Abstract
Purpose
To evaluate the role of multiparametric ultrasound (mpUS) in the characterization of focal breast lesions (FBLs).
Methods
This prospective study enrolled patients undergoing multiparametric breast ultrasound for FBLs. An experienced breast radiologist evaluated the following ultrasound features: US BI-RADS category, vascularization pattern (internal, vessels in rim and combined) and presence of penetrating vessels with each Doppler method (Color-Doppler, Power-Doppler, Microvascular imaging), strain ratio (SR) and Tsukuba score (TS) with Strain Elastography (SE), Emax, Emean, Emin and Eratio with 2D-shear wave elastography (2D-SWE). Core biopsy for all BI-RADS 4-5 FBLs and 24-month follow-up for all BI-RADS 2-3 FBLs were considered for standard of reference. The diagnostic performance was assessed with the area under curve (AUCs) and cut-off values were determined according to the Youden’s index.
Results
A total of 139 FBLs were included with 75/139 (53.9%) benign and 64/139 (46.1%) malignant FBLs. Internal vascularization patterns (p < 0.001), penetrating vessels (p < 0.001), TS 4-5 (p < 0.001) and all 2D-SWE parameters (p < 0.001) were significantly different between benign and malignant FBLs. The BI-RADS score provided an AUC of 0.876 (95% CI 0.810–0.926) for the diagnosis of malignant FBLs. Among the 2D-SWE measurements, an excellent diagnostic performance was observed for Emax with an AUC of 0.915 (95% CI 0.856–0.956) and Emean of 0.908 (95% CI 0.847–0.951). Optimal cutoff for the diagnosis of malignant FBLs were US BI-RADS > 3, Strain Ratio > 2.52, Tsukuba Score > 3, Emax > 82.6 kPa, Emean > 66.0 kPa, Emin > 54.4 kPa and Eratio > 330.8.
Multiparametric ultrasound, particularly SWE, can improve specificity in the characterization of FBLs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast ultrasound (US) is considered, in association with mammography and magnetic resonance imaging, a valuable tool in breast imaging [1], especially in young women with dense breasts [2].
Whereas B-Mode US features are still considered fundamental for the characterization of of focal breast lesions (FBLs), recently technological advances have enabled the spread of multiparametric ultrasound (mpUS), which allows the association of the conventional B-Mode assessment with other tools for analyzing specific features, such as the assessment of vascularization and elasticity of FBLs. The application of Color-Doppler (CD), Power-Doppler (PD) and new developments, such as Microvascular Flow Imaging (MFVI), provides important information on the vascularization of FBLs [3, 4], which is related to the underlying phenomenon neoangiogenesis [5]. Breast elastography [6] is a noninvasive method for measuring the elasticity of a FBLs through two different technique: the Strain Elastography (SE) [7], which allows qualitative assessment methods such as the Tsukuba Score (TS) [8] and semi-quantitative methods such as the Strain Ratio (SR) [9], and the shear-wave elastography (SWE) [10], which ensures a quantitative evaluation of the elasticity of each point of the area examined (2D-SWE) in terms of absolute data, such as maximum elasticity (Emax), mean elasticity (Emean) and minimum elasticity (Emin) expressed in kPa, or relative data, such as elasticity ratio (Eratio). Several studies have shown that the breast mpUS approach can improve diagnostic performance, particularly it increased the specificity and reduced the number of unnecessary biopsies in low-suspicion FBLs [11,12,13,14]. It can be also useful in the assessment of US BI-RADS 3 and 4 FBLs, suggesting a strategy for down- and up- grading [15, 16]. In addition, breast mpUS could be useful in the evaluation of some tumors that are sometimes difficult to distinguish from benign FBLs because both appear as smooth-surfaced masses, such as mucinous carcinoma [17] and triple negatives [18]. However, many of these studies did not evaluate and directly compare both qualitative and quantitative parameters in the multiparametric breast ultrasound approach.
The purpose of this study was to evaluate the role of mpUS in the characterization of FBLs by analyzing the multiparametric features, both qualitative and quantitative, of the US findings.
