Abstract
Purpose
This study aimed to assess the diagnostic accuracy of duplex sonography (DUS) compared with that of computed tomography angiography (CTA) in detecting occlusion and stenosis in peripheral arterial disease (PAD) in candidate patients for endovascular revascularization with intraprocedural digital subtraction angiography (DSA).
Methods
The study involved 94 patients suffering from PAD who were candidates for endovascular procedures requiring DSA. They were all submitted preoperatively to DUS and CTA. Based on image analysis, five segments of the arterial tree were assessed: iliac, common femoral, superficial femoral, popliteal, and infrageniculate.
According to the stenosis or occlusion degree, the arteries were rated as nonstenotic, stenotic, and occluded.
Results
The agreement between DUS and CTA findings using DSA as a reference modality was expressed as a Cohen’s kappa (κ) statistic agreement.
Our results show that DUS has been less accurate than CTA in evaluating iliac arterial diseases (Cohen’s κ agreement of 0.91 and 1.0, respectively) when measured against DSA.
We found good diagnostic concordance between DUS and DSA in detecting hemodynamic stenosis and occlusion of the femoro-popliteal axis (Cohen’s κ agreement between 0.96 and 0.93).
Below the knee, CTA showed even less concordance with DSA (Cohen’s κ 0.75).
Conclusions
Because of its accuracy, high-quality DUS performed by well-trained operators may therefore represent a good alternative to CTA in patients undergoing endovascular revascularization to minimize the use of contrast-enhanced radiological imaging.
Nevertheless, preoperative CTA imaging is required in cases of nondiagnostic DUS or when a more complete overview of the vascular tree is needed for complex invasive interventions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peripheral arterial disease (PAD) is a chronic vascular disease characterized by an impaired circulation in the lower extremities with a broad spectrum of symptoms.
PAD is diagnosed based on the patient’s history and physical examination. Although the selection of treatment strategies is based on severity of the disease, imaging plays a crucial role in planning interventions [1].
In clinical practice, hemodynamic stenosis and occlusion of the arterial tree can be detected and quantified using different imaging modalities, including digital subtraction angiography (DSA) contrast-enhanced CT angiography (CTA), contrast-enhanced magnetic resonance angiography (MRA), and duplex sonography (DUS).
DSA is still considered the gold standard for PAD diagnosis and grading as it displays the highest sensitivity and specificity. However, DSA is invasive and may be associated with well-documented limitations that make it unsuitable as a routine diagnostic technique. The main limitations of DSA include the need for catheterization, contrast allergic reactions, arterial injury, hemorrhage, atheroembolism, potential pseudoaneurysm formation at the puncture site and, most importantly, contrast-induced nephropathy despite the use of nonionic contrast media. Consequently, this investigation is currently conducted in the context of endovascular revascularization procedures [2].
Reduced equipment availability, contraindications as metal implants, claustrophobia, pacemakers, and overestimation of vascular narrowing accounts for the less frequent use of MRA in PAD diagnosis. Thus, CTA is currently used in evaluating atherosclerotic peripheral arterial occlusive disease and/or intervention planning. However, this imaging modality shares some important limitations with DSA since it always requires radiation and the use of intravenous iodine contrast to adequately assess the arterial lumen [3].
DUS is an extremely reliable noninvasive imaging modality for evaluating the lower-extremity arterial system [4]. It might be considered as a preinterventional diagnostic modality of PAD without resorting to more invasive and expensive methods such as CTA [5].
This study aimed to assess the diagnostic accuracy of DUS compared with CTA in detecting occlusion and quantification of flow patterns in stenotic areas of the arterial tree in PAD in candidate patients for endovascular revascularization with intraprocedural DSA serving as the gold standard.
Materials and methods
The study group included patients with chronic lower-limb ischemic disease who have come to our department.
The inclusion criteria were as follows:
-
Any age and both genders
-
Patients with intermittent claudication after less than 200 m or critical peripheral ischemia who were candidates for endovascular procedures requiring DSA despite risk factors including body habitus, IDM, comorbidities, dyslipidemia, diabetes, and smoking.
The exclusion criteria were contraindication for nonionic contrast media, asymptomatic patients, patients with intermittent claudication after more than 200 m of walking that does not interfere with lifestyle, previous vascular interventions, vascular malformations, or anatomical anomalies.
