlights the urgent need for transdiagnostic work if we are to better understand the specificity versus universality of particular alterations in brain responses to individuals with neurodevelopmental disorders. Thus, moving forward, it is imperative to include both individuals with ASD and SZ—and potentially those with other neurodevelopmental or psychiatric disorders or subthreshold traits—within a single study sample and to measure dimensional traits associated with both disorders across all participants. Studying children or adults at biological risk of ASD or SZ (i.e., siblings, parents, children of affected individuals) to see whether those at risk show altered neural response and/or behavior would also provide important information about intermediate phenotypes and risk states as they relate to brain functioning and clinical profiles. In addition, studies with a longitudinal design and an intervention (targeting either face or emotion processing circuits) may help better parse out causally relevant effects across diagnostic categories.
In summary, both the inconsistencies in findings within ASD and SZ samples and the overlap between findings across ASD and SZ samples make a clear case for the need to consider symptomatology in a dimensional fashion when designing studies and analyzing results in search of biologically meaningful and behaviorally relevant markers of face and emotion processing. Taking a cross-diagnostic, the RDoC approach allows for both direct comparison across diagnoses and more detailed sub-grouping or correlations featuring diagnostically cross-cutting, categorically agnostic phenotypic traits. In addition, this type of study would also allow for clinical characterization to be made along a continuum, rather than imposing an artificial dichotomy (i.e., clinical vs. TD). By taking this approach, future research can address whether and how each categorical disorder (or subset of individuals within a disorder) shows a unique neural signature of altered reception of facial communication, versus whether some alterations in ERP markers of face and emotion processing are reflective of broad clinical impairment in social functioning versus diagnosis or trait-specific pathology. In so doing, research will begin to make more effective headway toward understanding the complexity of distinct versus overlapping etiologies of associated neurodevelopmental disorders, which will in turn position the field to accumulate the precision and replicability of findings needed to develop more targeted treatment approaches.
References
Addington, J., & Addington, D. (1998). Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophrenia Research, 32(3), 171–181.
Akbarfahimi, M., Tehrani-Doost, M., & Ghassemi, F. (2013). Emotional face perception in patients with schizophrenia: an event-related potential study. Neurophysiology, 45(3), 249–257. https://doi.org/10.1007/s11062-013-9363-8.
Akechi, H., Senju, A., Kikuchi, Y., Tojo, Y., Osanai, H., & Hasegawa, T. (2010). The effect of gaze direction on the processing of facial expressions in children with autism spectrum disorder: an ERP study. Neuropsychologia, 48(10), 2841–2851. https://doi.org/10.1016/j.neuropsychologia.2010.05.026.
American Psychiatric Association, A. (1994). Diagnostic and statistical manual of mental disorders (DSM-IV).
Apicella, F., Sicca, F., Federico, R. R., Campatelli, G., & Muratori, F. (2013). Fusiform gyrus responses to neutral and emotional faces in children with autism spectrum disorders: a high density ERP study. Behavioural Brain Research, 251, 155–162. https://doi.org/10.1016/j.bbr.2012.10.040.
Association, A. P. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Pyschiatric Publishing.
Balconi, M., & Pozzoli, U. (2008). Event-related oscillations (ERO) and event-related potentials (ERP) in emotional face recognition. The International Journal of Neuroscience, 118(10), 1412–1424. https://doi.org/10.1080/00207450601047119.
Baron-Cohen, S., Wheelwright, S., Hill, J., Raste, Y., & Plumb, I. (2001). The "Reading the Mind in the Eyes" Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry, 42(2), 241–251.
Batty, M., & Taylor, M. J. (2003). Early processing of the six basic facial emotional expressions. Cognitive Brain Research, 17(3), 613–620.
Batty, M., Meaux, E., Wittemeyer, K., Roge, B., & Taylor, M. J. (2011). Early processing of emotional faces in children with autism: an event-related potential study. Journal of Experimental Child Psychology, 109(4), 430–444. https://doi.org/10.1016/j.jecp.2011.02.001.
Bediou, B., Henaff, M. A., Bertrand, O., Brunelin, J., d'Amato, T., Saoud, M., & Krolak-Salmon, P. (2007). Impaired fronto-temporal processing of emotion in schizophrenia. Neurophysiologie Clinique, 37(2), 77–87. https://doi.org/10.1016/j.neucli.2007.04.001.
Bellack, A. S., Blanchard, J. J., & Mueser, K. T. (1996). Cue availability and affect perception in schizophrenia. Schizophrenia Bulletin, 22(3), 535–544.
Bentin, S., & Peled, B.-S. (1990). The contributionof task-related factors to ERP repetition effects at short and long lags. Memory & Cognition, 18(4), 359–366. https://doi.org/10.3758/bf03197125.
Bentin, S., Allison, T., Puce, A., Perez, E., & McCarthy, G. (1996). Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience, 8(6), 551–565.
Blau, V. C., Maurer, U., Tottenham, N., & McCandliss, B. D. (2007). The face-specific N170 component is modulated by emotional facial expression. Behavioral and Brain Functions, 3(7), 1–13.
Brenner, C. A., Rumak, S. P., Burns, A. M., & Kieffaber, P. D. (2014). The role of encoding and attention in facial emotion memory: an EEG investigation. International Journal of Psychophysiology, 93(3), 398–410. https://doi.org/10.1016/j.ijpsycho.2014.06.006.
Brenner, C. A., Rumak, S. P., & Burns, A. M. (2015). Facial emotion memory in schizophrenia: from encoding to maintenance-related EEG. Clinical Neurophysiology.
Button, K. S., Ioannidis, J. P., Mokrysz, C., Nosek, B. A., Flint, J., Robinson, E. S., & Munafò, M. R. (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14(5), 365–376.
Caharel, S., Bernard, C., Thibaut, F., Haouzir, S., Di Maggio-Clozel, C., Allio, G., et al. (2007). The effects of familiarity and emotional expression on face processing examined by ERPs in patients with schizophrenia. Schizophrenia Research, 95(1–3), 186–196. https://doi.org/10.1016/j.schres.2007.06.015.
