Abstract
Spontaneous rectus sheath hematoma (SRSH) is an uncommon cause of acute abdominal pain characterized by bleeding within the rectus sheath; it is a benign condition and, in most cases, it is treated conservatively. Bleeding of the abdominal wall is an unusual condition that is quite challenging to identify promptly and can be easily overlooked during a routine physical examination. In daily practice, anticoagulant therapy is one of the main risk factors for hemorrhagic events. In this respect, we report a rare case of spontaneous hematoma of the abdominal wall (diagnosed and monitored through an ultrasound examination) that arose after sneezing in a patient receiving anticoagulant treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spontaneous rectus sheath hematoma (SRSH) is an uncommon cause of acute abdominal pain characterized by a focal collection of blood inside the rectus sheath [1]; it is a benign condition that usually occurs due to damage of the epigastric vessels in relation to intrinsic muscle injuries (e.g., intense rectus muscle contraction with fiber laceration) [2, 3]. Muscle injuries, with or without hematomas, can be classified as extrinsic or intrinsic. Extrinsic injuries (i.e., following direct trauma) can potentially involve all muscles of the human body and are clinically classified as mild, moderate, or severe on the basis of the functional impairment [4]. Intrinsic muscle injuries occur due to contraction and simultaneous elongation of the muscle–tendon unit (i.e., the eccentric mechanism), leading to a disruption of the muscular/collagen fibers; sports trauma and involuntary physiological reflexes, such as sneezing, coughing, or vomiting, are the most frequent causes of these kinds of injuries [2,3,4]. Anticoagulation therapy and pathologies that induce fragility of blood vessels and/or coagulation disorders are the most common predisposing factors for rectus sheath hematoma [2]. Coupled with an accurate medical history and physical examination, diagnostic imaging modalities—such as ultrasound imaging, computed tomography (CT), and magnetic resonance imaging (MRI)—are paramount to accurately identifying the size/location of the hematoma and better defining the differential diagnosis with other pathological conditions.
Case description
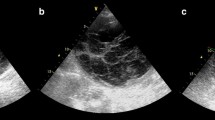
A 69-year-old woman presented to our ultrasound department complaining of the right lower quadrant (RLQ) abdominal pain. The acute abdominal pain had appeared, after a sneeze, 2 days before. She described her pain as sharp with an intensity of 7/10 on the visual analog scale (VAS), predominantly localized to the RLQ of the abdomen, without radiation of symptoms, and a positive Carnett’s sign. The latter is defined as an increase in abdominal pain when a patient, in a supine position, tenses their abdominal wall by lifting their head and shoulders off the exam table, indicating the abdominal wall and not the abdominal cavity as the source of pain. Our patient was unable to perform postural changes, from supine to sitting and from sitting to standing, due to intense discomfort. She had a medical history significant for chronic atrial fibrillation (CAF) and oral anticoagulation therapy. During the first-line medical evaluation in the emergency room, her vital signs were as follows: temperature 36.5 °C, blood pressure 130/80 mmHg, respiratory rate 21 breaths per minute, and oxygen saturation 99% on room air. The electrocardiogram revealed CAF (85 beats per minute) with no ST or T wave abnormalities. Other parameters—complete blood count (CBC), inflammatory markers (VES and PCR) tests, kidney function tests, liver function tests, cardiac enzymes, complete urine test, lipase and amylase, and urine screen—were all within normal limits. Physical examination showed tenderness in the RLQ of the abdomen (focal soft spot) and an active abdominal guarding reaction during deep palpation, but no palpable mass at the level of the abdominal wall was identified. A sonographic examination, using a linear multifrequency probe, was performed at the level of the abdominal wall, focusing on the clinically identified soft spot in the RLQ of the abdomen. The main ultrasonographic finding was a small, oval, and anechoic area, measuring 1.1 cm × 1.6 cm and located inside the rectus abdominis muscle (Fig. 1a–c); of note, there was no herniation of bowel contents with the Valsalva maneuver, and no disruption of the deep fascial planes was identified. A color Doppler examination showed the absence of intralesional vascular spots (Fig. 1d), and the surrounding abdominal muscles and fascia were unremarkable. Based on the clinical and ultrasonographic findings, the diagnostic hypothesis was a post-traumatic hematoma within the rectus sheath. However, given the small size of the blood collection, we decided not to stop the anticoagulant treatment. Within 2 days, we performed a strict ultrasonographic follow-up in order to evaluate its morphological evolution (Fig. 2a, b). At 12 days, the ultrasound examination revealed a remarkable reduction in the size of the hematoma and an initial healing process of the local soft tissues (Fig. 2c, d). Four weeks after the trauma (i.e., the sneeze), the patient no longer complained of any pain or discomfort in the abdominal region.
