Abstract
Objective
To evaluate the diagnostic value of shear-wave elastography (SWE) and colour Doppler ultrasound (US) for evaluation of breast non-mass lesions (NMLs) detected by B-mode US.
Methods
This retrospective study enrolled 116 NMLs (42 benign, 74 malignant). For each lesion, B-mode US, SWE and colour Doppler US were performed. Mean elasticity (E mean), maximum elasticity (E max) and vascularity were assessed by SWE and Doppler US. Diagnostic performances of B-mode US, SWE and Doppler US were calculated to differentiate benign and malignant NMLs.
Results
In benign NMLs, average E mean and E max were lower, and low vascularity (no flow or only one vessel flow) was more frequent (P < 0.001). When BI-RADS category 4a NMLs were downgraded to category 3 with ‘E mean of 85.1 kPa or less’ and/or ‘low vascularity’, specificities increased (69.0–90.5 %; P < 0.001), without significant loss in sensitivities (97.3–100 %). When these 4a NMLs were downgraded by the combination of SWE and Doppler US, all downgraded NMLs (59.3 %, 19/32) were confirmed as benign.
Conclusions
Addition of SWE and colour Doppler US to B-mode US improved diagnostic performances in differentiating benign and malignant NMLs. This study suggests that the combination of SWE and colour Doppler may help patients with BI-RADS category 4a NMLs avoid unnecessary biopsies.
Key Points
• B-mode US features of malignant and benign NMLs may overlap.
• SWE and colour Doppler provides useful information about breast NMLs.
• SWE and colour Doppler may decrease unnecessary biopsies of breast NMLs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast ultrasound (US) is widely used as a diagnostic tool for both detection and characterization of breast lesions [1–3]. The Breast Imaging Reporting and Data System (BI-RADS) for breast US provides standardized terminology to describe features of, assessments of and recommendations for the breast lesions detected on US [4], thus clarifying the indications for biopsy of particular lesions [5].
According to the BI-RADS lexicon for breast US, a “mass” is defined as a space-occupying lesion seen in two different planes and can be distinguished from normal anatomic structures [4]. However, we often encounter non-mass lesions (NMLs) that do not meet the strict criteria of a “mass” described by BI-RADS (e.g. ill-defined geographic hypoechoic lesions, tubular hypoechoic duct-like structures or architectural distortion), as the use of high-resolution US has increased [6, 7]. For example, ductal carcinoma in situ (DCIS) and invasive lobular carcinoma are known to manifest as NMLs on US [6–8]. However, there is considerable overlap between conventional B-mode US features of malignant NMLs and benign NMLs such as fibrocystic change, sclerosing adenosis, atypical ductal hyperplasia and intraductal papilloma [7–11]. These observations have raised the necessity for correct identification and accurate diagnosis of breast NMLs detected by US.
Utrasonographic elastography (USE) and colour Doppler US have been investigated as ancillary imaging tools to further characterize breast lesions. Among the variable USE techniques, shear-wave elastography (SWE) quantifies the tissue’s elasticity in kilopascals (kPa) or meters per second and also displays colour-coded images in real time by the local estimation of shear-wave propagation speed [12, 13]. Recent studies have shown that SWE improved the diagnostic performance of B-mode US in differentiating benign from malignant breast masses [13–16]. Colour or power Doppler US shows the vascularity of the mass by visualizing the blood vessels. Previous researchers have reported that combined use of colour Doppler US with B-mode US can improve diagnostic performances for the diagnosis of breast lesions [17, 18]. However, these studies about ancillary US have focused on breast masses, and therefore their results may not be directly applicable to the diagnosis of breast NMLs.
To our knowledge, there is one recent study that assessed the ancillary role of SWE for only 34 cases of breast NMLs [9]. Therefore, the purpose of this study was to evaluate SWE and colour Doppler US features of breast NMLs and to investigate the clinical benefit of adding SWE and colour Doppler US to B-mode US to distinguish benign and malignant NMLs and to decide the need for biopsy.
Materials and methods
Patients and breast non-mass lesions
The institutional review board approved this retrospective study, and informed consent was waived.
