Abstract
The underlying neural mechanisms of implicit and explicit facial emotion recognition (FER) were studied in children and adolescents with autism spectrum disorder (ASD) compared to matched typically developing controls (TDC). EEG was obtained from N = 21 ASD and N = 16 TDC. Task performance, visual (P100, N170) and cognitive (late positive potential) event-related-potentials, as well as coherence were compared across groups. TDC showed a task-dependent increase and a stronger lateralization of P100 amplitude during the explicit task and task-dependent modulation of intra-hemispheric coherence in the beta band. In contrast, the ASD group showed no task dependent modulation. Results indicate disruptions in early visual processing and top-down attentional processes as contributing factors to FER deficits in ASD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Facial emotion recognition (FER) is one of the most frequently studied neurocognitive functions in autism spectrum disorders (ASD), as it contributes to the social communication and interaction deficits characteristic for the condition (American Psychiatric Association 2013). Numerous studies have applied a variety of different methods, trying to quantify FER deficits in ASD, but results remain heterogeneous (Harms et al. 2010). Investigating which factors produce this variability is important to understand the nature of FER deficits in ASD. An important factor in this respect, that has rarely been examined, is the difference between implicit and explicit FER tasks. Neuroimaging studies have shown that different neural networks underlie implicit versus explicit FER (Critchley et al. 2000; Gorno-Tempini et al. 2001). Behavioral studies of affective reactivity suggest that FER might be more strongly affected during implicit tasks in ASD (Kamio et al. 2006; Mathersul et al. 2013). This might be related to a lack of perceived relevance of emotional stimuli and therefore less allocation of attentional resources to affective stimulus content. Furthermore individuals with ASD seem to be able to overcome these deficits when they are explicitly instructed to take emotional content into account (Begeer et al. 2006). This suggests that either implicit and explicit FER networks might not be affected to the same degree in ASD, or that FER deficits in ASD are related to reduced saliency of emotional stimuli during implicit processing which can be overcome by intentional allocation of attention during explicit FER.
To better understand underlying mechanisms of implicit versus explicit FER in ASD it is important to study their neural basis. Here, event-related potentials (ERPs) are particularly useful and have advantages over other imaging methods, as ERPs (1) have a very high temporal resolution, (2) are a direct measure of neural activity, and (3) can resolve the time course of discrete face processing steps. ERPs have frequently been used to examine general face processing abilities in ASD and several studies report abnormal ERP responses with regard to structural face processing and the processing of face familiarity (McPartland et al. 2004; Hileman et al. 2011; Churches et al. 2010; Webb et al. 2012).
However, only few studies have investigated FER deficits in children and adolescents with ASD using ERPs, and findings remain highly variable across studies. For instance, one study implementing an implicit FER task did not report differences in early ERPs regarding FER in children with ASD (Apicella et al. 2013). Another study, in contrast, found pronounced differences in P100 and N170 (Batty et al. 2011). The only study using an explicit FER task compared ERPs across adults and children with and without ASD and found differences in FER performance and early ERPs only between the adult groups (O’Connor et al. 2005).
To date, only one study has directly investigated the influence of implicit versus explicit FER processing on ERP correlates in ASD. Wong et al. (2008) compared children with ASD and TDC aged 6–10 years across an implicit (gender discrimination) and explicit FER task (emotional vs. neutral expressions). Both groups showed the same level of behavioral performance, and similar task dependent ERP modulation. Source analysis showed that activity of neural generators in brain regions relevant for face processing were significantly reduced and slower in participants with ASD relative to TDC for both conditions. However this pioneering study has several limitations: First, the explicit task used in this study was simple, only demanded the discrimination of neutral versus emotional faces. Thus, the lack of behavioral and ERP differences might have been due to ceiling effects. Second, the study only investigated early ERPs reflecting visual and structural processing (P100 and N170) but did not take into account later ERPs reflecting cognitive processing. The late positive potential (LPP) for example is sensitive to emotional content and perceived significance of stimuli and thereby also the allocation of attention related to these factors (Schupp et al. 2004; Hajcak and Olvet 2008; Ferrari et al. 2010).The LPP is consequently well suited to examine motivational and attentional aspects of FER in ASD. All in all, more detailed research is needed to provide a better understanding of the complex neural processing mechanisms underlying explicit and implicit FER in ASD. Comparing early visual and cognitive ERPs for implicit versus explicit FER will show whether deficits are actually more prominent during implicit processing, as suggested by behavioral studies, and reveal underlying mechanisms.
In addition to ERPs, which can illustrate the discrete time course of facial emotion processing, other methods need to be implemented to study network effects, as FER involves complex interaction of different brain areas (Haxby et al. 2000). For example, fMRI based studies have shown that not only the activity of single brain areas (Schultz et al. 2000; Koshino et al. 2008) but also connectivity between different areas is affected in general face processing (Koshino et al. 2008; Kleinhans et al. 2008; Lynn et al. 2016), as well as during explicit FER in ASD (Monk et al. 2010; Wicker et al. 2008).
EEG-based measures of coherence can quantify functional cortical connectivity by looking at the synchronization of oscillations between different areas (Nunez et al. 1997; Sporns et al. 2000). Like ERPs EEG-coherence relies on direct measure of neuronal activity and does not depend on indirect measures such as blood flow. The majority of EEG-based studies have focused on differences of connectivity patterns at rest (Murias et al. 2007; Coben et al. 2008; Duffy and Als 2012; Mathewson et al. 2012). In addition there is evidence suggesting that functional coherence is affected in ASD (e.g. Isler et al. 2010; Carson et al. 2014; Jaime et al. 2016).
Again, only few studies have investigated cortical coherence during face processing in ASD and none have directly compared implicit and explicit FER. One study used magnetoencephalography to investigate coherence during implicit FER in adolescents with ASD. Reduced interregional coherence between the right fusiform gyrus and insula were found in the beta band (Leung et al. 2014). The only existing study contrasting implicit and explicit processing looked at neutral faces compared to objects. Results showed reduced interhemispheric coherence as well as less task dependent modulation of coherence in the ASD group (Catarino et al. 2013). Taken together, these findings indicated that both differences in inter- and intrahemispheric connectivity might contribute to FER deficits in ASD: On the one hand interhemispheric coherence is found to be reduced during visual processing in ASD (Isler et al. 2010; Catarino et al. 2013) which is related to deficits in integration of visual features and impaired perceptual abilities in ASD (Peiker et al. 2015). This is also relevant for FER, as it typically relies on the integration of different facial features. On the other hand previous studies reported differences in intrahemispheric coherence (Leung et al. 2014). This finding indicates that the integration of information across brain areas and feedback between these areas are also affected during facial emotion processing in ASD. Both patterns of inter-and intrahemispheric coherence might differ for implicit versus explicit FER. As it has been suggested that implicit FER is more strongly affected in ASD, there might also be reduced patterns of cortical coherence for implicit FER.