Campanella, S., Montedoro, C., Streel, E., Verbanck, P., & Rosier, V. (2006). Early visual components (P100, N170) are disrupted in chronic schizophrenic patients: an event-related potentials study. Neurophysiologie Clinique, 36(2), 71–78. https://doi.org/10.1016/j.neucli.2006.04.005.
Carretié, L., Iglesias, J., Garcia, T., & Ballesteros, M. (1997). N300, P300 and the emotional processing of visual stimuli. Electroencephalography and Clinical Neurophysiology, 103(2), 298–303.
Carretié, L., Martin-Loeches, M., Hinojosa, J. A., & Mercado, F. (2001). Emotion and attention interaction studied through event-related potentials. Journal of Cognitive Neuroscience, 13(8), 1109–1128. https://doi.org/10.1162/089892901753294400.
Churches, O., Wheelwright, S., Baron-Cohen, S., & Ring, H. (2010). The N170 is not modulated by attention in autism spectrum conditions. Neuroreport, 21(6), 399–403.
Churches, O., Baron-Cohen, S., & Ring, H. (2012a). The psychophysiology of narrower face processing in autism spectrum conditions. Neuroreport, 23(6), 395–399. https://doi.org/10.1097/WNR.0b013e3283525bc8.
Churches, O., Damiano, C., Baron-Cohen, S., & Ring, H. (2012b). Getting to know you: the acquisition of new face representations in autism spectrum conditions. Neuroreport, 23(11), 668–672. https://doi.org/10.1097/WNR.0b013e3283556658.
Cloutier, M., Sanon, A. M., Guerin, A., Nitulescu, R., Ramanakumar, A. V., Kamat, S. A., et al. (2016). The economic burden of schizophrenia in the United States in 2013. The Journal of clinical psychiatry.
Conroy, M. A., & Polich, J. (2007). Affective valence and P300 when stimulus arousal level is controlled. Cognition and Emotion, 21(4), 891–901.
Conty, L., N'Diaye, K., Tijus, C., & George, N. (2007). When eye creates the contact! ERP evidence for early dissociation between direct and averted gaze motion processing. Neuropsychologia, 45(13), 3024–3037. https://doi.org/10.1016/j.neuropsychologia.2007.05.017.
Cristino, A., Williams, S., Hawi, Z., An, J., Bellgrove, M., Schwartz, C., et al. (2014). Neurodevelopmental and neuropsychiatric disorders represent an interconnected molecular system. Molecular Psychiatry, 19(3), 294–301.
Cygan, H. B., Tacikowski, P., Ostaszewski, P., Chojnicka, I., & Nowicka, A. (2014). Neural correlates of own name and own face detection in autism spectrum disorder. PLoS One, 9(1), e86020. https://doi.org/10.1371/journal.pone.0086020.
Davis, P. J., & Gibson, M. G. (2000). Recognition of posed and genuine facial expressions of emotion in paranoid and nonparanoid schizophrenia. Journal of Abnormal Psychology, 109(3), 445–450.
Dawson, G., Webb, S. J., & McPartland, J. (2005). Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychology, 27(3), 403–424. https://doi.org/10.1207/s15326942dn2703_6.
Donchin, E. (2008). The context for updating the "context updating" model of the P300. Psychophysiology, 45, S12–S12.
Donchin, E., & Coles, M. G. H. (1988). Is the P300 component a manifestation of context updating. Behavioral and Brain Sciences, 11(3), 357–374.
Donchin, E., & Coles, M. G. H. (1998). Context updating and the P300. Behavioral and Brain Sciences, 21(1), 152-+.
Dowd, E. C., & Barch, D. M. (2010). Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biological Psychiatry, 67(10), 902–911. https://doi.org/10.1016/j.biopsych.2009.10.020.
Eimer, M. (2000). Effects of face inversion on the structural encoding and recognition of faces: evidence from event-related brain potentials. Cognitive Brain Research, 10(1), 145–158.
Eimer, M., & Holmes, A. (2002). An ERP study on the time course of emotional face processing. Neuroreport, 13(4), 427–431.
Faja, S., Dawson, G., Aylward, E., Wijsman, E. M., & Webb, S. J. (2016). Early event-related potentials to emotional faces differ for adults with autism spectrum disorder and by serotonin transporter genotype. Clinical Neurophysiology, 127(6), 2436–2447. https://doi.org/10.1016/j.clinph.2016.02.022.
Fernandes, J. M., Cajão, R., Lopes, R., Jerónimo, R., & Barahona-Corrêa, J. B. (2018). Social cognition in schizophrenia and autism spectrum disorders: a systematic review and meta-analysis of direct comparisons. Frontiers in Psychiatry, 9, 504.
Ferrari, V., Bradley, M. M., Codispoti, M., & Lang, P. J. (2010). Detecting novelty and significance. Journal of Cognitive Neuroscience, 22(2), 404–411.
Feuerriegel, D., Churches, O., Hofmann, J., & Keage, H. A. (2015). The N170 and face perception in psychiatric and neurological disorders: a systematic review. Clinical Neurophysiology, 126(6), 1141–1158. https://doi.org/10.1016/j.clinph.2014.09.015.
First, M. B. (1995). Structured clinical interview for the DSM (SCID). Wiley Online Library.
Fujita, T., Kamio, Y., Yamasaki, T., Yasumoto, S., Hirose, S., & Tobimatsu, S. (2013). Altered automatic face processing in individuals with high-functioning autism spectrum disorders: Evidence from visual evoked potentials. Research in Autism Spectrum Disorders, 7(6), 710–720.
Fukuta, M., Kirino, E., Inoue, R., & Arai, H. (2014). Response of schizophrenic patients to dynamic facial expressions: an event-related potentials study. Neuropsychobiology, 70(1), 10–22.