Based on image analysis, five segments of the lower-limb arterial tree were assessed: iliac, common femoral, superficial femoral, popliteal, and infrageniculate.
According to the degree of stenosis or occlusion, the arteries were classified as nonstenotic, stenotic, and occluded.
The arterial diseases were also classified as isolated and multisegmental.
DUS was performed using a Toshiba Aplio duplex ultrasound machine, frequency transducers with a range of 5–13 MHz for the lower-limb artery, and a 3.5 MHz probe for the infrarenal aorta and iliac vessels. The lesions were located using two-dimensional (2-D) ultrasonography and color and power Doppler mapping. Doppler US was used to measure blood flow velocity.
Duplex ultrasound criteria for evaluating vessel patency were based on normal triphasic waveform pattern and color saturation, recorded throughout the lumen of the artery. A small incident angle (θ) of 60° or less (i.e., cos θ ≥ 0.5) is essential to minimize measurement error.
Degree of stenosis was evaluated by Doppler waveform, peak systolic velocity (PSV), and velocity ratio analysis. The criteria for identifying arterial hemodynamically significant stenosis > 70% were PSV > 200 cm/s, PSV ratio > 2.0, and aliasing and spectral broadening seen with color Doppler and/or monophasic poststenosis flow. PSV ratio was calculated by dividing the PSV at or immediately downstream from the stenosis by the PSV upstream from the stenosis. An increase in either PSV or PSV ratio indicates stenosis. Cutoff thresholds were nonhemodynamic stenosis for < 70% stenosis; PSV < 200 cm/s; PSV ratio < 2.0, with PSV the better parameter; hemodynamic stenosis for 70–99% stenosis; PSV ≥ 205 cm/s; PSV ratio ≥ 2.0, with ratio superior, and occlusion when no flow is detected in the vessel.
To minimize interobserver variation, all Doppler studies were done by the same operator; three measurements for each segment were made to improve diagnostic accuracy.
Computed tomography angiography (CTA) was performed with a multidetector row. The interpretation of CTA was based on the axial images and processed images used including multiplanar reformation (MPR) images, volume rendering (VR) images, maximum intensity projection (MIP) images, curved planar reformation (CPR) images, and volume-rendered 3-D reconstruction. CTA imaging included the entire arterial tree from the abdominal aorta to the foot. The image acquisition was triggered with a delay between 6 and 10 s to ensure that the distal arteries will be properly opacified. Centerlines in the vessel of interest were used to obtain both longitudinal and cross-sectional views of the vessel for quantitative measurements.
The images were analyzed based on transverse, MIP, and VR images.
All intraprocedural angiographic examinations (DSA) were performed using digital subtraction technique and an apparatus with digital imaging facilities. Water-soluble iodinated ionic or nonionic contrast media (Omnipaque) was used, and its doses and rate of injection varied according to the situation results. Multiple views were obtained at different angles for the arterial stenosis evaluation.
The Bollinger scoring system was used to assess both CTA imagens and the angiographies.
According to the severity of the disease, stenosis was graded according to the following criteria:
-
Nonhemodynamic stenosis: < 50% arterial narrowing
-
Hemodynamic stenosis: > 50% arterial narrowing
-
Occlusion: complete absence of flow in the vessel
Statistical analysis
The sample size consisted of several patients studied and treated in a defined time interval. Given our sample of fewer than 100 units, Cohen’s κ coefficient is generally thought to be a robust measure. The agreement between DUS and CTA findings using DSA as a reference modality was expressed as a Cohen’s κ statistic agreement using SAS Technical Support (MAGREE.SAS).
Results
The study involved 94 patients suffering from PAD, 54 men (64.2%) and 40 women (55.8%), with a mean age of 68.2 years and a standard deviation of ± 14.3. Eighteen patients complained of disabling intermittent claudication (stage 2B of Fontaine’s classification), 46 patients had rest pain (stage 3 of Fontaine’s classification), and 30 patients had tissue loss or gangrene (stage 4 of Fontaine’s classification).
The comorbidities and risk factors of the studied patients are reported in Table 1.