Methods
Study participants
The institutional Ethic Committee approved this prospective, single-center study and all regulatory approvals were granted (N°022019). All patients included gave their full written informed consent. Our study complied with the terms of the Declaration of Helsinki [19].
This study included 1200 patients women who underwent US evaluation between January 2019 and December 2019 by a radiologist with 20 years of experience in breast imaging, including ultrasound, at the Breast Unit of the Policlinico Universitario “P.Giaccone” in Palermo, Italy. Inclusion criteria were: (1) a palpable mass detected on physical examination; (2) a detected lesion from adjunct mammography examination; (3) dense breasts; (4) mastodynia; (5) young patients having family history or (6) in a follow-up for benign breast nodules.
Exclusion criteria included negative examination (BI-RADS 1), lack of adequate standard of reference, namely refusal to undergo biopsies for FBLs classified as BI-RADS 4–5 or inconsistent follow-up for FBLs classified as BI-RADS 3, and the lack of even multiparametric assessment techniques of vascular imaging, and elasticity, and 2D-SWE.
Standard of reference
The standard of reference of this study was considered US-guided core-biopsy for all the FBLs classified as BI-RADS 4 or 5, either before or after mpUS assessment, and US follow-up at 6, 12 and 24-months for all FBLs classified as BI-RADS 2 and 3. In particular, stability or size decrease during follow-up was considered typically benign US findings. FBLs with size increases during follow-up were considered malignant and US-guided core-biopsy was performed.
Ultrasound measurements
The breast radiologist assessed the following mpUS features of all FBLs: B-Mode, CD, PD, MVFI, SE, 2D-SWE, blinded to the pathological results. An ultrasound unit (RS80A+, Samsung Medison, Co. Ltd.) provided with high-frequency linear transducers (LA 5–12 MHz and LA 2–9 MHz) was used. For each FBL, the following parameters were recorded: US BI-RADS category, maximum diameter of the lesion, vascularization pattern and presence of penetrating vessels with each vascular imaging method (CD, PD, MVFI), Strain Ratio and Tsukuba Score evaluated with SE, Emax, Emean, Emin and Eratio acquired with by 2-D SWE analysis.
To obtain vascular information, a Doppler box was used and the parameters were adjusted to increase the sensitivity to low-velocity flow with a low wall filter and gain as high as possible but still minimizing flash artefacts. Soft pressure was applied to the transducer to preserve the flow of small blood vessels. The parameters for CD and PD US were velocity scale < 2.3 cm/s, dynamic range of 45 dB, and frame average rate of eight and nine frames per second. The parameters for MVFI were velocity scale < 1.3 cm/s, dynamic range of 56 dB and frame rate of 40 frames per second. The vascularization patterns recorded as “absent”, “internal” and “vessels in rim”, according to the BI-RADS US lexicon, and “combined”, with both internal and vessels in rim; the latter, although not included in US BI-RADS analysis, it is often encountered in clinical practice and widely reported in literature [4, 20]. In addition, the radiologist was asked to report the presence of a “penetrating vessel”, defined as a radially aligned or marginally oriented vessel within the tumor [20].
The SE was performed applying the probe only with soft pressure and placing a target lesion in the center of a box that superiorly included the subcutaneous fat, inferiorly included the pectoralis muscle and on both sides included more than 5 mm of normal breast parenchyma from the lesion’s borders. A real-time elastography image was obtained using a color map that showing the degree of displacement for all pixels within the box, representing the amount of strain in a scale ranging from red (greatest strain, softest area) to green (average strain, intermediate component) and blue (no strain, hardest area). To calculate the Strain Ratio, a ROI within the lesion and a ROI outside the lesion (in the context of surrounding fat tissue) were placed, in order to measure the relative density of the lesion.
The 2D-SWE was performed by placing the probe vertically on the skin, avoiding applying heavy pressure. A real-time assessment of the lesion was performed in a dual screen mode, displacing at the same time a conventional B-mode observation and a qualitative elasticity assessment. A specific box is superimposed on the lesion, allowing a two-dimensional colorimetric map in real time, ranging from dark blue (lower retyping) to red (higher rigidity). A circular ROI sized 2 mm was placed within the lesion in the stiffest area as depicted by a color-coded map in a rectangular box, ranging from 0 (dark blue: soft) to 180 kPa (red: stiff). To calculate Eratio, another circular ROI of the same size was placed at the same level in the surrounding breast tissue.