Taken together, the aim of the current study was to characterize the neural basis of FER deficits in ASD by comparing neural correlates of implicit versus explicit processing during early visual as well as later cognitive ERPs, and intra- and interhemispheric coherence. For this purpose we employed a task that exclusively examined negative emotions to avoid ceiling effects. Because negative and complex facial expressions are more difficult to process for ASD individuals than positive emotions (Corden et al. 2008; Wallace et al. 2008; Bal et al. 2010). Furthermore, we examined the LPP in addition to early visual ERP components to evaluate motivational and attentional aspects of FER deficits in ASD. Also, we aimed to examine possible differences between groups in EEG-coherence at occipital, temporal and parietal electrodes for implicit versus explicit FER.
It was hypothesized that compared to TDC, ASD individuals would show (1) more errors in the behavioral FER task, (2) greater ERP differences in the implicit versus the explicit condition, indicative of a lack of automatic allocation of attention and perceived emotional significance during implicit FER, and (3) aberrant patterns of intra- and interhemispheric cortical coherence.
Methods
Participants
Twenty-one individuals with ASD (12.5 ± 2.2 years) and 16 typically developing controls (TDC, 13.0 ± 2.9 years) were included in our study. Groups were matched for age, gender, handedness (Oldfield 1971), and IQ (see Table 1). Both groups included significantly more males than females (ASD-group: females N = 1, TDC-group: females N = 3), which reflects the male-bias in ASD prevalence in the general population. We excluded individuals with a learning disability (i.e. IQ ≥ 70), a history of neurological disorders (including epilepsy), and impaired eye-sight (except if this was corrected via glasses). Cognitive abilities (i.e. full scale IQ) were assessed by a short form of the WISC or WAIS respectively (“Wechsler Intelligence Scale for Children”, Petermann and Petermann 2010; and “Wechsler Adult Intelligence Scale”; Aster et al. 2006; German versions, four tasks were used: “Vocabulary”, “Letter-Number-Sequencing”, “Matrix Reasoning”, “Symbol Search”; Waldmann 2008).
Participants with ASD were diagnosed with either Autism (N = 6), Asperger Syndrome (N = 9) or atypical autism (N = 6) according to ICD-10 criteria (World Health Organization 1992). Both, the German version of the Autism Diagnostic Interview-Revised (ADI-R, Bölte et al. 2006), and the Autism Diagnostic Observation Schedule (ADOS, Rühl et al. 2004) were administered by experienced clinicians (psychiatrists, clinical psychologists). We excluded individuals with comorbid psychiatric disorders such as Attention-Deficit–Hyperactivity Disorder, schizophrenia, bipolar disorder, social phobia, obsessive–compulsive disorder, major depressive disorder with suicidal ideation, or any personality disorder according to ICD-10 as assessed by the Diagnostic Interview for Children and Adolescents (Kinder-DIPS; Schneider et al. 2009). Also none of the participants were taking any kind of psychotropic medication.
Typically developing controls (TDC) were included if they had no personal or family history of ASD, or any other psychiatric disorders. This was verified using the “Social Responsiveness Scale” (SRS, Bölte et al. 2005, mean raw score for TDC 16.31, SD 10.93), as well as the “Social Communication Questionnaire” (SCQ, Bölte and Poustka 2008, mean score for TDC 1.94, SD 2.18).
All participants and their caregivers gave informed consent. The study was approved by the local ethical committee in accordance with the declaration of Helsinki.
Stimuli and Procedure
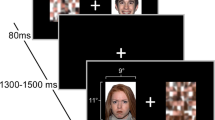
Participants were seated in a darkened room approximately 80 cm from a 30 × 37.5 cm screen on which the stimuli were presented. Facial expression stimuli consisted of color photographs of 50 young adults (25 male/25 female) each portraying angry, sad and disgusted emotional expressions, which were taken from a standardized set of facial expression stimuli (FACES database, Ebner et al. 2010). The experiment comprised two conditions. In both conditions the same emotional facial expression stimuli were shown, only in different randomized order. Two different task-instructions were used to shift the focus of attention either towards the emotional expressions in the explicit condition or away from them in the implicit condition. During the implicit task participants were instructed to attend to people’s hair color, and had to respond with a button press whenever they saw someone who was blond. In the explicit task the instruction was to concentrate on emotional expressions and respond whenever an angry facial expression was shown. Stimuli were shown for 3 s each with 1.5–2.5 s inter-trial interval, during which a fixation cross was shown. Stimuli were presented using Presentation™ software (Neurobehavioral Systems, Inc., Berkeley, CA, http://www.neurobs.com).
Participants’ responses on the explicit FER-task were recorded and analyzed with respect to reaction times (averaged for target/anger trials) and task performance. Performance on the facial emotion recognition task was computed as d′ (“D-PRIME”), which is defined as the difference of the z-transformed values of hit rate (H) and false alarm rate (F).
where hit rate (H) is defined as the number of correct responses divided by the number of target trials, and false alarm rate (F) as the ratio between the number of false positive responses and the number of trials where no response should have been given (Macmillan and Creelman 2004).
EEG Recordings
For data recording 64-channel equidistant electrode caps (Easycap GmbH, Herrsching, Germany) with sintered Ag–AgCl electrodes were used. Fpz was used as recording reference while impedances were kept below 10 kΩ. Data was recorded using Brain Vision Recorder software and Brain Vision MR-Plus amplifiers (Brain Products GmbH, Munich, Germany) with an online anti-aliasing low-pass filter with 250 Hz high cut-off and a sampling rate of 500 Hz. Eye movements and blinks were recorded by vertical and horizontal electro-occulograms from electrodes placed 1 cm above and below the left eye and lateral to the outer canthi.