Gaebel, W., & Wolwer, W. (1992). Facial expression and emotional face recognition in schizophrenia and depression. European Archives of Psychiatry and Clinical Neuroscience, 242(1), 46–52.
Gordon, E., Coyle, S., Anderson, J., Healey, P., Cordaro, J., Latimer, C., & Meares, R. (1992). Eye movement response to a facial stimulus in schizophrenia. Biological Psychiatry, 31(6), 626–629.
Gosling, A., & Eimer, M. (2011). An event-related brain potential study of explicit face recognition. Neuropsychologia, 49(9), 2736–2745. https://doi.org/10.1016/j.neuropsychologia.2011.05.025.
Grice, S. J., Halit, H., Farroni, T., Baron-Cohen, S., Bolton, P., & Johnson, M. H. (2005). Neural correlates of eye-gaze detection in young children with autism. Cortex, 41(3), 342–353.
Groom, M. J., Kochhar, P., Hamilton, A., Liddle, E. B., Simeou, M., & Hollis, C. (2017). Atypical processing of gaze cues and faces explains comorbidity between autism spectrum disorder (ASD) and attention deficit/hyperactivity disorder (ADHD). Journal of Autism and Developmental Disorders, 47(5), 1496–1509. https://doi.org/10.1007/s10803-017-3078-4.
Gunji, A., Inagaki, M., Inoue, Y., Takeshima, Y., & Kaga, M. (2009). Event-related potentials of self-face recognition in children with pervasive developmental disorders. Brain Dev, 31(2), 139–147. https://doi.org/10.1016/j.braindev.2008.04.011.
Gunji, A., Goto, T., Kita, Y., Sakuma, R., Kokubo, N., Koike, T., et al. (2013). Facial identity recognition in children with autism spectrum disorders revealed by P300 analysis: a preliminary study. Brain Dev, 35(4), 293–298. https://doi.org/10.1016/j.braindev.2012.12.008.
Happé, F., Ronald, A., & Plomin, R. (2006). Time to give up on a single explanation for autism. Nature Neuroscience, 9(10), 1218–1220.
Herrmann, M. J., Ellgring, H., & Fallgatter, A. J. (2004). Early-stage face processing dysfunction in patients with schizophrenia. The American Journal of Psychiatry, 161(5), 915–917.
Herrmann, M. J., Ehlis, A. C., Ellgring, H., & Fallgatter, A. J. (2005). Early stages (P100) of face perception in humans as measured with event-related potentials (ERPs). Journal of Neural Transmission, 112(8), 1073–1081. https://doi.org/10.1007/s00702-004-0250-8.
Hileman, C. M., Henderson, H., Mundy, P., Newell, L., & Jaime, M. (2011). Developmental and individual differences on the P1 and N170 ERP components in children with and without autism. Developmental Neuropsychology, 36(2), 214–236. https://doi.org/10.1080/87565641.2010.549870.
Hobson, R. P. (1986). The autistic child’s appraisal of expressions of emotion. Journal of Child Psychology and Psychiatry, 27(3), 321–342.
Ibanez, A., Riveros, R., Hurtado, E., Gleichgerrcht, E., Urquina, H., Herrera, E., et al. (2012). The face and its emotion: right N170 deficits in structural processing and early emotional discrimination in schizophrenic patients and relatives. Psychiatry Research, 195(1-2), 18–26. https://doi.org/10.1016/j.psychres.2011.07.027.
Insel, T. R. (2010). Rethinking schizophrenia. Nature, 468(7321), 187–193.
Itier, R. J., & Taylor, M. J. (2002). Inversion and contrast polarity reversal affect both encoding and recognition processes of unfamiliar faces: a repetition study using ERPs. Neuroimage, 15(2), 353–372.
Itier, R. J., & Taylor, M. J. (2004a). Effects of repetition and configural changes on the development of face recognition processes. Developmental Science, 7(4), 469–487.
Itier, R. J., & Taylor, M. J. (2004b). Effects of repetition learning on upright, inverted and contrast-reversed face processing using ERPs. Neuroimage, 21(4), 1518–1532.
Itier, R. J., & Taylor, M. J. (2004c). N170 or N1? Spatiotemporal differences between object and face processing using ERPs. Cerebral Cortex, 14(2), 132–142.
Itier, R. J., & Taylor, M. J. (2004d). Source analysis of the N170 to faces and objects. Neuroreport, 15(8), 1261–1265.
Jacques, C., Jonas, J., Maillard, L., Colnat-Coulbois, S., Koessler, L., & Rossion, B. (2018). The inferior occipital gyrus is a major cortical source of the face-evoked N170: evidence from simultaneous scalp and intracerebral human recordings. Human Brain Mapping.
Jetha, M. K., Zheng, X., Goldberg, J. O., Segalowitz, S. J., & Schmidt, L. A. (2013). Shyness and emotional face processing in schizophrenia: an ERP study. Biological Psychology, 94(3), 562–574. https://doi.org/10.1016/j.biopsycho.2013.10.001.
Johnston, V. S., Miller, D. R., & Burleson, M. H. (1986). Multiple P3s to emotional stimuli and their theoretical significance. Psychophysiology, 23(6), 684–694.
Johnston, P. J., Stojanov, W., Devir, H., & Schall, U. (2005). Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. The European Journal of Neuroscience, 22(5), 1221–1232. https://doi.org/10.1111/j.1460-9568.2005.04294.x.
Jones E. J. H., Dawson G., & Webb, S. J. (2018). Sensory hypersensitivity predicts enhanced attention capture by faces in the early development of ASD. Dev Cogn Neurosci, 29, 11–20.
Joseph, R. M., & Tanaka, J. (2003). Holistic and part-based face recognition in children with autism. Journal of Child Psychology and Psychiatry, 44(4), 529–542.