From October 2013 to December 2014, 1306 consecutive women with breast lesions were examined with B-mode US, SWE and colour Doppler US before undergoing US-guided core needle biopsy (US-CNB) or US-guided vacuum-assisted biopsy (US-VAB) at our institution. The B-mode US findings of these lesions were retrospectively reviewed and classified into “mass” or “NML” by two radiologists (J.S.C. and E.S.K.) in consensus. Among the 124 breast lesions classified as NML, eight NMLs were excluded (five malignant lesions for which neoadjuvant chemotherapy was performed before surgery; three with severe artefacts seen on SWE images [e.g. superficial lesion located less than 5 mm in depth or close to the nipple–areolar complex]). Finally, a total of 116 NMLs in 113 patients were included in this study. Three patients had two lesions: one patient had two malignant lesions, one patient had one benign and one malignant lesion, and one patient had two benign lesions.
Of 116 NMLs, 81 NMLs (74 malignant and seven benign) were surgically confirmed after US-guided biopsy. Two benign NMLs underwent both US-CNB and US-VAB. The remaining 33 benign NMLs diagnosed by US-CNB were followed up with US (mean duration 13.6 months, range 10–20 months), and all these showed no interval change in size on follow-up US. Final diagnosis was based on histopathological results of biopsy or surgical excision specimens. There were 74 malignant and 42 benign NMLs. Malignant NMLs included invasive ductal carcinoma (n = 36), microinvasive ductal carcinoma (n = 5), DCIS (n = 19), invasive lobular carcinoma (n = 10), invasive micropapillary carcinoma (n = 2), metaplastic carcinoma (n = 1) and breast metastasis from ovarian cancer (n = 1). Benign NMLs included stromal fibrosis (n = 10), fibrocystic change (n = 8), fibroadenoma (n = 5), fibroadenomatoid mastopathy (n = 4), granulomatous lobular mastitis (n = 4), intraductal papilloma (n = 2), sclerosing adenosis (n = 2), atypical ductal hyperplasia (n = 1), flat epithelial atypia (n = 1), apocrine metaplasia (n = 1), adenosis (n = 1), columnar cell change (n = 1), duct ectasia (n = 1) and fat necrosis (n = 1).
US examinations
All B-mode US, SWE and colour Doppler US images were obtained with one US system (Aixplorer; SuperSonic Imagine, Aix en Provence, France) equipped with a 15–4-MHz linear array transducer. US examinations were performed by one of three board-certified radiologists with more than 9 years of experience in breast imaging. After bilateral whole breast conventional B-mode US examinations, the radiologists obtained at least two orthogonal (either transverse and longitudinal or radial and antiradial planes) B-mode US images for each breast lesion. Subsequently, they measured lesion size (maximum diameter of the lesion) and noted in radiological reports the BI-RADS final assessment category (3, probably benign; 4a, low suspicion for malignancy; 4b, moderate suspicion for malignancy; 4c, high suspicion for malignancy; 5, highly suggestive of malignancy). They determined the BI-RADS final category with B-mode US findings alone [4], and the findings of other modalities such as mammography were not considered. Targeted SWE and colour Doppler US were performed for the lesion scheduled for US-guided biopsy by the same radiologists. Two-orthogonal view SWE and colour Doppler US images showing most suspicious features were obtained for the lesion scheduled for US-guided biopsy by the same radiologists. They used customized settings of the SWE parameters, with the preference for the penetration mode in cases of poor penetration. Tissue elasticity was displayed with a colour-coded map in kilopascals (kPa) at each pixel and a colour scale ranging from 0 (dark blue, soft) to 180 kPa (red, hard). Quantitative elasticity values were measured by applying a 2-mm circular quantification region of interest (Q box), which was carefully drawn over the stiffest portion of the lesion including the immediately adjacent stiffest tissue. Quantitative elasticity values including the maximum elasticity (E max) and mean elasticity (E mean) were automatically calculated. For colour Doppler US, a standard equipment setting was used. Doppler amplification was set to a level in which normal breast tissue did not display any noise and was just under the level in which random noise appeared. The following settings were used: medium wall filter, pulse repetition frequency 700 Hz, intermediate persistence, frame rate of 16 frames per second and a dynamic range of 50 dB.
US-CNB was performed with at least four passes using a 14-gauge dual-action semiautomatic core biopsy needle with a 22-mm throw (Stericut with coaxial; TSK Laboratory, Tochigi, Japan) or a 14-gauge automated biopsy gun (Acecut, TSK Laboratory, Soja, Japan). US-VAB was performed with an 8-gauge or 11-gauge needle (Mammotome; Devicor Medical, Cincinnati, OH, USA).