Statistical analysis
Continuous variables were reported medians and interquartile ranges after testing for normal distribution with the Shapiro–Wilk normality test and they were compared with the Mann–Whitney U test. Categorical variables were reported as numbers and percentages and they were compared with the Pearson’s chi-squared test of Fisher’s exact test. The diagnostic performance of mpUS measurements was assessed with the area under the receiver operating characteristics curve (AUC) with 95% confidence interval (95% CI). Optimal cutoffs for the diagnosis of malignant lesions were determined with the Younden index with their sensitivity and specificity [21].
Statistical significance level was set at p < 0.05. Statistical analyses were performed using IBM SPSS software version 26.0 (Armonk, NY, USA: IBM Corp) and MedCalc Statistical Software version 14.8.1 (Ostend, Belgium).
Results
The study included a total of 139 FBLs (size range 3.5–50 mm; mean size ± SD: 14.9 ± 8.3 mm) in 134 women (age range 21–87 years, mean age ± SD: 53.6 ± 14.5 years) who underwent mpUS. According to the standard of reference, 75/139 (53.9%) FBLs were benign and 64/139 (46.1%) were malignant (Table 1); among benign FBLs, the most prevalent lesion was fibroadenoma (n = 62), while among malignant FBLs, the most prevalent was invasive ductal carcinoma (n = 49).
According to BI-RADS assessment, two (1.4%) FBLs were classified as BI-RADS 2, 55 (39.6%) as BI-RADS 3, 29 (20.9%) as BI-RADS 4a, 12 (8.6%) as BI-RADS 4b, 14 (10.1%) as BI-RADS 4c and 27 (19.4%) as BI-RADS 5.
Regarding the vascular pattern (Table 2), presence of vascularization was significantly more common in malignant FBLs compared to benign FBLs for all the vascular imaging methods (p ≤ 0.003). The internal vascular pattern and the presence of penetrating vessels were significantly more common in malignant FBLs for all the vascular imaging methods (all p < 0.001). Contrarily, benign FBLs demonstrated more commonly the vessel in rim pattern in CD (p = 0.015) and in MVFI (p = 0.033) and the combined pattern in PD (p = 0.033) and in MVFI (p = 0.001).
All the quantitative measurements acquired with SE and 2D-SWE (Table 3) were significantly higher in malignant FBLs compared with benign FBLs (all p < 0.001). Regarding the qualitative assessment of SE there were significant differences in the TS, with 67/75 (89.3%) benign FBLs showing a TS 1-3, while 8/75 (10.7%) showing a TS 4-5; on the other hand, 46/64 (71.9%) malignant FBLs showed a TS 4–5, while 18/64 (28.1%) showed a TS 1–3 (p < 0.001).
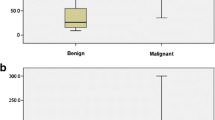
Table 4 reports the performance and the cut-off values obtained using the Youden method for the mpUS measurements with their sensitivity and specificity. The BI-RADS score provided an AUC of 0.876 (95% CI 0.810–0.926). In SE, TS had the highest diagnostic performance for the diagnosis of malignant FBLs with an AUC of 0.874 (95% CI 0.807–0.924). Among the 2D-SWE measurements, an excellent diagnostic performance was observed for Emax with an AUC of 0.915 (95% CI 0.856–0.956) and Emean of 0.908 (95% CI 0.847–0.951). AUCs are illustrated in Fig. 1. Optimal cutoff for the diagnosis of malignant lesions were US BI-RADS > 3, strain ratio > 2.52, Tsukuba score > 3, Emax > 82.6 kPa, Emean > 66.0 kPa, Emin > 54.4 kPa and Eratio > 330.8. Examples of lesions with mpUS assessment are provided in Figs. 2, 3 and 4.