Jung, H. T., Kim, D. W., Kim, S., Im, C. H., & Lee, S. H. (2012). Reduced source activity of event-related potentials for affective facial pictures in schizophrenia patients. Schizophrenia Research, 136(1-3), 150–159. https://doi.org/10.1016/j.schres.2011.10.023.
Kang, E., Keifer, C. M., Levy, E. J., Foss-Feig, J. H., McPartland, J. C., & Lerner, M. D. (2018). Atypicality of the N170 event-related potential in autism spectrum disorder: a meta-analysis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(8), 657–666.
Kang, E., McPartland, J. C., Keifer, C. M., Foss-Feig, J. H., Levy, E. J., & Lerner, M. D. (2019). Reply to: Can the N170 be used as an electrophysiological biomarker indexing face processing difficulties in autism spectrum disorder? Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(3), 324–325. https://doi.org/10.1016/j.bpsc.2018.09.004.
Kanwisher, N. (2001). Faces and places: of central (and peripheral) interest. Nature Neuroscience, 4(5), 455–456.
Kaufmann, J. M., Schweinberger, S. R., & MikeBurton, A. (2009). N250 ERP correlates of the acquisition of face representations across different images. Journal of Cognitive Neuroscience, 21(4), 625–641.
Kay, S. R., Flszbein, A., & Opfer, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261.
Kay, S. R., Opler, L. A., Spitzer, R. L., Williams, J. B., Fiszbein, A., & Gorelick, A. (1991). SCID-PANSS: two-tier diagnostic system for psychotic disorders. Comprehensive Psychiatry, 32(4), 355–361. https://doi.org/10.1016/0010-440x(91)90085-q.
Kesler-West, M. L., Andersen, A. H., Smith, C. D., Avison, M. J., Davis, C. E., Kryscio, R. J., & Blonder, L. X. (2001). Neural substrates of facial emotion processing using fMRI. Cognitive Brain Research, 11(2), 213–226. https://doi.org/10.1016/S0926-6410(00)00073-2.
Kestenbaum, R., & Nelson, C. A. (1992). Neural and behavioral correlates of emotion recognition in children and adults. Journal of Experimental Child Psychology, 54(1), 1–18.
Key, A. P., & Corbett, B. A. (2014). ERP responses to face repetition during passive viewing: a nonverbal measure of social motivation in children with autism and typical development. Developmental Neuropsychology, 39(6), 474–495.
Key, A. P. F., Dove, G. O., & Maguire, M. J. (2005). Linking brainwaves to the brain: an ERP primer. Developmental Neuropsychology, 27(2), 183–215.
Khorrami, A., Tehrani-Doost, M., & Esteky, H. (2013). Comparison between face and object processing in youths with autism spectrum disorder: an event related potentials study., 8(4), 179–187.
Kim, D.-W., Shim, M., Song, M. J., Im, C.-H., & Lee, S.-H. (2015). Early visual processing deficits in patients with schizophrenia during spatial frequency-dependent facial affect processing. Schizophrenia Research, 161(2), 314–321.
Kington, J. M., Jones, L. A., Watt, A. A., Hopkin, E. J., & Williams, J. (2000). Impaired eye expression recognition in schizophrenia. Journal of Psychiatric Research, 34(4-5), 341–347.
Kirihara, K., Kasai, K., Tada, M., Nagai, T., Kawakubo, Y., Yamasaki, S., et al. (2012). Neurophysiological impairment in emotional face processing is associated with low extraversion in schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 37(2), 270–275. https://doi.org/10.1016/j.pnpbp.2012.02.012.
Klin, A., Sparrow, S. S., de Bildt, A., Cicchetti, D. V., Cohen, D. J., & Volkmar, F. R. (1999). A normed study of face recognition in autism and related disorders. Journal of Autism and Developmental Disorders, 29(6), 499–508.
Klin, A., Jones, W., Schultz, R., Volkmar, F., & Cohen, D. (2002). Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry, 59(9), 809–816.
Komlosi, S., Csukly, G., Stefanics, G., Czigler, I., Bitter, I., & Czobor, P. (2013). Fearful face recognition in schizophrenia: an electrophysiological study. Schizophrenia Research, 149(1-3), 135–140. https://doi.org/10.1016/j.schres.2013.06.044.
Kozak, M. J., & Cuthbert, B. N. (2016). The NIMH research domain criteria initiative: Background, issues, and pragmatics. Psychophysiology, 53(3), 286–297.
Kuefner, D., De Heering, A., Jacques, C., Palmero-Soler, E., & Rossion, B. (2009). Early visually evoked electrophysiological responses over the human brain (P1, N170) show stable patterns of face-sensitivity from 4 years to adulthood. Frontiers in Human Neuroscience, 3.
Labuschagne, I., Croft, R., Phan, K., & Nathan, P. (2010). Augmenting serotonin neurotransmission with citalopram modulates emotional expression decoding but not structural encoding of moderate intensity sad facial emotional stimuli: an event-related potential (ERP) investigation. Journal of Psychopharmacology, 24(8), 1153–1164.
Law Smith, M. J., Montagne, B., Perrett, D. I., Gill, M., & Gallagher, L. (2010). Detecting subtle facial emotion recognition deficits in high-functioning autism using dynamic stimuli of varying intensities. Neuropsychologia, 48(9), 2777–2781. https://doi.org/10.1016/j.neuropsychologia.2010.03.008.
Lee, S., Kim, E., Kim, S., Im, W., Seo, H., Han, S., et al. (2007). Facial affect perception and event-related potential N170 in schizophrenia: a preliminary study. CLINICAL PSYCHOPHARMACOLOGY AND NEUROSCIENCE, 5(2), 76.
Lee, S.-H., Kim, E.-Y., Kim, S., & Bae, S.-M. (2010). Event-related potential patterns and gender effects underlying facial affect processing in schizophrenia patients. Neuroscience Research, 67(2), 172–180. https://doi.org/10.1016/j.neures.2010.03.001.
Lewis, S. F., & Garver, D. L. (1995). Treatment and diagnostic subtype in facial affect recognition in schizophrenia. Journal of Psychiatric Research, 29(1), 5–11.