ERP Analysis
Offline data was re-referenced to average reference and high pass filtered using Brain Vision Analyzer 2 software (Brain Products GmbH, Munich, Germany). A 0.1 Hz high pass filter (Butterworth zero phase filter, 24dB/octave) was applied for the further analysis of the LPP, while a 1 Hz high pass filter (Butterworth zero phase filter, 24 dB/oct) was used for the analysis of the P100 and N170 components to remove DC artefacts which could have hampered the analysis of these components. Artefacts were removed using independent component analysis (ICA) as implemented in EEGLAB (Delorme and Makeig 2004), a freely available open source software toolbox (EEGLAB toolbox for single-trial EEG data analysis, Swartz Center for Computational Neurosciences, La Jolla, CA; http://www.sccn.ucsd.edu/eeglab) running under MATLAB (The MathWorks, Inc., Natick, Massachusetts, United States). To facilitate independent component separation, the data was cleaned manually from gross artifacts such as excessive muscle tension. Afterwards, ICA was performed (using the “runica” command), and components representing artifacts caused by e.g. muscle tension, blinks or line noise were removed. In a next step data was epoched into 600 ms segments (100 ms baseline, 500 ms post stimulus) to assess P100 and N170 and 1300 ms segments for the LPP (300 ms baseline, 1000 ms post stimulus).In both cases a maximum of 150 segments could be obtained for each condition (implicit/explicit).
While a minimum of two-thirds of trials had to be artifact-free for a subject to be included in the analyses, the number of artifact free trials was also compared across groups using t-tests. On average participants with ASD and TDC had the same number of artifact-free trials for LPP [t(35) = 0.68, p = .50; TDC M = 93.2% SD = 5.6%, ASD M = 92.1% SD = 4.2%] and coherence analysis [t(35) = 0.70, p = .49; TDC M = 88.3% SD = 7.4%, ASD M = 86.8% SD = 5.8%], while there was a slight difference of less than 10% regarding P100/N170 analysis [t(35) = 3.0, p = .01;TDC M = 87.2% SD = 7.8%, ASD M = 80.0. % SD = 6.8%].
In a next step a 30 Hz low-pass filter was applied (Butterworth Zero Phase Filter, 48 dB/oct) and averages were computed for both conditions (implicit/explicit) respectively.
ERPs were analyzed using semi-automatic peak detection. Time- windows and electrodes of interest were derived both from previous literature (especially on children and individuals with ASD, Batty et al. 2011; Hileman et al. 2011), and observed maxima of grand average data. P100 peaks were examined at O1 and O2 and observed latencies ranged from 76 to 162 ms. N170 peaks were inspected at left and right temporo-parietal clusters consisting of P7/TP7 and P8/TP8, and peaks were found between 142 and 238 ms. The broad range in peak latencies likely reflects the specific sample of this study that comprises a relatively large age range as well as a patient group, which are known for more variable peak latency (Milne 2011). Individual peaks were determined separately for each hemisphere/channel and peak amplitude was exported as a mean value of ±6 ms around the peak.
The LPP was assessed as the mean activity of an occipito-parietal cluster of electrodes (O1, O2, Oz, PO1, PO2) in a time window of 400–600 ms after stimulus onset. Electrodes and time-widow of interest were based on grand average data and previous literature on the LPP in children (Bal et al. 2010).
Coherence Analysis
For the analysis of frequency content and coherence, implicit and explicit conditions were contrasted to assess the influence of intentional processing. Data were re-referenced to average reference, filtered with a 1 Hz high-pass filter, gross artifacts were rejected manually and the data was then additionally cleaned using ICA, analogous to the preprocessing of the ERP data. Afterwards 1 s segments were extracted which started at stimulus onset. A low-pass filter of 40 Hz was used and a fast fourier transform (FFT) with a 10% Hanning Window and a maximum resolution of 0.977 Hz was applied. Afterwards power and magnitude squared coherence (MSC) were computed in the alpha (8–12 Hz) and beta (13–25 Hz) frequency range, as implemented in BrainVision Analyzer 2. Where MSC is defined as:
With Gxy(ω) being the cross power spectrum at frequency ω between signals x(t) and y(t), and Gxx(ω) and Gyy(ω) being the autospectra of x(t) and y(t) (Challis and Kitney 1991).
Alpha and beta band were chosen as they are closely related to attentional and cognitive processes (e.g., Rowland et al. 1985; van Ede et al. 2011; Klimesch 2012). Electrode pairs of interest, evaluating intra-hemispheric (O1–P7, O1–TP7, P7–TP7 left and O2–P8, O2–TP8, P8–TP8 right) as well as inter-hemispheric (O1–O2, P7–P8, TP7–TP8) coherence, were chosen to assess coherence between the areas generating the observed ERPs typical for FER in the visual system. Thereby we aimed to examine coherence between areas of early visual processing, namely the primary/secondary visual cortex and the fusiform gyrus, as a lack of connectivity between these areas has been suggested in fMRI studies (Lynn et al. 2016; Bird et al. 2006) and it is thought that social perceptual deficits in ASD already begin during early visual processing. Studies on cortical coherence during visual processing also suggest reduced interhemispheric connectivity in ASD (Isler et al. 2010; Catarino et al. 2013), which was therefore also assessed at the electrodes of interest.
Statistical Analysis
Behavioral performance measured as d′ and reaction times were compared across groups using independent sample t-tests. Sample data was approximately normally distributed. When the assumption of homogeneity of variances was violated corrected values are reported.
P100 and N170 amplitudes and latencies were analyzed with repeated measures analysis of variance (ANOVA), with the between-subject factor GROUP (ASD/TDC) and within-subject factors CONDITION (implicit/explicit) and HEMISPHERE (left/right). For the LPP mean activity during the time window of interest was also analyzed using a repeated measures ANOVA with between-subject factor GROUP (ASD/TDC) and within-subject factor CONDITION (implicit/explicit).
MSC was analyzed separately for each frequency band (alpha/beta) and the type of coherence (inter-hemispheric/intra-hemispheric). Looking at inter-hemispheric coherence a repeated measure ANOVA was performed with GROUP (ASD/TDC) as between-subject factor and within-subject factors CONDITION (implicit/explicit) and ELECTRODE-PAIR (O1–O2/P7–P8/TP7–TP8). For intra-hemispheric coherence the between-subject factor GROUP (ASD/TDC) and within-subject factors CONDITION (implicit/explicit) as well as ELECTRODE-PAIR (occipital-parietal/occipital-temporo-parietal/parietal-temporo-parietal) and HEMISPHERE (left/right). Emotion type was not taken into account for these analyses, as no differences were expected.