Image analysis
Two radiologists (J.S.C. and E.S.K.), with 6 and 9 years of experience in breast imaging, retrospectively reviewed the B-mode US, SWE and colour Doppler US images in consensus. They were blinded to any information regarding patient history, findings with other imaging modalities or histopathologic diagnosis.
On the basis of B-mode US findings, the reviewers classified lesions that did not meet the strict criteria of a “mass” according to the BI-RADS lexicon for US as NMLs in consensus. If there was any discrepancy between the readers’ reviews, the lesion was not classified as NML. For the lesions that were finally classified as NMLs, the reviewers evaluated the presence or absence of associated calcifications for each lesion. NMLs were considered to have associated calcifications only when all the reviewers recognized the existence of echogenic foci suggestive of calcifications based on B-mode US. The maximum stiffness colour scale on the SWE colour code map was classified into two categories: soft colour (from dark blue to light blue) and stiff colour (from green to red). Vascularity on colour Doppler US was determined according to the number of the vessels (circumferential, central or penetrating) of the lesion [19] and divided into two categories: low (no flow or only one vessel flow signal observed) and high (more than two vessel flow signals are observed).
Statistical analysis
Age, lesion size on B-mode US, SWE parameters (E max, E mean and maximum stiffness colour) and vascularity on colour Doppler US of malignant and benign NMLs were compared by using the independent two-sample t test, chi-square test or Fisher’s exact test.
The malignancy rate of each BI-RADS category based on B-mode US was calculated in terms of percentage of malignancy. In the analysis of diagnostic performance, positive test results for malignancy were defined as BI-RADS category 4a or higher for B-mode US and high vascularity (more than two vessel flow signals) for colour Doppler US, respectively. In terms of SWE parameters, stiff colour on the maximum stiffness colour map was considered positive for malignancy, and positive results of quantitative elasticity values (E max and E mean) were determined using the cut-off values obtained with the Youden index [20]. The sensitivities, specificities, accuracies, positive predictive values (PPV) and negative predictive values (NPV) of B-mode US, SWE parameters and colour Doppler US were calculated for differentiating malignant NMLs from benign NMLs. The areas under the curves (AUCs) were also calculated from construction of the receiver operating characteristic curve.
In addition, we evaluated the hypothetical effect of SWE and colour Doppler US when BI-RADS category 4a NMLs were downgraded to category 3 on the basis of additional information from SWE and colour Doppler US. The sensitivity and specificity of B-mode US alone were compared with those of the combination of B-mode and SWE and/or colour Doppler US, with the criteria hypothetically applied, by using the McNemar test.
Analysis was performed using SPSS® statistical software (SPSS Inc., Chicago, IL, ver. 22.0), and statistical significance was accepted as P values less than 0.05.
Results
The mean age of all 113 patients was 48.4 years (standard deviation [SD] 10.0, range 29–85 years). The mean US size of the 116 NMLs was 27.5 mm (SD 15.7, range 6–60 mm). The clinical and imaging characteristics of the benign and malignant groups are shown in Table 1. The mean lesion size of malignant NMLs was significantly larger than benign NMLs (P < 0.001). BI-RADS categories based on B-mode US were higher in the malignant group than in the benign group (P < 0.001). Calcifications were more frequently seen in malignant NMLs than in benign NMLs. Both E mean and E max were significantly higher in malignant NMLs (E mean, 134.6 vs. 38.7 kPa; E max, 152.0 vs. 44.0 kPa; P < 0.001) (Fig. 1). The proportions of maximum stiffness colour on SWE and vascularity on colour Doppler US were also significantly different between the two groups. Soft colour (from dark blue to light blue) on SWE and low vascularity (no vascularity or one vessel) on colour Doppler US were more frequent in benign NMLs (P < 0.001).
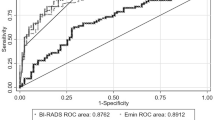
The malignancy rate of each BI-RADS category was 0 % (0/10) for category 3, 9.4 % (3/32) for category 4a, 78.6 % (11/14) for category 4b, 100 % (23/23) for category 4c and 100 % (37/37) for category 5. To differentiate malignant NMLs from benign NMLs, the diagnostic performances of B-mode US, the SWE parameters and colour Doppler US are summarized in Table 2. E mean showed the highest AUC (0.924) among the SWE parameters and colour Doppler US. AUCs of the combined use of SWE and colour Doppler US were 0.801 for the criterion ‘E mean of higher than 85.1 kPa or high vascularity’ (Fig. 2). SWE, colour Doppler US and the combined use of these two modes showed lower sensitivities (70.3–95.9 %) and higher specificities (64.3–95.2 %) compared to B-mode US (100 % sensitivity, 23.8 % specificity).