Liu, T., Pinheiro, A. P., Zhao, Z., Nestor, P. G., McCarley, R. W., & Niznikiewicz, M. (2016). Simultaneous face and voice processing in schizophrenia. Behavioural Brain Research, 305, 76–86. https://doi.org/10.1016/j.bbr.2016.01.039.
Lord, C., DiLavore, P. C., & Gotham, K. (2012). Autism diagnostic observation schedule: Western Psychological Services Torrance, CA.
Luckhardt, C., Kroger, A., Cholemkery, H., Bender, S., & Freitag, C. M. (2017). Neural correlates of explicit versus implicit facial emotion processing in ASD. Journal of Autism and Developmental Disorders, 47(7), 1944–1955. https://doi.org/10.1007/s10803-017-3141-1.
Luckhardt, C., Kröger, A., Elsuni, L., Cholemkery, H., Bender, S., & Freitag, C. M. (2018). Facilitation of biological motion processing by group-based autism specific social skills training. Autism Research, 11(10), 1376–1387.
Luo, W., Feng, W., He, W., Wang, N., & Luo, Y. (2009). Three stages of facial expression processing: ERP study with rapid serial visual presentation. Neuroimage. https://doi.org/10.1016/j.neuroimage.2009.09.018.
Luyster, R. J., Bick, J., Westerlund, A., & Nelson 3rd., C. A. (2017). Testing the effects of expression, intensity and age on emotional face processing in ASD. Neuropsychologia. https://doi.org/10.1016/j.neuropsychologia.2017.06.023.
Lynn, S. K., & Salisbury, D. F. (2008). Attenuated modulation of the N170 ERP by facial expressions in schizophrenia. Clinical EEG and Neuroscience, 39(2), 108–111.
Maestro, S., Muratori, F., Cavallaro, M. C., Pei, F., Stern, D., Golse, B., & Palacio-Espasa, F. (2002). Attentional skills during the first 6 months of age in autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 41(10), 1239–1245.
Magnée, M. J., de Gelder, B., van Engeland, H., & Kemner, C. (2011). Multisensory integration and attention in autism spectrum disorder: evidence from event-related potentials. PLoS One, 6(8), e24196. https://doi.org/10.1371/journal.pone.0024196.
Maher, S., Mashhoon, Y., Ekstrom, T., Lukas, S., & Chen, Y. (2016). Deficient cortical face-sensitive N170 responses and basic visual processing in schizophrenia. Schizophrenia Research, 170(1), 87–94.
Mangun, G. R. (1995). Neural mechanisms of visual selective attention. Psychophysiology, 32(1), 4–18.
Marder, S. R., & Fenton, W. (2004). Measurement and treatment research to improve cognition in schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophrenia Research, 72(1), 5–9.
Massie, H. N. (1978). Blind ratings of mother-infant interaction in home movies of prepsychotic and normal infants. The American Journal of Psychiatry, 135(11), 1371–1374.
McMahon, C. M., & Henderson, H. A. (2015). Error-monitoring in response to social stimuli in individuals with higher-functioning autism spectrum disorder. Developmental Science, 18(3), 389–403.
McPartland, J. C. (2016). Considerations in biomarker development for neurodevelopmental disorders. Current Opinion in Neurology, 29(2), 118–122.
McPartland, J., Dawson, G., Webb, S. J., Panagiotides, H., & Carver, L. J. (2004). Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry, 45(7), 1235–1245. https://doi.org/10.1111/j.1469-7610.2004.00318.x.
McPartland, J. C., Wu, J., Bailey, C. A., Mayes, L. C., Schultz, R. T., & Klin, A. (2011). Atypical neural specialization for social percepts in autism spectrum disorder. Social Neuroscience, 6(5-6), 436–451. https://doi.org/10.1080/17470919.2011.586880.
Mitchell, K. J. (2011). The genetics of neurodevelopmental disease. Current Opinion in Neurobiology, 21(1), 197–203. https://doi.org/10.1016/j.conb.2010.08.009.
Mohamed, T. N., Neumann, M. F., & Schweinberger, S. R. (2009). Perceptual load manipulation reveals sensitivity of the face-selective N170 to attention. Neuroreport, 20(8), 782–787.
Mori, K., Morita, K., Shoji, Y., Matsuoka, T., Fujiki, R., & Uchimura, N. (2012). State and trait markers of emotionally charged visual event-related potentials (P300) in drug-naïve schizophrenia. Psychiatry and Clinical Neurosciences, 66(4), 261–269.
Morrison, R. L., Bellack, A. S., & Mueser, K. T. (1988). Deficits in facial-affect recognition and schizophrenia. Schizophrenia Bulletin, 14(1), 67–83.
Mueser, K. T., Doonan, R., Penn, D. L., Blanchard, J. J., Bellack, A. S., Nishith, P., & DeLeon, J. (1996). Emotion recognition and social competence in chronic schizophrenia. Journal of Abnormal Psychology, 105(2), 271–275.
Müller, V. I., Kellermann, T. S., Seligman, S. C., Turetsky, B. I., & Eickhoff, S. B. (2012). Modulation of affective face processing deficits in schizophrenia by congruent emotional sounds. Social cognitive and affective neuroscience, nss107.
Murashko, A. A., & Shmukler, A. (2019). EEG correlates of face recognition in patients with schizophrenia spectrum disorders: a systematic review. Clinical Neurophysiology, 130(6), 986–996. https://doi.org/10.1016/j.clinph.2019.03.027.
Murphy, D., & Spooren, W. (2012). EU-AIMS: a boost to autism research. Nature Reviews Drug Discovery, 11(11), 815.
Neuhaus, E., Kresse, A,. Faja, S., Bernier, R. A. (2016). Webb SJ. Face processing among twins with and without autism: social correlates and twin concordance. Soc Cogn Affect Neurosci, 11(1) 44–54.