Diagnostic Phase. Short (a) and long (b) axis B-mode ultrasound images show the anechoic area (white arrowheads) located inside the rectus abdominis muscle (RAM) and superficial to the deep lamina of the rectus sheath (yellow arrowhead). A soft pattern of the mass was identified with the strain elastography modality (c), and the absence of intralesional vascular spots was demonstrated with the color Doppler examination (d). The schematic drawing shows the supposed pathomechanism of squeezing and stretching of the perforating branches of the epigastric vessels causing the sheath hematoma of the RAM (e). sc subcutaneous tissue
Follow-Up Phase. At the 2 days’ follow-up, the short (a) and long (b) axis B-mode ultrasound images show no structural and dimensional variations of the intramuscular hematoma (white arrowheads). At the 12 days’ follow-up, the short (c) and long (d) axis B-mode ultrasound images display a substantial reduction of the sizes of the hypo-/isoechoic areas (white arrowheads) and initial healing signs of the rectus abdominis muscle (RAM) and of its sheath (yellow arrowhead). sc subcutaneous tissue
Discussion
The anterior abdominal wall is characterized by a superficial layer consisting of skin and adipose tissue; a middle layer (i.e., the myofascial layer), which consists of muscles and their fascial envelopes; and a deep layer formed by the transversalis fascia, preperitoneal fat, and parietal peritoneum [6,7,8]. The rectus abdominis muscles extend from the last costal cartilage to the upper edge of the pubis, and they are composed of several muscular bellies separated by three or four tendinous intersections. The pyramidal muscles are small and variable triangular muscles that are localized between the pubis and linea alba. Laterally, the external oblique, internal oblique, and transversus abdominis muscles extend from the lateral edge of the rectus to the flanks with three overlapping layers. Sonographically, the outer muscle fascia (i.e., epimysium) appears as a well-delineated echogenic envelope circumscribing the hypoechoic muscular belly. The aponeuroses of the aforementioned muscles merge with each other to form a unique structure—the rectus sheath—that envelops the rectus abdominis muscle reaching the linea alba [9, 10]. During an ultrasound, intramuscular vessels can be clearly visualized with the color and power Doppler modalities; of note, these vessels are localized close to the hyperechoic septa of the muscular belly [11].
SRSH is a rare cause of acute abdominal pain, developing after an accumulation of blood in the rectus sheath due to a tear of the rectus abdominis muscle or damage to the epigastric vessels; in the latter case, the epigastric arteries/veins or their perforating branches can be involved [1]. Anticoagulation therapy and several medical conditions that cause fragility of blood vessels and/or coagulation disorders are the most common predisposing factors for SRSH [12]. Involuntary reflexes, such as coughing, vomiting, or sneezing, can cause an intense contraction of the thoracic and abdominal muscles; thus, mechanical transmission of high pressure to different parts of the human body may result in peculiar injuries. Sneezing can be caused by a number of local irritants, chronic inflammatory diseases, and other stimuli. Intranasal triggers can include mucus, infectious pathogens, dust, allergens, or particulate/chemical irritants [13]. A sneeze is characterized by an explosive exhalation with a strong concentric contraction of the rectus abdominis muscles and, often, a sudden forward inclination of the trunk in an upright posture [14]; biomechanically speaking, the combination of contraction and shortening of the rectus abdominis muscle may result in a squeezing and stretching of the epigastric vessels and their branches. The aforementioned mechanism of the abdominal muscles during sneezing, in patients receiving anticoagulant therapy, may generate a SRSH.[14]. It most commonly occurs in the lower abdominal wall because of the weaker support of the rectus abdominis muscle by the transversalis fascia and peritoneum below the level of the linea semicircularis. Additionally, considering the firm attachments of the perforating branches of the inferior epigastric artery—which pierce the rectus abdominis from a deep plane—and the changes in muscle length during movements that predominantly occur in the lower half of the rectus abdominis muscle, the retro-/intramuscular epigastric vessels are exposed to extra shearing forces exactly at the level of lower abdominal quadrants [15]. SRSH occurs more frequently in women than in men and usually in the sixth and seventh decades of life [15].