A 47-year-old woman diagnosed with invasive ductal carcinoma by US-guided biopsy and surgical excision. a The B-mode US image shows a 4.5-cm non-mass lesion assessed as BI-RADS category 4b (arrows) in the left upper inner breast, b the colour Doppler US image shows high vascularity, c the SWE image shows an E mean of 85.2 kPa, an E max of 97.5 kPa and yellow colour on the maximum stiffness colour map and d this lesion was not detected on the mammogram at the time of diagnosis
We evaluated the theoretical performance of adding SWE and Doppler US to B-mode US when downgrading BI-RADS category 4a NMLs because there were no malignancies among the NMLs with BI-RADS category 3. When BI-RADS category 4a NMLs were downgraded to category 3 with the separate criterion of either ‘E mean of 85.1 kPa or less’ or ‘low vascularity’, the specificity increased to 90.5 % or 71.4 %, respectively, without significant loss in sensitivity (Table 3). Application of ‘E mean of 85.1 kPa or less’ or ‘low vascularity’ led to two DCIS (6.3 %, 2/32) or one DCIS (3.1 %, 1/32), respectively, being incorrectly downgraded from category 4a to 3. When BI-RADS category 4a NMLs were strictly downgraded by the combination of SWE and colour Doppler US using a combined criterion of ‘E mean of 85.1 kPa or less, and low vascularity’, the specificity increased to 69.0 % without change in sensitivity of 100 %. All 19 NMLs (59.4 %, 19/32) downgraded by this criterion were confirmed as benign (Fig. 3).
A 57-year-old woman diagnosed with fibroadenomatoid mastopathy by US-guided biopsy. a The B-mode US image shows a 2.8-cm non-mass lesion assessed as BI-RADS category 4a (arrows), b the colour Doppler US image shows low vascularity and c the SWE image shows E mean of 23.6 kPa, E max of 25.3 kPa and dark blue colour on the maximum stiffness colour map
Discussion
Each US operator might recognize and describe breast NMLs on US with variations, because there is currently no standardized guideline for the interpretation of these lesions. Also, US-guided biopsies are frequently recommended to manage NMLs, because B-mode US findings of these lesions are often too subtle to differentiate between malignant and benign lesions. Therefore, we hypothesized that functional information about elasticity and vascularity may help characterize breast NMLs better than using morphological features. In this study, benign NMLs showed significantly lower values of SWE quantitative parameters (E mean and E max) and soft colour on the maximum stiffness colour map, which is in accordance with the preliminary results of a recent study that assessed the utility of SWE for the diagnosis of breast NMLs [9]. We also assessed the colour Doppler US features of breast NMLs by using a simple scoring system based on the number of vessels [17, 19], which can be easily applicable to clinical practice. Our results showed that malignant NMLs showed significantly higher vascularity (more than two vessels) than benign NMLs. However, a substantial portion (35.7 %, 15/42) of our benign NMLs also showed high vascularity.
In terms of diagnostic performance for differentiating between malignant and benign NMLs, SWE, colour Doppler US and the combined use of the two modes showed higher specificities (64.3–95.2 %) and lower sensitivities (70.3–95.9 %) compared to B-mode US (23.8 % specificity, 100 % sensitivity). These findings are consistent with those of previous studies that applied SWE or Doppler US to diagnose breast masses [15–17]. Considering the high specificities of SWE and colour Doppler US, we speculated that addition of these ancillary techniques to B-mode US may reduce unnecessary biopsies with benign results for BI-RADS category 4a NMLs showing a low malignancy rate (9.4 %). When BI-RADS category 4a NMLs were hypothetically downgraded to category 3 if they showed ‘E mean of 85.1 kPa or less’ and/or ‘low vascularity’, the specificity (69.0–90.5 %) of B-mode US was significantly improved without loss in sensitivity (97.3–100 %). In particular, when BI-RADS category 4a NMLs were strictly downgraded by the combination of SWE and colour Doppler US using the criterion of ‘E mean of 85.1 kPa or less, and low vascularity’, 59.4 % (19/32) of category 4a NMLs could have avoided unnecessary biopsies and there were no downgraded malignancies. Our results indicate that additional information about elasticity and vascularity of breast NMLs can improve the low specificity of B-mode US for distinguishing benign lesions from malignant lesions and thus may reduce unnecessary biopsies of benign NMLs. In addition, we believe that diagnostic value of SWE and colour Doppler US may be higher when evaluating breast NMLs than breast masses, because B-mode US features associated with malignancy have not yet been established for breast NMLs while they have been for breast masses [4].