CTA imaging of the iliac axis showed total occlusion in 7 patients, stenosis in 35, and no stenosis in the remaining 52. In DUS, total occlusion was detected in 6 patients, stenosis in 36, and no stenosis in 52. DSA showed total occlusion in 7 patients, stenosis in 35, and no stenosis in 52. DUS misinterpreted one occlusion, which was a subocclusive stenosis on the DSA in an obese patient (Cohen’s κ agreement for CTA: 1.0—perfect agreement; Cohen’s κ agreement for DUS: 0.91—almost perfect agreement).
For the common femoral artery (CFA), CTA diagnosed occlusion in 4 patients, stenosis in 18, and no stenosis in 72 while DUS diagnosed total occlusion in 4 patients, stenosis in 20, and no stenosis in the remaining 70. DSA demonstrated total occlusion in 4 patients, stenosis in 21, and no stenosis in 69. An understaging of arterial stenosis occurred in 3 cases for CTA (3 false negatives) and 1 case for DUS (1 false negative) (Cohen’s κ agreement for CTA: 0.90—almost perfect agreement; Cohen’s κ agreement for DUS: 0.96—almost perfect agreement).
In CTA imaging, the superficial femoral artery (SFA) was occluded in 19 patients, hemodynamic stenosis was detected in 58, and no stenosis in 17; DUS assessment showed total occlusion in 19 patients, stenosis in 60, and no stenosis in 16. DSA imaging confirmed total occlusion in 19 patients, stenosis in 61 cases, and no stenosis in 14 patients. When compared with DSA, CTA improperly indicated 3 nonstenotic SFA lesions which were hemodynamic eccentric stenoses with surrounding mural calcification (3 false negatives). In one case, DUS underestimated a hemodynamic stenosis because of a calcified plaque that was in proximity to the Hunter channel and combined with a proximal occlusion of the ipsilateral external iliac artery (Cohen’s κ agreement for CTA: 0.87—almost perfect agreement; Cohen’s κ agreement for DUS: 0.95—almost perfect agreement).
At the popliteal level, CTA diagnosed occlusion in 11 patients, stenosis in 27, and no popliteal artery stenosis in 56 while DUS diagnosed total occlusion in 12 patients, stenosis in 29, and patent or nonstenotic popliteal artery in 53. DSA documented occlusion in 11 patients, stenosis in 28, and no stenosis in 55.
CTA underestimated the narrowing of the popliteal artery in one case while DUS misinterpreted an occlusion that was a subocclusive disease with surrounding mural calcification at the origin of this vessel (false positive) and understaged narrowing in one case (Cohen’s κ agreement for CTA 0.97—almost perfect agreement; Cohen’s κ agreement for DUS: 0.95—almost perfect agreement).
Regarding the infrageniculate arteries, CTA demonstrated occlusion in 29 patients, stenosis in 46, and no stenotic vessels in 19; DUS detected total occlusion in 24 patients, stenosis in 51, and no stenosis in 19; and DSA imaging showed occlusion in 24 patients, stenosis in 52, and no stenosis in the remaining 18.
CTA overstaging occurred in five cases of multisegmental disease, and understaging by DUS occurred in one case (Cohen’s κ agreement for CTA 0.75—substantial agreement; Cohen’s κ agreement for DUS: 0.96—almost perfect agreement).
The comparison of CTA and DUS results with intraprocedural DSA findings is reported in Tables 2 and 3. PAD involved one segment in 41% of the patients, two segments in 47%, and three or more segments in 35%.
Discussion
Management strategies for PAD depend on its severity. Any hemodynamic vascular testing should provide information about the anatomic distribution, degree and length of the lesion, and the configuration of the inflow and runoff vessels. In this context, DUS, CTA, or MRA imaging methods have good sensitivity and specificity compared with invasive angiography in delineating anatomy and planning revascularization [3, 6] including assistance in selecting vascular access sites, identification of significant lesions, and determination of the feasibility of and modality for invasive treatment. All contribute to minimize the risks and the discomfort for the patient. The diagnostic accuracy of DUS in several vascular pathologies has been widely recognized [7, 8].
According to the latest ESVS and ESC guidelines, all these diagnostic modalities are recommended to develop an individualized management of PAD patients (evidence class I, level A), without designating superiority to any of them [9].
Notwithstanding, DUS is usually considered for treatment planning solely in patients with noncritical ischemia of the lower limb; it is not yet accepted as the sole preinterventional imaging tool to plan the most appropriate therapeutic strategy in clinical practice. Thus, patients predicted by DUS to require peripheral arterial are still submitted to CTA or MRA to confirm ultrasound results [10].