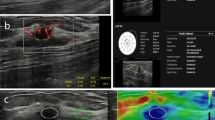
Asymptomatic 38-year-old woman; on B-Mode US examination (A): hypoechoic oval mass with circumscribed margins, parallel orientation and no posterior findings, classified as BI-RADS 3. The microvascular flow imaging shows mild internal vascularization (B). Strain elastography (C) characterizes the lesion as soft, with a predominant green pattern (Tsukuba score 1). 2D-shear wave elastography colorimetric map (D) shows a soft lesion with a peripheral stiffer area (green), with an Emax of 82.6 kPa. The multiparametric US features described are consistent with the diagnosis of fibroadenoma
79-year-old woman with known calcific fibroadenoma. On B-mode US examination (A): hypoechoic oval mass with circumscribed margins and some course calcifications (indicated by arrowhead), responsible for a posterior shadowing (thin arrows). The microvascular flow imaging (B) reveals the absence of internal or vessels in rim vascularization. Strain elastosonography (C) shows a predominantly blue pattern with green areas at the periphery (Tsukuba score 3); on 2D-shear wave elastography analysis (D), the colorimetric map (lower right) shows heterogeneity of stiffness with the highest values corresponding to intralesional calcifications
A 59-year-old woman with a palpable lump in her left breast. B-mode US examination (A) shows the presence of a hypoechoic mass with irregular shape and indistinct, spiculated margins. Intralesional microcalcifications (thin arrows), posterior shadowing (thick arrows) and a perilesional "echogenic halo" (arrowheads) are present. The study of vascularization shows the presence of flow within the lesion with all three techniques Color Doppler (B), Power Doppler (C) and microvascular flow imaging (D): the latter technique also shows the presence of a “penetrating vessel” (arrowhead) penetrating from the periphery into the lesion. Elasticity assessment with strain (E) and 2D shear wave elastography (F) reveals a lesion harder than the surrounding tissue, with Emin, Emean, Emax and Eratio values above the identified cut-offs. Histopathology of US core biopsy: invasive ductal carcinoma
Discussion
US B-mode imaging is a widely used technique for the characterization of FBLs, and the anatomic morphologic descriptors provided by this technique are included in the BI-RADS lexicon of the American College of Radiology [22]. The exclusively morphologic assessment guaranteed by the B-mode approach, however, does not allow the evaluation of the many phenotypic features acquired by a heterogeneous disease such as breast cancer [23]. The latest available edition of the BI-RADS lexicon (dated 2013), however, considers associated features both vascularization (absent, internal vascularization, vessels in rim) and elasticity assessment (soft, intermediate, hard), although it specifies not to use them as the only diagnostic feature in interpretation nor to override morphologic features more predictive of malignancy for patient management. On the other hand, in accordance with the updated 2018 EFSUMB guidelines and the 2015 WFUMB guidelines, biopsy is suggested for a FBLs classified BI-RADS 3 that shows high stiffness on breast ultrasound elastography [15, 24]. In addition, the 2015 WFUMB guidelines indicate the downgrading of a US BI-RADS 3 or 4a FBLs on the basis of stiffness on breast ultrasound elastography, while downgrading of a US BI-RADS 4b, 4c, or 5 FBLs is not recommended; this approach confirmed by recent prospective studies [16, 25] The application of such arrangements in clinical practice could reduce unnecessary biopsies and allow to avoid expensive and relatively invasive procedures.
The results of this study demonstrated a significant difference between benign and malignant FBLs in quantitative 2D-SWE parameters, with significantly higher values in malignant FBLs (all p < 0.001), confirming previously reported data performed with different ultrasound equipment [26, 27]. The cut-off values for Emin–Emean–Emax and their corresponding AUC were higher than BI-RADS assessment alone; these results were in accordance with the results of the multicentric study of Berg et al. [26]. The 2D-SWE analysis also resulted in increased specificity for all four parameters studied compared to BI-RADS assessment alone, particularly for the Emax parameter, with a decrease in sensitivity only for Eratio. Consequently, these data suggest that 2D-SWE assessment would have led to a significant reduction in unnecessary biopsies, without a significant increase in breast cancer missed, except for the Eratio parameter alone.
TS was the mpUS parameter with the highest specificity, and despite the suboptimal sensitivity, the data on correlation with benign and malignant FBLs agree with those in the literature [28].