To establish that possible group differences in MSC were not caused by group differences in overall strength in alpha and beta power an ANOVA was also carried out for alpha and beta power respectively with GROUP (ASD/TDC) as a between-subject factor and within-subject factors CONDITION (implicit/explicit) as well as ELECTRODE-LOCATION (occipital/parietal/ temporo-parietal) and HEMISPHERE (left/right).
When interaction effects were observed, Bonferroni corrected post-hoc tests were subsequently applied. As frequency and coherence data were not normally distributed log transformation was applied to ensure that the distribution of the data and its residuals fulfilled the prerequisites for the statistical analyses. Otherwise residuals were approximately normally distributed. In the case that the assumption of sphericity was violated Greenhouse-Geisser correction was applied. Statistical analyses were conducted using STATISTICA software (StatSoft, Tulsa, USA).
Results
Behavioral Measures
The TDC group performed significantly better on the FER-task than the ASD group [t(35) = −2.5, p = .01]. A similar pattern was observed when examining reaction times [t(31.8) = 2.5, p = .02], with the ASD group showing slower responses than controls (see Table 1).
ERP Measures
P100
While no significant effects were observed in P100 latency (see Supplementary Table 1), we found a main-effect of CONDITION (F1,35 = 4.5, p = .04), i.e. a larger P100 amplitude was present in the explicit compared to the implicit condition. Additionally, an interaction between GROUP, CONDITION and HEMISPHERE was found (F1,35 = 4.81, p = .04). This interaction was caused by a larger P100 amplitude in the right compared to the left hemisphere during the explicit task in TDC (p = .03), which was absent in the ASD group (p = 1.00). Furthermore we observed a larger P100 in the right hemisphere in TDC during the explicit condition compared to the implicit condition (p = .01). No significant effects between implicit and explicit conditions were found in the ASD group (p = 1.00) (see Fig. 1 and Supplementary Table 2).
N170
There were no significant main- or interaction effects in N170 latency (Supplementary Table 3; Supplementary Fig. 1). For the N170 amplitude a trend for a main effect of CONDITION was observed (F1,35 = 3.8, p = .06, Supplementary Table 4), which indicates a higher N170 amplitude in the explicit condition.
LPP
For the LPP, there were no significant main or interaction effects. However, there was a trend for an interaction of GROUP and CONDITION (F1,35 = 3.63; p = .07), with the ASD group showing a tendency towards slightly higher amplitudes in the implicit compared to the explicit condition (Fig. 2, Supplementary Table 5). Post hoc tests however showed that these differences did not reach significance (ASD implicit versus explicit p = .32, all other comparisons p = 1.00).
Frequency and Coherence Analysis
Frequency
For the alpha frequency band a main effect of ELECTRODE (F1.5,52.1 = 77.7, p < .001) was evident. Alpha activity was stronger at occipital compared to parietal and temporo-parietal electrodes (O > P with p < .001, O > TP with p < .001), and at parietal compared to temporo-parietal sites (P > TP with p < .001). Additionally, an interaction between the factors CONDITION, ELECTRODE and HEMISPHERE (F2,70 = 3.3, p = .04) showed a slightly different pattern of alpha activity during the explicit condition between hemispheres (see Supplementary Table 6).
A main effect of ELECTRODE was also evident in the beta range (F1.441,50.429 = 47.6, p > .001) with higher beta power at occipital compared to parietal and temporo-parietal electrodes (O > P with p = .03, O > TP with p < .001), and parietal compared to temporo-parietal electrodes (P > TP with p < .001, Supplementary Table 7).
No group differences were found for any analyses regarding alpha and beta frequency.
MSC Interhemispheric
Looking at interhemispheric coherence in the alpha range a main effect of ELECTRODE-PAIR was observed (F1.7,60.0 = 370.8, p < .001). Coherence was higher between occipital electrodes compared to parietal (p < .001) and temporo-parietal ones (p < .001, Supplementary Table 8). The same pattern was found for inter-hemispheric coherence in the beta range (F1.6,56.6 = 212.0, p < .001, Supplementary Table 9).
MSC Intrahemispheric
Intra-hemispheric coherence in the alpha range also showed a main effect of ELECTRODE-PAIR (F1.7,60.0 = 370.81, p < .001; O-P > P-TP > O-TP with p < .001 for all comparisons, Supplementary Table 10).
The same pattern was observed for the beta range (F2,70 = 558.8, p > .001). Additionally, an interaction of HEMISPHERE by GROUP (F1,35 = 4.5, p = .04, Supplementary Table 11) was found. Post-hoc tests revealed a trend towards stronger intra-hemispheric MSC in the right hemisphere in the ASD group (p = .054 for left versus right hemisphere, TDC p = 1.0).
A three-way interaction was found between CONDITION, ELECTRODE-PAIR and GROUP (F2,70 = 3.2, p = .05). The TDC showed a task dependent modulation in coherence. During the implicit condition coherence was equally strong between occipital and parietal and between parietal and tempo-parietal electrodes (O–P ≈ P–TP), and lowest between occipital and tempo-parietal electrodes (O–P > O–TP, p < .001 and P–TP > O–TP, p < .001). During the explicit condition coherence between occipital and parietal electrodes increased and became stronger than the coherence between the other electrodes (O–P > P–TP > O–TP; all p < .001). The ASD group showed no task dependent modulation, but the same coherence pattern for both conditions (O–P > P–TP > O–TP; with p < .001 for all comparisons, Supplementary Fig. 2).
Discussion
The present study examined the neural correlates of implicit versus explicit processing of FER in medication free participants with ASD compared to age, IQ, and gender matched TDC. We observed behavioral differences in task performance between ASD individuals and controls, which were accompanied by between-group differences on the neural level.
On the behavioral level, we found impaired task performance and increased reaction time during the explicit FER task. This shows that individuals with ASD had problems to identify facial expressions even when they were explicitly instructed to pay attention to emotional content. Hence, FER deficits in ASD cannot be explained by a lack of attention to affective content of facial stimuli alone. Our results rather indicate that, among other factors, task difficulty plays an important role. Similarly previous investigations have shown behavioral deficits in FER to be especially prominent in ASD when challenging tasks (i.e. more difficult tasks) are used (Harms et al. 2010; Enticott et al. 2014). Our findings also highlight that the particular choice of task is of importance when examining FER in ASD individuals, and that the paradigm employed in our study was sufficiently powered (in terms of task difficulty) to detect between-group differences in FER abilities.