In contrast to this trend, in the last decades, several published validation studies have raised the possibility of using DUS as the sole investigation method to assess the most suitable treatment plan for PAD [11,12,13,14].
In this comparison analysis between CTA and DUS, DSA has been considered the standard of reference for quantifying arterial stenosis to be treated endovascularly because it yields findings that are easy to interpret and can depict the whole target artery with high spatial resolution and accurate lumen evaluation in calcified vessels.
This study suggests that when used appropriately, DUS provides most of the essential anatomical information and hemodynamic data for extensive peripheral arterial mapping. Using DSA as a reference point, our data demonstrated the high diagnostic accuracy of DUS for both occlusions and stenoses at any level of the entire peripheral arterial tree. It has been proven to distinguish between tight stenosis and occlusion and determine the severity of arterial stenosis. This is consistent with the data of previous studies that showed DUS reliability in detecting and rating lower extremity arterial diseases both in the aorto-iliac and femoro-distal tract when compared with DSA [15, 16].
In a study by Adiseshiah et al., duplex imaging was compared with angiography and has been shown to detect arterial disease with an overall sensitivity of 92%, a specificity of 99%, a positive predictive value of 91%, and a negative predictive value of 100% [17].
Our results have confirmed the diagnostic accuracy of CTA when compared with intraprocedural DSA. This concordance of findings has been almost perfect for detecting iliac stenosis and occlusion with a Cohen’s κ agreement of 1.0; meanwhile, an underestimation of the degree of stenosis by CTA was observed at the femoro-popliteal level with a Cohen’s κ agreement between 0.97 and 0.87. This understaging may be due to eccentric plaques and/or circular calcifications that may reduce the accuracy of stenosis degree assessment due to beam-hardening artifacts. Although bones and circular calcifications that can obscure the true lumen of the arteries can be erased from the CT projections, this process may result in a false diagnosis of high-grade stenosis or occlusion.
The reported data are consistent with the results of Osama et al., who found an agreement between DSA and multidetector-row CTA in 98,1% of cases [18].
Below the knee, CTA showed even less concordance with DSA (Cohen’s κ 0.75) since it misinterpreted as occlusion five sub-occlusive stenoses, which were all associated with proximal steno-obstructive lesions of the arterial tree. Possible reasons for this overstaging are motion artifacts, different rates of calf filling, and insufficient arterial opacification distal to occlusions [19, 20].
A calcified vessel can again be mistaken for an occluded one because of the blooming effect of calcium [21].
In this study, DUS has been less accurate than CTA in assessing iliac arterial diseases (Cohen’s κ agreement of 0.91 and 1.0, respectively) when measured against DSA.
Bowel gas and the deep location of arteries are the main reasons for the lower accuracy of DUS in the iliac region. Despite its feasibility, direct aorto-iliac ultrasound evaluation requires patient preparation and may yield false-negative results in 5–25% of cases [22].
US may not be the best choice of imaging in certain patients. The majority mentioned that a small amount of body fat (normal body mass index—BMI) was best in obtaining good-quality abdominal DUS images whereas a large amount of body fat (BMI > 30.0) produces the worst image quality [23].
To minimize this limit, more innovative transducers or software are currently available to obtain high-quality abdominal US images in patients with varying amounts of adipose tissue.
Additionally, ultrasound spectral waveform analysis of ipsilateral common femoral arterial flow may be employed as a complementary, quick and reliable indirect diagnostic criterion to detect aorto-iliac occlusive disease.
According to Shaalan, a PSV detection of 45 cm/s or less and a monophasic waveform at the CFA level helps discriminate hemodynamic stenosis of the proximal iliac axis with an accuracy of 88% [24].
Bilateral diseased iliac arteries and/or distal superficial femoral artery outflow disease do not adversely influence the predictive value of both CFA waveform and PSV parameters because of the runoff of the unaffected profunda femoris artery, which is involved in PAD in less than 10% of cases as reported by Haimovici et al. [25].
This means that normal DUS findings reliably exclude significant iliac occlusive disease.
The use of a wide range of transducers (5–13 MHz) offers simultaneous morphological and hemodynamic information of the femoro-distal arteries as a unique feature.