To assess the diagnostic performance of quantitative SWE parameters, we derived cut-off values of 85.1 kPa and 92.5 kPa for E mean and E max, respectively. For the combination of SWE and colour Doppler US, E mean with this cut-off value was used as it resulted in the highest AUC value. Our cut-off value of E mean was higher than that of a recent study for breast NMLs [9]. The relatively low cut-off value (41.6 kPa) of that study may be a consequence of the small number of samples (n = 34). When applying their cut-off value of 41.6 kPa, 40.5 % (17/42) of our benign group were incorrectly classified as positive for malignancy. Our study included a larger number of breast NMLs (n = 116) compared to this previous study, and SWE is thought to improve the diagnostic performance of B-mode US if the cut-off values derived from our study samples are applied. However, further study with a larger number of patients is needed to determine the best cut-off value of quantitative SWE parameters to distinguish benign NMLs from malignant NMLs.
Recently, some studies analysed B-mode US features of benign and malignant breast NMLs [7, 8]. These studies reported that NMLs with associated calcifications detected by B-mode US had higher malignancy rates (62.8–81.8 %). In this study, calcified NMLs detected by B-mode US also had a high malignancy rate (97.4 %, 38/39), and all of these NMLs showed suspicious calcifications on mammography at the time of diagnosis. Considering the results of previous studies along with our own results, the presence of calcifications may be considered a B-mode US feature suggestive of malignancy in breast NMLs. However, the morphology of calcifications cannot be fully interpreted with US compared with mammography, despite the development of high-resolution US which depicts calcifications as echogenic foci better than past US techniques [4]. Therefore, our opinion is that patients with calcified NMLs detected by B-mode US have to undergo mammography for further evaluation and then the higher BI-RADS category found of the two imaging modalities can be used to decide how to manage these lesions. Meanwhile, 66.4 % (77/116) of our study sample was non-calcified NMLs, and 46.8 % of non-calcified NMLs were confirmed as malignant. To diagnose non-calcified NMLs, associated architectural distortion and orientation of duct-like structures were suggested as features that could help distinguish benign NMLS from malignant NMLs in previous studies [7, 8]. However, these B-mode US findings may have limited value in clinical practice, because they can be difficult to assess as a result of the ill-defined margin of breast NMLs [6]. On the other hand, all BI-RADS category 4a NMLs that could be hypothetically downgraded to category 3 by the combination of SWE and colour Doppler US in this study were found to be non-calcified NMLs. Also, when we applied the SWE criterion ‘E mean of higher than 85.1 kPa’, 88.9 % (32/36) of the non-calcified malignant NMLs were correctly diagnosed, whereas 68.4 % (26/38) of the calcified malignant NMLs were correctly diagnosed. On the basis of these results, we think that SWE and colour Doppler US may have greater value in the evaluation of non-calcified NMLs.
This study had several limitations. First, this study was a retrospective, single-institution study. A future prospective study will be needed to verify the clinical benefit of SWE and colour Doppler US for differentiating benign and malignant NMLs. Second, B-mode US, SWE and colour Doppler US were performed by three radiologists in this study. Interobserver variability may have existed among the radiologists. However, all radiologists who participated in the SWE data acquisition were instructed on the level of compression and probe positioning needed to reduce technical errors. Consequently, there were few cases that were excluded because of artefacts (n = 3) in our case selection. Third, we did not assess the mammographic findings of the NMLs in this study, because we were focused on evaluating the features of SWE and colour Doppler US of breast NMLs detected by B-mode US. However, for patients who underwent both US and mammography during the study period, the higher BI-RADS category of the BI-RADS categories found with these two imaging modalities was used to decide whether or not to perform a biopsy. Fourth, our institution is a referral centre which in itself might affect the study population as we have a higher concentration of patients with malignancy. Therefore, the proportion of malignant NMLs in this cohort might be higher than that of the general population. Finally, the period of imaging follow-up after biopsies with benign results was relatively short (range 10–20 months), whereas 2 years of follow-up is generally recommended.