Looking at early visual ERPs we found two differences in the P100 response between individuals with ASD and TDC, namely (1) a lateralization-effect of the P100 during explicit processing with higher amplitudes in the right compared to left hemisphere in the TDC but not the ASD group. (2) TDC showed task dependent modulation of the P100 amplitude, with higher amplitudes during the explicit versus implicit condition in the right hemisphere. Participants with ASD did also not show this effect.
These findings confirm that between group differences can be observed when comparing implicit versus explicit FER. However results do not show stronger deficits in the ASD group during implicit processing, but rather point towards a lack of a task-dependent increase in neural activation.
Similar results have been reported regarding a lack of attention dependent modulation of the N170 in ASD for tasks shifting attention between neutral faces and other objects (Churches et al. 2010). A comparable lack of attention-dependent modulation during face processing has also been found in an fMRI study, which suggested decreased connectivity between V1 and extrastriate areas as possible underlying mechanism (Bird et al. 2006). The P100 represents early stages of holistic face processing which are sensitive to top-down attentional processes (Taylor 2002) and can therefore be modulated by task demands. The observed lack of task-dependent P100 modulation might also be related to behavioral FER deficits in ASD, as top down modulation of the P100 has been linked to other important cognitive processes such as working memory performance (Rutman et al. 2010). Similar disruptions in the P100 and lack of top-down control have also been reported for non-social visual stimuli in ASD (Maekawa et al. 2011) pointing towards a general deficit in this domain.
Furthermore TDC showed a strong right lateralization during the explicit task, which the ASD group did not. Lateralization abnormalities during face processing are possible markers of ASD emerging early during development (McCleery et al. 2009; Keehn et al. 2015) and indicate atypical development of hemispheric specialization.
Interestingly both lack of task dependent modulation and lateralization are evident in the P100 and not the N170, suggesting that attention dependent modulation might happen already during very early visual processing steps for FER. Taken together our findings highlight the importance of comparing implicit and explicit processing when FER deficits are examined in ASD.
We did not only examine early visual processing but also later occurring cognitive components, associated with attentional and emotional relevance of stimuli. Regarding the LPP, there were no between group differences, but a trend towards abnormal LPP modulation with higher amplitudes during the implicit task in ASD. Previous studies have reported reduced motivational response to facial stimuli in ASD with decreased LPP amplitudes for facial stimuli but increased amplitudes for objects in ASD (Benning et al. 2016). The LPP is a potential, which represents motivational and attentional processes in the context of the perception of emotional stimuli (Schupp et al. 2000), and is therefore an interesting component for studying implicit versus explicit FER. However, the LPP is usually evaluated in the context of highly emotional (positive or negative) compared to neutral stimuli (Schupp et al. 2004; Hajcak and Olvet 2008; Ferrari et al. 2010). As no neutral or positive emotional stimuli were used in the current study it was not possible to make this comparison. Future studies investigating LPP differences for implicit versus explicit FER should therefore also include neutral and positive facial stimuli, to examine these effects in more detail.
Moreover, inter- and intra-hemispheric coherence was examined in the context of implicit versus explicit FER. Overall observed differences in coherence between TDC and ASD group were not as marked as in previous studies (Murias et al. 2007; Coben et al. 2008; Carson et al. 2014; Jaime et al. 2016), and again we did not find the expected pattern of result. We were not able to confirm our hypothesis that coherence would be reduced during the implicit but not the explicit FER task in ASD.
Also, there were no group differences regarding interhemispheric coherence, indicating that the integration of visual information between hemispheres was not altered in ASD. Instead an interaction effect illustrated different task dependent intra-hemispheric coherence patterns in the beta frequency range for the ASD and TDC group. TDC showed an increase in coherence between occipital and parietal electrodes during the explicit compared to the implicit condition. Participants with ASD on the other hand showed no difference in coherence patterns between conditions. These results again point towards a lack of task dependent modulation in the ASD group, while integration between occipital and parietal regions seems to be modulated in TDC during explicit processing. Similar patterns of lacking task-dependent modulation in connectivity have been reported in studies where attention was shifted between faces and objects (Catarino et al. 2013; Bird et al. 2006), suggesting that this pattern applies to face processing in general and not only FER. These findings might also be related to attentional deficits, as the beta band has been linked to top-down control mechanisms (Buschman and Miller 2007). Interestingly, similar results were observed for FER in the theta range reflecting affective processing, showing that children and adolescents with ASD lacked modulation of coherence for emotional compared to neutral facial expressions (Yeung et al. 2014). Evidence that the frontal lobe plays an important role in disrupted connectivity in ASD (Kitzbichler et al. 2015) further strengthens the hypothesis of disrupted top-down control mechanisms in this context.
Limitations
To increase task difficulty and avoid ceiling effects in behavioral FER performance no neutral expressions were used as comparison categories in the current study. However, this did not allow us to study whether the effects that were found were specific to FER, or can be applied to face processing in general. But similar patterns of results have been reported in studies comparing neutral face and object processing, suggesting that effects are comparable for neutral face processing and FER (Churches et al. 2010; Catarino et al. 2013).
Another limitation is that eye-tracking was not used during EEG acquisition and therefore viewing patterns cannot be compared between groups. It is possible that our results were influenced by this factor, as it is known that individuals with ASD tend to avoid looking at the eye region of facial stimuli (Kliemann et al. 2010). The increased P100 in TDC might have been related to the control group focusing more strongly on the eye-region during the explicit task, while the ASD group might not have done so. Still, studies do not agree on whether the P100 is affected by which facial feature is fixated (Zerouali et al. 2013) or not (McPartland et al. 2010). A direct study on the correlation between fixation and ERP amplitude would be needed to confirm a link between the two in the context of implicit versus explicit FER.
Moreover FER is an ability that undergoes rapid development in early childhood and continues throughout adolescence, with different developmental trajectories for different emotional categories (see Golarai et al. 2006 for an overview). TDC typically improve at recognizing negative emotions such as fear or disgust (Herba et al. 2006), but individuals with ASD do not improve at the same rate (Lozier et al. 2014; Rump et al. 2009) and show developmental delay of theses abilities. Even though our data did not suggest any group differences with regard to age related improvement in FER abilities or its neurophysiological correlates, investigating this factor could elucidate the progress of typical and impaired development of FER.