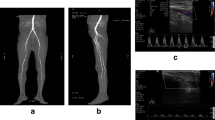
In agreement with the results of Ubbink and colleagues, we found good diagnostic concordance between DUS and DSA in detecting hemodynamic stenosis and occlusion of the femoro-popliteal axis (Cohen’s κ agreement between 0.96 and 0.93) (Fig. 1); [26].
The higher DUS diagnostic accuracy than CTA can be related to the quantitative measurement of the degree of stenosis based on ultrasound parameters (Fig. 2).
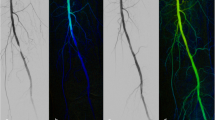
The reported data have documented the ability of color Doppler scanning to visualize patent infrageniculate arteries, also allowing flow detection in suboccluded infrapopliteal arteries not detected by CTA (Figs. 3 and 4). Although the most proximal anterior tibial and the peroneal arteries are the most difficult infrapopliteal segments to study by US, in our experience, they can be adequately visualized using different approaches (a medial approach for most proximal anterior tibial and peroneal arteries; the posterior or medial approach allows to detect the mid-peroneal artery, and the posterior or lateral approach may assist in the imaging of the distal peroneal artery and its branches). The depth of the tibio-peroneal trunk and the origin of its branches may require the use of a lower-frequency probe that reduces image resolution. In those cases, examining the vessels in transverse section, increasing the gain and changing the color box angle, allows for a better visualization of the arterial lumen. The collateral pathways can be mistaken for patent native vessels. However, identification of the satellite veins can help find a native artery instead of a large collateral vessel.
Additionally, setting the pulse repetition at 150–300 Hz, reducing the wall filter and increasing the persistence and the sensitivity of color-flow imaging, can be useful in determining the patency of very-low-flow arteries (< 20 cm/s) and avoid misinterpretation because of the potential pressure of the Doppler probe on the most distal superficial vessels that may mimic stenotic lesions as stated by Koelemay and colleagues [27].
In one case of this series, DUS understaged the stenosis degree of an anterior tibial artery in mixed multisegmental pathology (stenosis and occlusion); this stenosis was graded only on the PSV value. The calculated PSV ratio instead of PSV alone might have minimized the risk of error.
The quantitative assessment accuracy for the hemodynamic relevance of stenosis has been significantly improved by PSV ratio measurement especially in patients with multilevel segmental occlusions or diffuse stenotic disease. When ultrasound evaluation is equivocal for stenosis exceeding 70%, the PSV ratio value helps determine the actual significance of the lesion. PSV ratio instead of PSV may partially eliminate some pitfalls of blood flow velocity measurement, such as the influence of cardiac function on arterial velocity and false-negative findings at the distal stenosis because of decreased proximal flow due to tandem stenoses or obstructions. Additionally, PSV ratio can minimize the interobserver variance of ultrasound findings.
When the ultrasound assessment of the infrageniculate arteries is difficult, additional techniques can be employed. Power Doppler and echo enhancement techniques can help evaluate preocclusive and calcified vessels.
Main limitations to DUS include the lack of a panoramic view, operator-interpretation dependency, and high interobserver variability at the different lower-limb levels [28, 29].
It must also be noted that heavily calcified arteries, especially in diabetics and in patients with chronic renal insufficiency, cannot be visualized along their entire length by DUS. Nevertheless, incomplete ultrasound hemodynamic evaluation of these vessels can have less impact on therapeutic decision-making. When a distal artery is too calcified or narrow to be reliably detected on DUS, in most cases, it will be unfit for effective revascularization despite being assessed by intraoperative DSA. In a review of 71 studies comparing DSA with DUS, Koelemay and colleagues demonstrated that referral for endovascular treatment of PAD based on DUS was appropriate in 84% of patients and that interventional arteriography never changed the intended treatment [30] More recently, Wong et al. confirmed that DUS is accurate enough to guide the initial clinical management of patients with PAD [31].
Conclusion
DUS has proven to have high diagnostic accuracy in evaluating PAD. A crucial advantage of ultrasound investigation techniques is that it produces images of arterial disease and provides simultaneous Doppler measurements to estimate the degree of stenosis and the quality of inflow and outflow. The latter parameters are fundamental for assessing the technical feasibility and the potential outcomes of any procedures.
DUS findings can even surpass those by CTA in usefulness when imaging infrageniculate arteries by CTA is incomplete, as contrast agents within the most distal vessels fall below the sensitivity threshold.