Compared with BI-RADS assessment alone, SR showed lower sensitivity and overlapping specificity. Elia et al., using a similar cut-off (2.49) for SR, reported comparable sensitivity and specificity values [29].
MVFI showed a superior ability to visualize microvessels and, in general, in identifying the presence of vascularization, compared with conventional CD and PD, in different clinical settings, including the breast [4, 30, 31]. Therefore, the MVFI was considered, in this study and in previous studies [31], the most reliable vascular imaging technique in assessing the vascularization patterns most frequently associated with benign FBLs and malignant FBLs. The results of the current study showed a statistically significant prevalence of the absence of vascularization, vessels in rim pattern and combined pattern in benign FBLs compared with malignant FBLs. In our experience, lack of vascularization demonstrated by MVFI outperformed the CD findings of the prospective study by Watanabe et al. in the characterization of benign FBLs [20]. On the other hand, in our series the presence of internal vascularization as revealed by MVFI improved breast cancer characterization when compared to the study of Svensson et.al, performed with CD assessment alone [3]. Regarding the combined pattern, in our series, it was significantly more frequent in benign FBLs. These data are in accordance with the study by Watanabe [20].
According to standard of reference, a small but not negligible number of breast carcinomas (N = 4) would have been missed with exclusive use of the US BI-RADS lexicon; in particular, one triple negative invasive ductal carcinoma, one luminal B/Her negative invasive ductal carcinoma, one lobular carcinoma in situ (CLIS) and one mucinous carcinoma would have been missed. The first two cases, initially classified as BI-RADS 3, appeared suspicious on both vascular (particularly on MVFI) and elastographic evaluations. CLIS appeared suspicious on SE (TS 5) and on 2D-SWE (Emin, Emean, Emax of 59.3, 80.45, 101.6, respectively). Mucinous carcinoma, on the other hand, also initially classified as BI-RADS 3, showed values of the quantitative 2D-SWE parameters below the cut-offs identified in our case series and a combined vascularization, in the absence of penetrating vessels; however, it appeared suspicious on SE (TS 4 and an SR 3.8). Lower values of Emax and Emean than for other types have already been reported in the literature [32].
In our series, 22 unnecessary biopsies of benign FBLs would have occurred using the US BI-RADS lexicon alone. Among them, a number ranging from 6 to 16 would have been suspicious taking into consideration the quantitative parameters 2D-SWE and SR, particularly fibroadenomas; indeed, previous studies have stated that fibroadenomas, especially if calcific, giving false-positive SWE [33]. On the other hand, among the above false positives, a TS greater than 3 was recorded in only 2 FBLs, confirming that an exclusively qualitative assessment sometimes correlates better with the BI-RADS classification [33].
Our study has some limitations. Firstly, we did not perform a direct comparison of vascularization and tumor elasticity by histologic analysis. Such analyses could have provided more information about the true correspondence between vascularization pattern and/or presence of penetrating vessels with the true degree of angiogenesis, or between SE and 2D-SWE elastographic analyses with tumor stiffness, but would not have directly affected the results of our study. In addition, we did not perform histologic analysis for all FBLs, because for clearly benign FBLs on imaging the standard of reference was follow-up imaging. Second, inter- and intra-reader agreement reproducibility was not calculated. Finally, it is a single-center study that included a limited population of women; therefore, further studies, involving multiple centers and operators, are needed to include different populations to evaluate the reproducibility of our results in other geographical areas.
In conclusion, mpUS enables noninvasive real-time analysis of focal breast lesions. The multiparametric approach, with the technological improvement of B-mode imaging, the refinement of microvascular imaging systems and the introduction of elastography techniques enable the physician to obtain quantitative and reproducible parameters, improving diagnostic accuracy. In our experience, among the various methods, the Emax parameter of 2D-SWE showed the best results in terms of specificity, with similar sensitivity compared with BI-RADS classification.
Data availability
Not applicable.