Ninomiya, H., Onitsuka, T., Chen, C. H., Sato, E., & Tashiro, N. (1998). P300 in response to the subject's own face. Psychiatry and Clinical Neurosciences, 52(5), 519–522.
Obayashi, C., Nakashima, T., Onitsuka, T., Maekawa, T., Hirano, Y., Hirano, S., et al. (2009). Decreased spatial frequency sensitivities for processing faces in male patients with chronic schizophrenia. Clinical Neurophysiology, 120(8), 1525–1533. https://doi.org/10.1016/j.clinph.2009.06.016.
O'Connor, K., Hamm, J. P., & Kirk, I. J. (2005). The neurophysiological correlates of face processing in adults and children with Asperger's syndrome. Brain and Cognition, 59(1), 82–95. https://doi.org/10.1016/j.bandc.2005.05.004.
O'Connor, K., Hamm, J. P., & Kirk, I. J. (2007). Neurophysiological responses to face, facial regions and objects in adults with Asperger's syndrome: an ERP investigation. International Journal of Psychophysiology, 63(3), 283–293. https://doi.org/10.1016/j.ijpsycho.2006.12.001.
Onitsuka, T., Ninomiya, H., & Sato, E. (2001). The effect of subject's own face on visual P300 in schizophrenia. Biological Psychiatry, 49(8), 133S–133S.
Onitsuka, T., Niznikiewicz, M. A., Spencer, K. M., Frumin, M., Kuroki, N., Lucia, L. C., et al. (2006). Functional and structural deficits in brain regions subserving face perception in schizophrenia. The American Journal of Psychiatry, 163(3), 455–462. https://doi.org/10.1176/appi.ajp.163.3.455.
Onitsuka, T., Spencer, K. M., Lucia, L. C., Shenton, M. E., McCarley, R. W., & Niznikiewicz, M. A. (2009). Abnormal asymmetry of the face n170 repetition effect in male patients with chronic schizophrenia. Brain Imaging and Behavior, 3(3), 240–245. https://doi.org/10.1007/s11682-009-9066-3.
Onitsuka, T., Spencer, K. M., Nakamura, I., Hirano, Y., Hirano, S., McCarley, R. W., et al. (2020). Altered P3a modulations to emotional faces in male patients with chronic schizophrenia. Clinical EEG and Neuroscience, 51(4), 215–221. https://doi.org/10.1177/1550059419896723.
Osterling, J. A., & Dawson, G. (1994). Early recognition of children with autism: a study of first birthday home videotapes. Journal of Autism and Developmental Disorders, 24(3), 247–257.
Pelphrey, K. A., Sasson, N. J., Reznick, J. S., Paul, G., Goldman, B. D., & Piven, J. (2002). Visual scanning of faces in autism. Journal of Autism and Developmental Disorders, 32(4), 249–261.
Phillips, M. L., & David, A. S. (1997). Visual scan paths are abnormal in deluded schizophrenics. Neuropsychologia, 35(1), 99–105.
Picton, T. W. (1992). The P300 wave of the human event-related potential. Journal of Clinical Neurophysiology, 9(4), 456–479.
Picton, T. (1995). P300 - review and reconciliation. Psychophysiology, 32, S8–S8.
Polich, J., Friedman, D., Donchin, E., Johnson, R., Polich, J., & Picton, T. (1995). 30 years of P300. Psychophysiology, 32, S7–S7.
Ramos-Loyo, J., Gonzalez-Garrido, A. A., Sanchez-Loyo, L. M., Medina, V., & Basar-Eroglu, C. (2009). Event-related potentials and event-related oscillations during identity and facial emotional processing in schizophrenia. International Journal of Psychophysiology, 71(1), 84–90. https://doi.org/10.1016/j.ijpsycho.2008.07.008.
Ravden, D., & Polich, J. (1999). On P300 measurement stability: habituation, intra-trial block variation, and ultradian rhythms. Biological Psychology, 51(1), 59-76. doi:doi:10.1016/S0301-0511(99)00015-0
Rossion, B., & Caharel, S. (2011). ERP evidence for the speed of face categorization in the human brain: disentangling the contribution of low-level visual cues from face perception. Vision Research, 51(12), 1297–1311.
Rossion, B., Gauthier, I., Tarr, M. J., Despland, P., Bruyer, R., Linotte, S., & Crommelinck, M. (2000). The N170 occipito-temporal component is delayed and enhanced to inverted faces but not to inverted objects: an electrophysiological account of face-specific processes in the human brain. Neuroreport, 11(1), 69–72.
Rossion, B., Joyce, C. A., Cottrell, G. W., & Tarr, M. J. (2003). Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage, 20(3), 1609–1624.
Rousselet, G. A., & Pernet, C. R. (2011). Quantifying the time course of visual object processing using ERPs: it's time to up the game. Frontiers in Psychology, 2, 107.
Sadeh, B., Podlipsky, I., Zhdanov, A., & Yovel, G. (2010). Event-related potential and functional MRI measures of face-selectivity are highly correlated: a simultaneous ERP-fMRI investigation. Human Brain Mapping, 31(10), 1490–1501.
Salisbury, D. F., Krompinger, J. W., Lynn, S. K., Onitsuka, T., & McCarley, R. W. (2019). Neutral face and complex object neurophysiological processing deficits in long-term schizophrenia and in first hospitalized schizophrenia-spectrum individuals. International Journal of Psychophysiology, 145, 57–64. https://doi.org/10.1016/j.ijpsycho.2019.06.002.
Sato, W., Kochiyama, T., Yoshikawa, S., & Matsumura, M. (2001). Emotional expression boosts early visual processing of the face: ERP recording and its decomposition by independent component analysis. Neuroreport, 12(4), 709–714.
Schultz, R. T. (2005). Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience, 23(2-3), 125–141. https://doi.org/10.1016/j.ijdevneu.2004.12.012.