Because of its accuracy in stenosis localization and hemodynamic evaluation, high-quality DUS performed by well-trained vascular operators may therefore represent a good alternative to CTA in patients undergoing endovascular revascularization with intraprocedural DSA to reduce the use of contrast-enhanced radiological imaging.
Nevertheless, preoperative CTA imaging is required in nondiagnostic DUS cases or when a more complete overview of the vascular tree is needed for complex invasive interventions.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Koheler TR (1993) Duplex scanning for the evaluation of the lower limb disease. In: Bernstein EF (ed) Vascular Diagnosis. St Luis Mosby-year book inc.
Duan Y, Wang X, Yang X, Wu D, Cheng Z, Wu L (2013) Diagnostic efficiency of low-dose CT angiography compared with conventional angiography in peripheral arterial occlusions. Am J Roentgenol 201:W906–W914
Jens S, Koelemay MJ, Reekers JA, Bipat S (2013) Diagnostic performance of computed tomography angiography and contrast-enhanced magnetic resonance angiography in patients with critical limb ischaemia and intermittent claudication: systematic review and meta-analysis. Eur Radiol 23(11):3104–3114
Antignani PL, Benedetti-Valentini F, Aluigi L, Baroncelli TA, Camporese G, Failla G, Martinelli O, Palasciano GC, Pulli R, Rispoli P, Amato A, Amitrano M, Dorigo W, Gossetti B, Irace L, Laurito A, Magnoni F, Minucci S, Pedrini L, Righi D, Verlato F (2012) Diagnosis of vascular diseases. Ultrasound investigations—guidelines. Int Angiol 31:1–77
Collins R, Burch J, Cranny G, Aguiar-Ibanez R, Craig D, Wright K, Berry E, Gough M, Kleijnen J, Westwood M (2007) Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease. BMJ 16:1257–1273
Collins R, Cranny G, Burch J, Aguiar-Ibanez R, Craig D, Wright K et al (2007) A systematic review of duplex scanning ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease. Health Technol Assess 11:1–202
Tufano A, Minelli R, Rossi E et al (2020) Inferior epigastric artery pseudoaneurysm secondary to port placement during a robot-assisted laparoscopic radical cystectomy. J Ultrasound. https://doi.org/10.1007/s40477-020-00442-1
Corvino A, Catalano O, de Magistris G, Corviino F, Giurazza F, Raffaella N, Vallone G (2020) Usefulness of doppler techniques in the diagnosis of peripheral iatrogenic pseudoaneurysms secondary to minimally invasive interventional and surgical procedures: imaging findings and diagnostic performance study. J Ultrasound. https://doi.org/10.1007/s40477-020-00475-6
Aboyans V, Ricco JB, Bartelink ML, Björck M, Brodmann M, Cohnert T et al (2018) Editor’s choice—2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 55(3):305–368. https://doi.org/10.1016/j.ejvs.2017.07.018
Favaretto E, Pili C, Amato A, Losinno F, Rossi C, Faccioli L, Palareti G (2007) Analysis of agreement between duplex ultrasound scanning and arteriography in patients with lower limb artery disease. J Cardiovasc Med 8(5):337–341
Bostrom A, Ljungman C, Hellberg A, Logason T, Barlin T, Astholm G, Karacagil S (2002) Duplex scanning as the sole preoperative imaging method for infrainguinal arterial surgery. Eur J Vasc Endovasc Surg 23:140–145
Hingorani A, Ascher E, Marks N (2007) Preprocedural imaging: new options to reduce the need for contrast angiography. Semin Vasc Surg 20:15–28
Katsamouris AN, Giannoukas AD, Tsetis D, Kostas T, Petinarakis I, Gourtsoyiannis N (2001) Can ultrasound replace arteriography in the management of chronic arterial occlusive disease of the lower limb. Eur J Vasc Endovasc Surg 21(2):155–159
Herique Rossi F, Puech-Leao P, Mitsuro IN (2006) Color-flow duplex hemodynamic assessment of runoff in ischemic lower limb revascularization. Vascular 14:149–155
Pemberton M, London NJ (1997) Colour flow duplex imaging of occlusive arterial disease of the lower limb. Br J Surg 84:912–919
Lingush J Jr, Reavis SW, Preissler JS, Hansen KJ (1998) Duplex ultrasound scanning defines operative strategies for patients with limb-threatening ischemia. J Vasc Surg 28:482–491