Finally, we used static facial expressions in our study which are less ecologically valid than dynamic facial stimuli. As social impairments in ASD occur during dynamic social interactions in real live, using dynamic stimuli might give even more insight into disrupted FER mechanisms. Also studies have shown that face processing deficits in ASD are linked to disrupted integration of movement of different facial features (Shah et al. 2016). Moreover FER deficits become more pronounced when dynamic expressions are examined, as individuals with ASD lack modulation of activity in parts of the social brain related to dynamic stimulus processing (Pelphrey et al. 2007). Therefore the use of dynamic facial stimuli might further add to a better understanding of differences in FER abilities and its neural correlates when examining implicit and explicit processing strategies in ASD.
Conclusion
The current study is the first to examine FER linking task performance to ERP abnormalities and EEG coherence measures in the context of implicit versus explicit processing strategies. By comparing well characterized, matched and un-medicated ASD and TDC individuals, differences were found on the behavioral as well as neural levels. While reduced task performance indicates impairments in identifying the target emotion in ASD, ERP results point towards deficits in early visual perception and attentional top-down processing. Moreover, results of the coherence analysis indicate a lack of task dependent modulation in ASD. The study highlights the importance of taking implicit versus explicit processing into account, while also confirming that early visual perceptual processes and deficits in attentional top-down processing play an important role in FER deficits in ASD.
References
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Washington, DC: American Psychiatric Publishing.
Apicella, F., Sicca, F., Federico, R. R., Campatelli, G., & Muratori, F. (2013). Fusiform gyrus responses to neutral and emotional faces in children with autism spectrum disorders: A high density ERP study. Behavioural Brain Research, 251, 155–162.
Bal, E., Harden, E., Lamb, D., van Hecke, A. V., Denver, J. W., & Porges, S. W. (2010). Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders, 40(3), 358–370.
Batty, M., Meaux, E., Wittemeyer, K., Rogé, B., & Taylor, M. J. (2011). Early processing of emotional faces in children with autism: An event-related potential study. Journal of Experimental Child Psychology, 109(4), 430–444.
Begeer, S., Rieffe, C., Terwogt, M. M., & Stockmann, L. (2006). Attention to facial emotion expressions in children with autism. Autism: The International Journal of Research and Practice. doi:10.1177/1362361306057862.
Benning, S. D., Kovac, M., Campbell, A., Miller, S., Hanna, E. K., Damiano, C. R., et al. (2016). Late positive potential ERP responses to social and nonsocial stimuli in youth with autism spectrum disorder. Journal of Autism and Developmental Disorders. doi:10.1007/s10803-016-2845-y.
Bird, G., Catmur, C., Silani, G., Frith, C., & Frith, U. (2006). Attention does not modulate neural responses to social stimuli in autism spectrum disorders. NeuroImage, 31(4), 1614–1624.
Bölte, S., & Poustka, F. (2008). Skala zur Erfassung sozialer Reaktivität (SRS). Bern: Huber.
Bölte, S., Poustka, F., Constantino, J. N., & Gruber, C. P. (2005). SRS: Skala zur Erfassung sozialer Reaktivität: dimensionale Autismus-Diagnostik. Bern: Verlag Hans Huber.
Bölte, S., Rühl, D., Schmötzer, G., & Poustka, F. (2006). Diagnostisches interview für autismus-revidiert (ADI-R). Bern: Huber.
Buschman, T. J., & Miller, E. K. (2007). Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science, 315(5820), 1860–1862.
Carson, A. M., Salowitz, N. M. G., Scheidt, R. A., Dolan, B. K., & van Hecke, A. V. (2014). Electroencephalogram coherence in children with and without autism spectrum disorders: Decreased interhemispheric connectivity in autism. Autism Research: Official Journal of the International Society for Autism Research, 7(3), 334–343.
Catarino, A., Andrade, A., Churches, O., Wagner, A. P., Baron-Cohen, S., & Ring, H. (2013). Task-related functional connectivity in autism spectrum conditions: An EEG study using wavelet transform coherence. Molecular autism, 4(1), 1.
Challis, R. E., & Kitney, R. I. (1991). Biomedical signal processing (in four parts). III, The power spectrum and coherence function. Medical & Biological Engineering & Computing, 29(3), 225–241.
Churches, O., Wheelwright, S., Baron-Cohen, S., & Ring, H. (2010). The N170 is not modulated by attention in autism spectrum conditions. NeuroReport, 21(6), 399–403.
Coben, R., Clarke, A. R., Hudspeth, W., & Barry, R. J. (2008). EEG power and coherence in autistic spectrum disorder. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 119(5), 1002–1009.
Corden, B., Chilvers, R., & Skuse, D. (2008). Avoidance of emotionally arousing stimuli predicts social-perceptual impairment in Asperger’s syndrome. Neuropsychologia, 46(1), 137–147. doi:10.1016/j.neuropsychologia.2007.08.005.
Critchley, H., Daly, E., Phillips, M., Brammer, M., Bullmore, E., Williams, S., et al. (2000). Explicit and implicit neural mechanisms for processing of social information from facial expressions: A functional magnetic resonance imaging study. Human Brain Mapping. doi:10.1002/(SICI)1097-0193(200002)9:2<93:AID-HBM4>3.0.CO;2-Z.
Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21.
Duffy, F. H., & Als, H. (2012). A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls—a large case control study. BMC Medicine, 10, 64.
Ebner, N. C., Riediger, M., & Lindenberger, U. (2010). FACES–a database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behavior Research Methods, 42(1), 351–362.
Enticott, P. G., Kennedy, H. A., Johnston, P. J., Rinehart, N. J., Tonge, B. J., Taffe, J. R., & Fitzgerald, P. B. (2014). Emotion recognition of static and dynamic faces in autism spectrum disorder. Cognition & Emotion, 28(6), 1110–1118.
Ferrari, V., Bradley, M. M., Codispoti, M., & Lang, P. J. (2010). Detecting novelty and significance. Journal of Cognitive Neuroscience, 22(2), 404–411.
Golarai, G., Grill-Spector, K., & Reiss, A. L. (2006). Autism and the development of face processing. Clinical Neuroscience Research. doi:10.1016/j.cnr.2006.08.001.
Gorno-Tempini, M. L., Pradelli, S., Serafini, M., Pagnoni, G., Baraldi, P., Porro, C., et al. (2001). Explicit and incidental facial expression processing: An fMRI study. NeuroImage. doi:10.1006/nimg.2001.0811.