References
Hooley RJ, Scoutt LM, Philpotts LE (2013) Breast ultrasonography: state of the art. Radiology 268(3):642–659
Berg WA (2004) Supplemental screening sonography in dense breasts. RadiolClin North Am. 42(5):845–851
Svensson WE, Pandian AJ, Hashimoto H (2010) The use of breast ultrasound color Doppler vascular pattern morphology improves diagnostic sensitivity with minimal change in specificity. Ultraschall Med 31(5):466–474. https://doi.org/10.1055/s-0028-1109478
Bartolotta TV, Orlando AAM, Schillaci MI, Spatafora L, Marco MD, Matranga D, Firenze A, Cirino A, Ienzi R (2021) Ultrasonographic detection of vascularity of focal breast lesions: microvascular imaging versus conventional color and power doppler imaging. Ultrason Imaging 43(5):273–281. https://doi.org/10.1177/01617346211029542. (Epub 2021 Jul 8)
Schneider BP, Miller KD (2005) Angiogenesis of breast cancer. J ClinOncol 23(8):1782–1790
Ricci P, Maggini E, Mancuso E, Lodise P, Cantisani V, Catalano C (2014) Clinical application of breast elastography: state of the art. Eur J Radiol 83(3):429–437. https://doi.org/10.1016/j.ejrad.2013.05.007
Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X (1991) Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging 13:111–134
Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T et al (2006) Breast disease: clinical application of US elastography for diagnosis. Radiology 239:341–350
Park HJ, Kim SM, Yun B, Jang M, Kim B, Lee SH, Ahn HS (2020) Comparison of one- and two-region of interest strain elastography measurements in the differential diagnosis of breast masses. Korean J Radiol 21(4):431–441. https://doi.org/10.3348/kjr.2019.0479
Youk JH, Gweon HM, Son EJ (2017) Shear-wave elastography in breast ultrasonography: the state of the art. Ultrasonography 36(4):300–309. https://doi.org/10.14366/usg.17024
Cho N, Jang M, Lyou CY et al (2012) Distinguishing benign from malignant masses at breast US: combined US elastography and color doppler US–influence on radiologist accuracy. Radiology 262:80–90
Li DD, Xu HX, Guo LH et al (2016) Combination of two-dimensional shear wave elastography with ultrasound breast imaging reporting and data system in the diagnosis of breast lesions: a new method to increase the diagnostic performance. Eur Radiol 36:3290–3300
Kapetas P, Clauser P, Woitek R, Wengert GJ, Lazar M, Pinker K, Helbich TH, Baltzer PAT (2019) Quantitative multiparametric breast ultrasound: application of contrast-enhanced ultrasound and elastography leads to an improved differentiation of benign and malignant lesions. Investig Radiol 54(5):257–264. https://doi.org/10.1097/RLI.0000000000000543
Cantisani V, David E, Barr RG, Radzina M, de Soccio V, Elia D, De Felice C, Pediconi F, Gigli S, Occhiato R, Messineo D, Fresilli D, Ballesio L, D’Ambrosio F (2021) US-elastography for breast lesion characterization: prospective comparison of US BIRADS, strain elastography and shear wave elastography. Ultraschall Med 42(5):533–540. https://doi.org/10.1055/a-1134-4937. (Epub 2020 Apr 24; English)
Barr RG, Nakashima K, Amy D et al (2015) WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 2: breast. Ultrasound Med Biol 41:1148–1160
Bartolotta TV, Orlando AAM, Dimarco M, Zarcaro C, Ferraro F, Cirino A, Matranga D, Vieni S, Cabibi D (2022) Diagnostic performance of 2D-shear wave elastography in the diagnosis of breast cancer: a clinical appraisal of cutoff values. Radiol Med 127(11):1209–1220. https://doi.org/10.1007/s11547-022-01546-w. (Epub 2022 Sep 17)
Mori M, Tsunoda H, Kawauchi N, Kikuchi M, Honda S, Suzuki K, Yamauchi H (2012) Elastographic evaluation of mucinous carcinoma of the breast. Breast Cancer 19(1):60–63. https://doi.org/10.1007/s12282-011-0268-3. (Epub 2011 Jun 4)
Dogan BE, Turnbull LW (2012) Imaging of triple-negative breast cancer. Ann Oncol 23(Suppl 6):vi63–vi69. https://doi.org/10.1093/annonc/mds191