Schweinberger, S. R., Pickering, E. C., Jentzsch, I., Burton, A. M., & Kaufmann, J. M. (2002). Event-related brain potential evidence for a response of inferior temporal cortex to familiar face repetitions. Cognitive Brain Research, 14(3), 398–409.
Senju, A., Tojo, Y., Yaguchi, K., & Hasegawa, T. (2005). Deviant gaze processing in children with autism: an ERP study. Neuropsychologia, 43(9), 1297–1306. https://doi.org/10.1016/j.neuropsychologia.2004.12.002.
Shah, D., Knott, V., Baddeley, A., Bowers, H., Wright, N., Labelle, A., et al. (2018). Impairments of emotional face processing in schizophrenia patients: evidence from P100, N170 and P300 ERP components in a sample of auditory hallucinators. International Journal of Psychophysiology, 134, 120–134. https://doi.org/10.1016/j.ijpsycho.2018.10.001.
Shen, I. H., Lin, S. C., Wu, Y. Y., & Chen, C. L. (2017). An event-related potential study on the perception and the recognition of face, facial features, and objects in children with autism spectrum disorders. Perceptual and Motor Skills, 124(1), 145–165.
Shibata, T., Nishijo, H., Tamura, R., Miyamoto, K., Eifuku, S., Endo, S., & Ono, T. (2002). Generators of visual evoked potentials for faces and eyes in the human brain as determined by dipole localization. Brain Topography, 15(1), 51–63.
Spitzer, R. L., & Endicott, J. (1979). Schedule for affective disorders and schizophrenia, SADS. New York State Psychiatric Institute, Department of Research Assessment and Training.
Streit, M., Ioannides, A. A., Liu, L., Wolwer, W., Dammers, J., Gross, J., et al. (1999). Neurophysiological correlates of the recognition of facial expressions of emotion as revealed by magnetoencephalography. Brain Research. Cognitive Brain Research, 7(4), 481–491.
Streit, M., Wolwer, W., Brinkmeyer, J., Ihl, R., & Gaebel, W. (2001). EEG-correlates of facial affect recognition and categorisation of blurred faces in schizophrenic patients and healthy volunteers. Schizophrenia Research, 49(1-2), 145–155.
Sysoeva, O. V., Constantino, J. N., & Anokhin, A. P. (2018). Event-related potential (ERP) correlates of face processing in verbal children with autism spectrum disorders (ASD) and their first-degree relatives: a family study. Molecular Autism, 9, 41. https://doi.org/10.1186/s13229-018-0220-x.
Tanaka, J. W., Curran, T., Porterfield, A. L., & Collins, D. (2006). Activation of preexisting and acquired face representations: the N250 event-related potential as an index of face familiarity. Journal of Cognitive Neuroscience, 18(9), 1488–1497.
Taylor, M. J., Edmonds, G. E., McCarthy, G., & Allison, T. (2001). Eyes first! Eye processing develops before face processing in children. Neuroreport, 12(8), 1671–1676.
Thoma, P., Soria Bauser, D., Norra, C., Brune, M., Juckel, G., & Suchan, B. (2013). Do you see what I feel? - Electrophysiological correlates of emotional face and body perception in schizophrenia. Clinical Neurophysiology. https://doi.org/10.1016/j.clinph.2013.10.046.
Trevisan, D. A., Foss-Feig, J. H., Naples, A. J., Srihari, V., Anticevic, A., & McPartland, J. C. (2020). Autism spectrum disorder and schizophrenia are better differentiated by positive symptoms than negative symptoms. Frontiers in Psychiatry, 11, 548.
Tseng, Y.-L., Yang, H. H., Savostyanov, A. N., Chien, V. S. C., & Liou, M. (2015). Voluntary attention in Asperger's syndrome: brain electrical oscillation and phase-synchronization during facial emotion recognition. Research in Autism Spectrum Disorders, 13–14, 32–51. https://doi.org/10.1016/j.rasd.2015.01.003.
Tso, I. F., Calwas, A. M., Chun, J., Mueller, S. A., Taylor, S. F., & Deldin, P. J. (2015). Altered attentional and perceptual processes as indexed by N170 during gaze perception in schizophrenia: relationship with perceived threat and paranoid delusions. Journal of Abnormal Psychology, 124(3), 519.
Tsunoda, T., Kanba, S., Ueno, T., Hirano, Y., Hirano, S., Maekawa, T., & Onitsuka, T. (2012). Altered face inversion effect and association between face N170 reduction and social dysfunction in patients with schizophrenia. Clinical Neurophysiology, 123(9), 1762–1768. https://doi.org/10.1016/j.clinph.2012.01.024.
Turetsky, B. I., Kohler, C. G., Indersmitten, T., Bhati, M. T., Charbonnier, D., & Gur, R. C. (2007). Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophrenia Research, 94(1-3), 253–263. https://doi.org/10.1016/j.schres.2007.05.001.
Tye, C., Mercure, E., Ashwood, K. L., Azadi, B., Asherson, P., Johnson, M. H., et al. (2013). Neurophysiological responses to faces and gaze direction differentiate children with ASD, ADHD and ASD + ADHD. Developmental Cognitive Neuroscience, 5, 71–85. https://doi.org/10.1016/j.dcn.2013.01.001.
Tye, C., Battaglia, M., Bertoletti, E., Ashwood, K. L., Azadi, B., Asherson, P., et al. (2014). Altered neurophysiological responses to emotional faces discriminate children with ASD, ADHD and ASD + ADHD. Biological Psychology. https://doi.org/10.1016/j.biopsycho.2014.08.013.
Ueno, T., Morita, K., Shoji, Y., Yamamoto, M., Yamamoto, H., & Maeda, H. (2004). Recognition of facial expression and visual P300 in schizophrenic patients: differences between paranoid type patients and non-paranoid patients. Psychiatry and Clinical Neurosciences, 58(6), 585–592. https://doi.org/10.1111/j.1440-1819.2004.01307.x.