Hajcak, G., & Olvet, D. M. (2008). The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion (Washington, D. C.), 8(2), 250.
Harms, M. B., Martin, A., & Wallace, G. L. (2010). Facial emotion recognition in autism spectrum disorders: A review of behavioral and neuroimaging studies. Neuropsychology Review, 20(3), 290–322.
Haxby, J. V., Hoffman, E. A., & Gobbini, M. (2000). The distributed human neural system for face perception. Trends in Cognitive Sciences. doi:10.1016/S1364-6613(00)01482-0.
Herba, C. M., Landau, S., Russell, T., Ecker, C., & Phillips, M. L. (2006). The development of emotion-processing in children: Effects of age, emotion, and intensity. Journal of Child Psychology and Psychiatry, and Allied Disciplines. doi:10.1111/j.1469-7610.2006.01652.x.
Hileman, C. M., Henderson, H., Mundy, P., Newell, L., & Jaime, M. (2011). Developmental and individual differences on the P1 and N170 ERP components in children with and without autism. Developmental Neuropsychology, 36(2), 214–236.
Isler, J. R., Martien, K. M., Grieve, P. G., Stark, R. I., & Herbert, M. R. (2010). Reduced functional connectivity in visual evoked potentials in children with autism spectrum disorder. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 121(12), 2035–2043.
Jaime, M., McMahon, C. M., Davidson, B. C., Newell, L. C., Mundy, P. C., & Henderson, H. A. (2016). Brief report: Reduced temporal-central EEG alpha coherence during joint attention perception in adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders, 46(4), 1477–1489.
Kamio, Y., Wolf, J., & Fein, D. (2006). Automatic processing of emotional faces in high-functioning pervasive developmental disorders: An affective priming study. Journal of Autism and Developmental Disorders. doi:10.1007/s10803-005-0056-z.
Keehn, B., Vogel-Farley, V., Tager-Flusberg, H., & Nelson, C. A. (2015). Atypical hemispheric specialization for faces in infants at risk for autism spectrum disorder. Autism Research, 8(2), 187–198.
Kitzbichler, M. G., Khan, S., Ganesan, S., Vangel, M. G., Herbert, M. R., Hamalainen, M. S., & Kenet, T. (2015). Altered development and multifaceted band-specific abnormalities of resting state networks in autism. Biological Psychiatry, 77(9), 794–804.
Kleinhans, N. M., Richards, T., Sterling, L., Stegbauer, K. C., Mahurin, R., Johnson, L. C., et al. (2008). Abnormal functional connectivity in autism spectrum disorders during face processing. Brain: A Journal of Neurology. doi:10.1093/brain/awm334.
Kliemann, D., Dziobek, I., Hatri, A., Steimke, R., & Heekeren, H. R. (2010). Atypical reflexive gaze patterns on emotional faces in autism spectrum disorders. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. doi:10.1523/JNEUROSCI.0688-10.2010.
Klimesch, W. (2012). alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16(12), 606–617.
Koshino, H., Kana, R. K., Keller, T. A., Cherkassky, V. L., Minshew, N. J., & Just, M. A. (2008). fMRI investigation of working memory for faces in autism: Visual coding and underconnectivity with frontal areas. Cerebral cortex (New York, N.Y.: 1991). doi:10.1093/cercor/bhm054.
Leung, R. C., Ye, A. X., Wong, S. M., Taylor, M. J., & Doesburg, S. M. (2014). Reduced beta connectivity during emotional face processing in adolescents with autism. Molecular Autism. doi:10.1186/2040-2392-5-51.
Lozier, L. M., Vanmeter, J. W., & Marsh, A. A. (2014). Impairments in facial affect recognition associated with autism spectrum disorders: A meta-analysis. Development and Psychopathology. doi:10.1017/S0954579414000479.
Lynn, A. C., Padmanabhan, A., Simmonds, D., Foran, W., Hallquist, M. N., Luna, B., et al. (2016). Functional connectivity differences in autism during face and car recognition: Underconnectivity and atypical age-related changes. Developmental Science. doi:10.1111/desc.12508.
Macmillan, N. A., & Creelman, C. D. (2004). Detection theory: A user’s guide (p. 7). New York: Psychology press.
Maekawa, T., Tobimatsu, S., Inada, N., Oribe, N., Onitsuka, T., Kanba, S., & Kamio, Y. (2011). Top-down and bottom-up visual information processing of non-social stimuli in high-functioning autism spectrum disorder. Research in Autism Spectrum Disorders, 5(1), 201–209.
Mathersul, D., McDonald, S., & Rushby, J. A. (2013). Automatic facial responses to briefly presented emotional stimuli in autism spectrum disorder. Biological Psychology. doi:10.1016/j.biopsycho.2013.08.004.
Mathewson, K. J., Jetha, M. K., Drmic, I. E., Bryson, S. E., Goldberg, J. O., & Schmidt, L. A. (2012). Regional EEG alpha power, coherence, and behavioral symptomatology in autism spectrum disorder. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 123(9), 1798–1809.
McCleery, J. P., Akshoomoff, N., Dobkins, K. R., & Carver, L. J. (2009). Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biological Psychiatry, 66(10), 950–957.
McPartland, J., Cheung, C. H. M., Perszyk, D., & Mayes, L. C. (2010). Face-related ERPs are modulated by point of gaze. Neuropsychologia. doi:10.1016/j.neuropsychologia.2010.07.020.
McPartland, J., Dawson, G., Webb, S. J., Panagiotides, H., & Carver, L. J. (2004). Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry, 45(7), 1235–1245.
Milne, E. (2011). Increased intra-participant variability in children with autistic spectrum disorders: Evidence from single-trial analysis of evoked EEG. Frontiers in Psychology, 2, 51.
Monk, C. S., Weng, S. J., Wiggins, J. L., Kurapati, N., Louro, H. M., Carrasco, M., et al. (2010). Neural circuitry of emotional face processing in autism spectrum disorders. Journal of Psychiatry & Neuroscience: JPN, 35(2), 105.
Murias, M., Webb, S. J., Greenson, J., & Dawson, G. (2007). Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry, 62(3), 270–273.
Nunez, P. L., Srinivasan, R., Westdorp, A. F., Wijesinghe, R. S., Tucker, D. M., Silberstein, R. B., & Cadusch, P. J. (1997). EEG coherency: I: Statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalography and Clinical Neurophysiology, 103(5), 499–515.