In conclusion, the addition of SWE and colour Doppler US to B-mode US improved diagnostic performance with increased specificity in distinguishing benign NMLs from malignant breast NMLs. This study suggests that the combined use of SWE and colour Doppler may help patients with BI-RADS category 4a NMLs avoid unnecessary biopsies.
Abbreviations
- CNB:
-
Core needle biopsy
- E mean :
-
Mean elasticity
- E max :
-
Maximum elasticity
- NML:
-
Non-mass lesion
- SWE:
-
Shear-wave elastography
- US:
-
Ultrasound
- USE:
-
Ultrasonographic elastography
- VAB:
-
Vacuum-assisted biopsy
References
Abdullah N, Mesurolle B, El-Khoury M, Kao E (2009) Breast imaging reporting and data system lexicon for US: interobserver agreement for assessment of breast masses. Radiology 252:665–672
Bassett LW (2000) Imaging of breast masses. Radiol Clin North Am 38:669–691, vii–viii
Moy L, Slanetz PJ, Moore R et al (2002) Specificity of mammography and US in the evaluation of a palpable abnormality: retrospective review. Radiology 225:176–181
D’Orsi C, Sickles E, Mendelson E, Morris E (2013) ACR BI-RADS® Atlas, breast imaging reporting and data system. American College of Radiology, Reston
Hong AS, Rosen EL, Soo MS, Baker JA (2005) BI-RADS for sonography: positive and negative predictive values of sonographic features. AJR Am J Roentgenol 184:1260–1265
Uematsu T (2012) Non-mass-like lesions on breast ultrasonography: a systematic review. Breast Cancer 19:295–301
Wang ZL, Li N, Li M, Wan WB (2015) Non-mass-like lesions on breast ultrasound: classification and correlation with histology. Radiol Med 120:905–910
Ko KH, Hsu HH, Yu JC et al (2015) Non-mass-like breast lesions at ultrasonography: feature analysis and BI-RADS assessment. Eur J Radiol 84:77–85
Ko KH, Jung HK, Kim SJ, Kim H, Yoon JH (2014) Potential role of shear-wave ultrasound elastography for the differential diagnosis of breast non-mass lesions: preliminary report. Eur Radiol 24:305–311
Shin HJ, Kim HH, Kim SM, Kwon GY, Gong G, Cho OK (2008) Screening-detected and symptomatic ductal carcinoma in situ: differences in the sonographic and pathologic features. AJR Am J Roentgenol 190:516–525
Izumori A, Takebe K, Sato A (2010) Ultrasound findings and histological features of ductal carcinoma in situ detected by ultrasound examination alone. Breast Cancer 17:136–141
Bercoff J, Tanter M, Fink M (2004) Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control 51:396–409
Youk JH, Son EJ, Park AY, Kim JA (2014) Shear-wave elastography for breast masses: local shear wave speed (m/sec) versus Young modulus (kPa). Ultrasonography 33:34–39
Chang JM, Moon WK, Cho N et al (2011) Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res Treat 129:89–97
Berg WA, Cosgrove DO, Dore CJ et al (2012) Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 262:435–449
Lee SH, Chang JM, Kim WH et al (2014) Added value of shear-wave elastography for evaluation of breast masses detected with screening US imaging. Radiology 273:61–69
Cho N, Jang M, Lyou CY, Park JS, Choi HY, Moon WK (2012) Distinguishing benign from malignant masses at breast US: combined US elastography and color Doppler US–influence on radiologist accuracy. Radiology 262:80–90
Ozdemir A, Ozdemir H, Maral I, Konus O, Yucel S, Isik S (2001) Differential diagnosis of solid breast lesions: contribution of Doppler studies to mammography and gray scale imaging. J Ultrasound Med 20:1091–1101, quiz 1102
Raza S, Baum JK (1997) Solid breast lesions: evaluation with power Doppler US. Radiology 203:164–168
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Acknowledgments
The scientific guarantor of this publication is Boo-Kyung Han. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was waived by the institutional review board. No study subjects or cohorts have been previously reported. Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, J.S., Han, BK., Ko, E.Y. et al. Additional diagnostic value of shear-wave elastography and color Doppler US for evaluation of breast non-mass lesions detected at B-mode US. Eur Radiol 26, 3542–3549 (2016). https://doi.org/10.1007/s00330-015-4201-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4201-6