Vettori, S., Jacques, C., Boets, B., & Rossion, B. (2019). Can the N170 be used as an electrophysiological biomarker indexing face processing difficulties in autism spectrum disorder? Biol Psychiatry Cogn Neurosci Neuroimaging, 4(3), 321–323. https://doi.org/10.1016/j.bpsc.2018.07.015.
Wagner, J. B., Hirsch, S. B., Vogel-Farley, V. K., Redcay, E., & Nelson, C. A. (2013). Eye-tracking, autonomic, and electrophysiological correlates of emotional face processing in adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders, 43(1), 188–199. https://doi.org/10.1007/s10803-012-1565-1.
Wang, Q., She, S., Luo, L., Li, H., Ning, Y., Ren, J., et al. (2020). Abnormal contingent negative variation drifts during facial expression judgment in schizophrenia patients. Frontiers in Human Neuroscience, 14, 274. https://doi.org/10.3389/fnhum.2020.00274.
Webb, S. J., Dawson, G., Bernier, R., & Panagiotides, H. (2006). ERP evidence of atypical face processing in young children with autism. Journal of Autism and Developmental Disorders, 36(7), 881–890. https://doi.org/10.1007/s10803-006-0126-x.
Webb, S. J., Jones, E. J., Merkle, K., Murias, M., Greenson, J., Richards, T., et al. (2010). Response to familiar faces, newly familiar faces, and novel faces as assessed by ERPs is intact in adults with autism spectrum disorders. International Journal of Psychophysiology, 77(2), 106–117. https://doi.org/10.1016/j.ijpsycho.2010.04.011.
Webb, S. J., Merkle, K., Murias, M., Richards, T., Aylward, E., & Dawson, G. (2012). ERP responses differentiate inverted but not upright face processing in adults with ASD. Social Cognitive and Affective Neuroscience, 7(5), 578–587. https://doi.org/10.1093/scan/nsp002.
Wolff, S. (2004). The history of autism. European Child & Adolescent Psychiatry, 13(4), 201–208.
Wolwer, W., Brinkmeyer, J., Stroth, S., Streit, M., Bechdolf, A., Ruhrmann, S., et al. (2012). Neurophysiological correlates of impaired facial affect recognition in individuals at risk for schizophrenia. Schizophrenia Bulletin, 38(5), 1021–1029. https://doi.org/10.1093/schbul/sbr013.
Wong, T. K., Fung, P. C., Chua, S. E., & McAlonan, G. M. (2008). Abnormal spatiotemporal processing of emotional facial expressions in childhood autism: dipole source analysis of event-related potentials. The European Journal of Neuroscience, 28(2), 407–416. https://doi.org/10.1111/j.1460-9568.2008.06328.x.
Wong, T., Fung, P., McAlonan, G., & Chua, S. (2009). Spatiotemporal dipole source localization of face processing ERPs in adolescents: a preliminary study. Behavioral and Brain Functions, 5(1), 16. https://doi.org/10.1186/1744-9081-5-16.
Wynn, J. K., Lee, J., Horan, W. P., & Green, M. F. (2008). Using event related potentials to explore stages of facial affect recognition deficits in schizophrenia. Schizophrenia Bulletin, 34(4), 679–687. https://doi.org/10.1093/schbul/sbn047.
Wynn, J. K., Jahshan, C., Altshuler, L. L., Glahn, D. C., & Green, M. F. (2013). Event-related potential examination of facial affect processing in bipolar disorder and schizophrenia. Psychological Medicine, 43(1), 109–117. https://doi.org/10.1017/s0033291712001006.
Yamasaki, T., Maekawa, T., Miyanaga, Y., Takahashi, K., Takamiya, N., Ogata, K., & Tobimatsu, S. (2017). Enhanced fine-form perception does not contribute to gestalt face perception in autism spectrum disorder. PLoS One, 12(2), e0170239. https://doi.org/10.1371/journal.pone.0170239.
Yang, C., Zhang, T., Li, Z., Heeramun-Aubeeluck, A., Liu, N., Huang, N., et al. (2017). Changes in event-related potentials in patients with first-episode schizophrenia and their siblings. BMC Psychiatry, 17(1), 20. https://doi.org/10.1186/s12888-016-1189-7.
Yovel, G., Sadeh, B., Podlipsky, I., Hendler, T., & Zhdanov, A. (2008). The face-selective ERP component (N170) is correlated with the face-selective areas in the fusiform gyrus (FFA) and the superior temporal sulcus (fSTS) but not the occipital face area (OFA): a simultaneous fMRI-EEG study. Journal of Vision, 8(6), 401–401. https://doi.org/10.1167/8.6.401.
Zhang, D., Zhao, Y., Liu, Y., & Tan, S. (2016). Perception of the duration of emotional faces in schizophrenic patients. Scientific Reports, 6.
Zheng, Y., Li, H., Ning, Y., Ren, J., Wu, Z., Huang, R., et al. (2016). Sluggishness of early-stage face processing (N170) is correlated with negative and general psychiatric symptoms in schizophrenia. Frontiers in Human Neuroscience, 10, 615.
Jones, E. J. H., Dawson, G., & Webb, S. J. (2018). Sensory hypersensitivity predicts enhanced attention capture by faces in the early development of ASD. Developmental Cognitive Neuroscience, 29, 11–20.
Funding
The authors’ work was supported by the National Institutes of Health (NIMH U19 MH108206, R01-MH107426, R01-MH100173, R01-MH103831, RC1-MH088971, 1DP5-OD012109), the Brain and Behavior Research Foundation (NARSAD Young Investigator Award), and the Autism Science Foundation (Accelerator Grant).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Levy, E.J., Isenstein, E.L., Foss-Feig, J. et al. Electrophysiological Studies of Reception of Facial Communication in Autism Spectrum Disorder and Schizophrenia. Rev J Autism Dev Disord 9, 521–554 (2022). https://doi.org/10.1007/s40489-021-00260-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40489-021-00260-z