Aly S, Sommerville K, Adiseshiah M, Raphael M, Coleridge Smith PD, Bishop CC (1998) Comparison of duplex imaging and arteriography in the evaluation of lower limb arteries. Br J Surg 85(8):1099–1102
Osama A, Zaytoun H, Soliman HH (2012) Role of multi-slice CT angiography versus doppler ultrasonography and conventional angiography in assessment of aorto-iliac arterial disease. Egypt J Radiol Nuclear Med 43(4):561–573
Martin ML, Tay KH, Flak B, Fry PD, Doyle DL, Taylor DC, Hsiang YN, Machan LS (2003) Multidetector CT angiography of the aortoiliac system and lower extremities: a prospective comparison with digital subtraction angiography. AJR Am J Roentgenol 180(4):1085–1091
Bui TD, Gelfand D, Whipple S, Wilson SE, Fujitani RM, Conroy R, Pham H, Gordon IL (2005) Comparison of CT and catheter arteriography for evaluation of peripheral arterial disease. Vasc Endovasc Surg 39:481–490
Sarwar A, Rieber J, Mooyaart EA, Seneviratne SK, Houser SL, Bamberg F, Raffel OC, Gupta R, Kalra MK, Pien H, Lee H, Brady TJ, Hoffmann U (2008) Calcified plaque: measurement of area at thin-section flat-panel CT and 64-section multidetector CT and comparison with histopathologic findings. Radiology 249(1):301–306
Edwards JM, Coldwell DM, Goldman ML, Strandnness DE Jr (1991) The role of duplex scanning in the selection of patients for transluminal angioplasty. J Vasc Surg 13:69–74
Brahee DD, Ogedegbe C, Hassler C (2013) Body mass index and abdominal ultrasound image quality: a pilot survey of sonographers. J Diagn Med Sonogr 29(2):66–72
Shaalan WE, French-Sherry E, Castilla M, Lozanski L, Bassiouny HS (2003) Reliability of common femoral artery haemodynamics in assessing the severity of aortoiliac inflow disease. J Vasc Surg 37:960–969
Haimovici H, Shapiro JH, Jacobson HG (1960) Serial femoral arteriography in occlusive disease: clinical-roentgenologic considerations with a new classification of occlusive disease. Am J Roentgenol 83:1042
Ubbink DT, Fidler M, Legemate DA (2001) Interobserver variability in aortoiliac and femoropopliteal duplex scanning. J Vasc Surg 33:540–545
Koelemay MJ, Legemate DA, Van Grup JA, De Vos H, Balm R, Jacobs MJ (2001) Interobserver variation of colour duplex scanning of the popliteal, tibial and pedal arteries. Eur J Endovasc Surg 21:160–164
Larch E, Minar E, Ahmadi R, Schnürer G, Schneider B, Stümpflen A, Ehringer H (1997) Value of duplex sonography for evaluation of tibioperoneal arteries in patients with femoropopliteal occlusions: a prospective comparison with anterograde intraarterial digital subtraction angiography. J Vasc Surg 25:629–636
Kallen AJ, Jhung MA, Cheng S (2008) Gadolinium-containing magnetic resonance imaging contrast and nephrogenic systemic fibrosis: a case-control study. Am J Kidney Dis 51(6):966–975
Koelemay MJ, Legemate DA, De Vos H, Van Grup JA, Balm R, Reekers JA, Jacobs MJ (2001) Duplex scanning allows selective use of arteriography in the management of patients with severe lower leg arterial disease. J Vasc Surg 34:661–667
Wong TH, Tay KH, Sebastian MG, Tan SG (2013) Duplex ultrasonography arteriography as first-line investigation for peripheral vascular disease. Singapore Med J 54:271–274
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not conflicts of interest or competing interests.
Ethical approval
Approval was obtained from our ethics committee. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Informed consent
Each patient has signed the informed consent, required by our hospital, for diagnostic tests and for therapeutic procedures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martinelli, O., Alunno, A., Drudi, F.M. et al. Duplex ultrasound versus CT angiography for the treatment planning of lower-limb arterial disease. J Ultrasound 24, 471–479 (2021). https://doi.org/10.1007/s40477-020-00534-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-020-00534-y