Bleeding of the abdominal wall is an unusual injury that is quite difficult to recognize promptly and can be easily overlooked during initial medical examinations, especially if it develops following minor trauma such as a sneeze [12]. The clinical presentation of the SRSH varies according to the size of the hematoma and the patient’s comorbidities; it may present with abdominal pain (84–97% of the cases), palpable abdominal wall mass (63–92%), tenderness (71%), abdominal guarding reaction (49%), nausea (23%), vomiting (15%), and, rarely, fever and chills [12,13,14,15,16]. SRSH can be challenging to diagnose, because it mimics several abdominal pathologies, such as appendicitis, sigmoid diverticulitis, perforated ulcer, ovarian cyst torsion, intestinal obstruction, ectopic pregnancy, abdominal wall endometriosis, abruptio placenta, tumor, or hernias [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Evaluating underlying diseases/conditions and precipitating events, in conjunction with a detailed physical examination of the abdomen, is paramount to a better definition of the diagnosis. Indeed, the diagnostic pathway to correct identification of a rectus sheath hematoma should always start with a careful history analysis and physical examination [3]. Ultrasound imaging of the abdominal wall is useful for confirming the clinically suspected local blood collection and for ruling out other causes of abdominal pain [5]. Sonography has an overall sensitivity of 87.6% in correctly identifying muscle injuries, and it is essential to an accurate assessment of the severity of the lesion to exclude important complications [18]; furthermore, it is paramount to correctly planning the treatment and better defining the prognosis [18]. An assessment of muscle injuries/hematomas of the abdominal wall typically requires high-frequency linear probes (from 9 to 18 MHz); however, if the fluid collection is large and deep, panoramic views or convex probes (from 1 to 5 MHz) may be useful. The main objective is to obtain a good resolution and diagnostic image [19]. Ultrasonography allows for the dynamic evaluation of muscular conditions that demonstrate excellent spatial resolution and a high definition of the soft tissues in motion [5, 18, 19]. On ultrasonography, the normal architecture of the muscular tissue is characterized by multiple, parallel, hypoechoic fibers surrounded by echogenic fibrofatty septa; in this respect, the pennate (long axis) and starry night (short axis) patterns are commonly used to describe the spatial distribution of the muscular/connective tissue technically [5, 11, 18, 19]. Ultrasound scans of the abdominal muscles should have transverse and longitudinal views from the proximal to the distal attachment, including the myotendinous junction and enthesis. On sonography, muscle injuries appear as a disruption of the normal echogenic fibrillar pattern with the presence of anechoic clefts and irregular linear bands with or without fluid collections [4, 11,12,13,14,15,16,17,18,19]. The power Doppler modality is used in clinical practice to differentiate quickly the hematoma (i.e., not vascularized) from local hyperemia, which presents multiple vascular spots of local angiogenesis [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19].