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194
Watanabe T, Kaoku S, Yamaguchi T et al (2019) Multicenter prospective study of color Doppler ultrasound for breast masses: utility of our color Doppler method. Ultrasound Med Biol 45(6):1367–1379. https://doi.org/10.1016/j.ultrasmedbio.2019.01.021
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Mendelson EB, Böhm-Vélez M, Berg WA et al (2013) ACR BI-RADS® ultrasound. In: ACR BI-RADS® Atlas, breast imaging reporting and data system. American College of Radiology, Reston
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Săftoiu A, Gilja OH, Sidhu PS et al (2019) The EFSUMB guidelines and recommendations for the clinical practice of elastography in non-hepatic applications: update 2018. Ultraschall Med 40(4):425–445. https://doi.org/10.1055/a-0838-9937
Tang L, Wang Y, Chen P, Chen M, Jiang L (2022) Clinical use and adjustment of ultrasound elastography for breast lesions followed WFUMB guidelines and recommendations in the real world. Front Oncol 24(12):1022917. https://doi.org/10.3389/fonc.2022.1022917
Berg WA, Cosgrove DO, Doré CJ, Schäfer FK, Svensson WE, Hooley RJ, Ohlinger R, Mendelson EB, Balu-Maestro C, Locatelli M, Tourasse C, Cavanaugh BC, Juhan V, Stavros AT, Tardivon A, Gay J, Henry JP, Cohen-Bacrie C, BE1 Investigators (2012) Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 262(2):435–449. https://doi.org/10.1148/radiol.11110640
Pesce K, Binder F, Chico MJ, Swiecicki MP, Galindo DH, Terrasa S (2020) Diagnostic performance of shear wave elastography in discriminating malignant and benign breast lesions: our experience with QelaXtoTM software. J Ultrasound 23(4):575–583. https://doi.org/10.1007/s40477-020-00481-8. (Epub 2020 Jun 11)
Duma MM, Chiorean AR, Chiorean M, Bolboaca SD, Florea M, Feier DS, Rusu GM, Sfrangeu SA (2014) Breast diagnosis: concordance analysis between the BI-RADS classification and tsukuba sonoelastography score. Clujul Med. 87(4):250–257. https://doi.org/10.15386/cjmed-362. (Epub 2014 Nov 12)
Elia D, Fresilli D, Pacini P, Cardaccio S, Polti G, Guiban O, Celletti I, Kutrolli E, De Felice C, Occhiato R, De Vito C, Amabile MI, De Luca A, D’Andrea V, Vergine M, Pediconi F, D’Ambrosio F, Cantisani V (2021) Can strain US-elastography with strain ratio (SRE) improve the diagnostic accuracy in the assessment of breast lesions? Preliminary results. J Ultrasound 24(2):157–163. https://doi.org/10.1007/s40477-020-00505-3. (Epub 2020 Jul 10)
Cannella R, Pilato G, Mazzola M et al (2023) New microvascular ultrasound techniques: abdominal applications. Radiol med 128:1023–1034. https://doi.org/10.1007/s11547-023-01679-6
Diao X, Zhan J, Chen L, Chen Y, Cao H (2020) Role of superb microvascular imaging in differentiating between malignant and benign solid breast masses. Clin Breast Cancer 20(6):e786–e793. https://doi.org/10.1016/j.clbc.2020.06.009. (Epub 2020 Jul 3)
Ganau S, Andreu FJ, Escribano F et al (2015) Shear-wave elastography and immunohistochemical profiles in invasive breast cancer: evaluation of maximum and mean elasticity values. Eur J Radiol 84(4):617–622. https://doi.org/10.1016/j.ejrad.2014.12.020
Elseedawy M, Whelehan P, Vinnicombe S et al (2016) Factors influencing the stiffness of fibroadenomas at shearwave elastography. ClinRadiol 71(1):92–95
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Trial registration
Trial registration number and date of registration for prospectively registered trials: N°022019.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zarcaro, C., Orlando, A.A.M., Ferraro, F. et al. Breast multiparametric ultrasound: a single-center experience. J Ultrasound (2024). https://doi.org/10.1007/s40477-024-00944-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40477-024-00944-2