O’Connor, K., Hamm, J. P., & Kirk, I. J. (2005). The neurophysiological correlates of face processing in adults and children with Asperger’s syndrome. Brain and Cognition, 59(1), 82–95.
Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113.
Peiker, I., David, N., Schneider, T. R., Nolte, G., Schöttle, D., & Engel, A. K. (2015). Perceptual integration deficits in autism spectrum disorders are associated with reduced interhemispheric gamma-band coherence. Journal of Neuroscience, 35(50), 16352–16361.
Pelphrey, K. A., Morris, J. P., McCarthy, G., & Labar, K. S. (2007). Perception of dynamic changes in facial affect and identity in autism. Social Cognitive and Affective Neuroscience. doi:10.1093/scan/nsm010.
Petermann, F., & Petermann, U. (2010). HAWIK-IV. Hamburg-Wechsler-Intelligenztest für Kinder-IV (Wechsler Intelligence Scale for Children (WISC-IV)-German version). Bern: Huber.
Rowland, N., Meile, M. J., & Nicolaidis, S. (1985). EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science, 228, 750–752.
Rühl, D., Bölte, S., Feineis-Matthews, S., & Poustka, F. (2004). ADOS, autism diagnostic observation schedule for autistic disorders. Bern: Huber (German)
Rump, K. M., Giovannelli, J. L., Minshew, N. J., & Strauss, M. S. (2009). The development of emotion recognition in individuals with autism. Child Development. doi:10.1111/j.1467-8624.2009.01343.x.
Rutman, A. M., Clapp, W. C., Chadick, J. Z., & Gazzaley, A. (2010). Early top-down control of visual processing predicts working memory performance. Journal of Cognitive Neuroscience, 22(6), 1224–1234. doi:10.1162/jocn.2009.21257.
Schneider, S., Unnewehr, S., & Margraf, J. (Eds.). (2009). Kinder-DIPS: Diagnostisches Interview bei psychischen Störungen im Kindes- und Jugendalter (2., aktualisierte und erw. Aufl.). Heidelberg: Springer.
Schultz, R. T., Gauthier, I., Klin, A., Fulbright, R. K., Anderson, A. W., Volkmar, F., et al. (2000). Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. doi:10.1001/archpsyc.57.4.331.
Schupp, H., Cuthbert, B., Bradley, M., Hillman, C., Hamm, A., & Lang, P. (2004). Brain processes in emotional perception: Motivated attention. Cognition and Emotion, 18(5), 593–611.
Schupp, H. T., Cuthbert, B. N., Bradley, M. M., Cacioppo, J. T., Ito, T., & Lang, P. J. (2000). Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology, 37(2), 257–261.
Shah, P., Bird, G., & Cook, R. (2016). Face processing in autism: Reduced integration of cross-feature dynamics. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. doi:10.1016/j.cortex.2015.11.019.
Sporns, O., Tononi, G., & Edelman, G. M. (2000). Theoretical neuroanatomy: Relating anatomical and functional connectivity in graphs and cortical connection matrices. Cerebral Cortex, 10(2), 127–141.
Taylor, M. J. (2002). Non-spatial attentional effects on P1. Clinical Neurophysiology, 113(12), 1903–1908.
van Ede, F., de Lange, F., Jensen, O., & Maris, E. (2011). Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha- and beta-band oscillations. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(6), 2016–2024.
von Aster, M., Neubauer, A., & Horn, R. (2006). Wechsler-Intelligenztest für Erwachsene: Ubersetzung und Adaptation der WAIS-III von David Wechsler [Wechsler intelligence test for adults: translation and adaptation of WAIS-III by David Wechsler]. Harcourt Test Services, Frankfurt/M.
Waldmann, H.-C. (2008). Kurzformen des HAWIK-IV: Statistische Bewertung in verschiedenen Anwendungsszenarien. Diagnostica, 54(4), 202–210.
Wallace, S., Coleman, M., & Bailey, A. (2008). An investigation of basic facial expression recognition in autism spectrum disorders. Cognition & Emotion, 22(7), 1353–1380.
Webb, S. J., Merkle, K., Murias, M., Richards, T., Aylward, E., & Dawson, G. (2012). ERP responses differentiate inverted but not upright face processing in adults with ASD. Social Cognitive and Affective Neuroscience. doi:10.1093/scan/nsp002.
Wicker, B., Fonlupt, P., Hubert, B., Tardif, C., Gepner, B., & Deruelle, C. (2008). Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Social Cognitive and Affective Neuroscience. doi:10.1093/scan/nsn007.
Wong, T. K. W., Fung, P. C. W., Chua, S. E., & McAlonan, G. M. (2008). Abnormal spatiotemporal processing of emotional facial expressions in childhood autism: Dipole source analysis of event-related potentials. The European Journal of Neuroscience, 28(2), 407–416.
World Health Organization (1992). The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: World Health Organization.
Yeung, M. K., Han, Y. M., Sze, S. L., & Chan, A. S. (2014). Altered right frontal cortical connectivity during facial emotion recognition in children with autism spectrum disorders. Research in Autism Spectrum Disorders, 8(11), 1567–1577.
Zerouali, Y., Lina, J.-M., & Jemel, B. (2013). Optimal eye-gaze fixation position for face-related neural responses. PLoS ONE. doi:10.1371/journal.pone.0060128.
Acknowledgments
We gratefully thank our participants and their parents for taking part in our study. We also thank Jennifer Zimmermann for her valuable support in EEG data acquisition and preparation, Heiko Zerlaut for his valuable help with phenotypic data preparation and Heike Althen for her helpful comments on an earlier draft of the manuscript. The study was supported by German Research Foundation (DFG)-Grant FR2069/2-1 to CMF.
Author Contributions
CL collected data, performed data analysis, and drafted the manuscript. AK was involved in study design and data collection. HC participated in study coordination and data collection. SB designed the study, supervised data analysis and interpretation of results, and critically revised the manuscript. CMF designed the study, supervised data analysis, interpretation of results and writing of the manuscript, and critically revised the manuscript. All co-authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
CL, AK, HC and CMF have no conflicts of interest to declare. SB has received support from Shire, Lilly, Actelion, Medice for symposia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luckhardt, C., Kröger, A., Cholemkery, H. et al. Neural Correlates of Explicit Versus Implicit Facial Emotion Processing in ASD. J Autism Dev Disord 47, 1944–1955 (2017). https://doi.org/10.1007/s10803-017-3141-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-017-3141-1