Furthermore, an ultrasound evaluation is useful for monitoring the “sonohistologic” evolution of the fluid collection within the target soft tissues of the abdominal wall [18]. The sonographic appearance of muscle hematomas depends on multiple factors, such as the spatial dimension of the collection, the time elapsed between the injury and examination, the patient’s age and possible bleeding disorders, or anticoagulant therapy. In the first 24–48 h, the hematoma generally appears as a hypoechoic fluid collection; however, during this period, it may solidify and become hyperechoic with respect to the surrounding muscles [4, 19, 20]. After 48–72 h, the hematoma develops a well-defined hypoechoic core with an echogenic margin that gradually enlarges itself and “fills in” the hematoma in a centripetal fashion [4, 19, 20]. Histologically, the hemorrhage and muscle fiber necrosis from the first phase are progressively replaced by fibroblast cells and connective tissue, which contribute to the formation of the scar during the healing phase. On ultrasound, an intramuscular scar can appear hyperechoic, heterogeneous hypoechoic, linear, or stellate, and it is usually located close to the musculotendinous junction [21]. The stellate pattern of the scar is usually related to a muscle contusion with a direct blow, whereas the linear pattern typically occurs after a muscle strain. It is important to note that if during the ultrasound follow-up a progressive enlargement of the hematoma is identified, causing intense pain and/or exerting a local mass effect on adjacent neurovascular structures (or placing the limb at risk of compartment syndrome), an ultrasound-guided evacuation of it may be necessary [4].
The CT scan is a panoramic, second-level diagnostic modality suitable for assessing the extension of the hematoma, evaluating the presence of active bleeding, and excluding other types of abdominal wall disease; the examination can be performed before and after the administration of a contrast medium. The protocol with the contrast agent includes arterial and venous phases, and the diagnosis of arterial active bleeding is confirmed in the presence of a focal/diffuse area of high attenuation that is isodense with the adjacent arterial vessels [22].
On the other hand, the MRI is a diagnostic technique widely used in assessing the different stages of the organization of the blood collection, from the acute to the chronic phase of the hemorrhagic disease. On the MRI, the intramuscular hematoma is easily recognized due to the distortion of the histological architecture, appearing as a mass of variable signal intensity depending on the stage of blood degradation. In the acute phase, the hematoma exhibits low T1 and T2 signal intensity related to intracellular deoxyhemoglobin, and in the early subacute phase, deoxyhemoglobin converts to intracellular methemoglobin, increasing the T1 signal intensity. In the late subacute phase, red cells lyse and methemoglobin becomes extracellular with an increase in T2 signal intensity. The final degradation products are hemosiderin and ferritin, which are the substances responsible for the low signal intensity of chronic hemorrhage [23].
SRSH is usually a benign condition that is rarely life-threatening (i.e., the size and location of the bleed must be carefully assessed), and the approach is generally conservative in the absence of hemodynamic repercussions [24]. In the presence of hemodynamic imbalance, the treatment includes resuscitation with intravenous fluids/blood products and normalization of the coagulation status with fresh frozen plasma and vitamin K. Surgical management, which is extremely challenging, or embolization by angiographic specialists is necessary for patients who fail conservative measures [25].
Conclusion
SRSH after sneezing is a rare condition and can occur especially in patients receiving anticoagulant therapy. In most cases, the anatomical arrangement of the sheath of the rectus abdominis muscle prevents the local extension of the fluid collection, but the high pressure inside the abdominal wall can cause acute pain. The supposed pathomechanism of this injury is a sudden combination of squeezing and stretching of the perforating branches of the epigastric vessels during the contraction/shortening of the rectus abdominis muscle during a sneeze—mechanical forces that, in patients with vasculopathy and/or anticoagulant therapy, may lead to a vascular injury with local hematoma. Ultrasonography is widely considered as the first-line examination for a fast assessment of the soft tissues of the abdominal wall. It is rapid, easily accessible, inexpensive, noninvasive, and repeatable. The multiplanar approach of ultrasound imaging provides an excellent spatial resolution of the abdominal wall structures; moreover, the dynamic scans allow the physician to rapidly evaluate the behavior of the intramuscular hematoma during an active contraction of the target muscle. Last but not the least, abdominal wall sonography is a suitable diagnostic tool during follow-up to monitor the evolution of the blood collection (e.g., dimension and echo-structure) in the absence of hemodynamic repercussions.
References
Klingler PJ, Wetscher G, Glaser K et al (1999) The use of ultrasound to differentiate rectus sheath hematoma from other acute abdominal disorders. Surg Endosc 13(11):1129–1134
Sanchis-Moysi J, Idoate F, Dorado C et al (2010) Large asymmetric hypertrophy of rectus abdominis muscle in professional tennis players. PLoS ONE 5:e15858
Ozaras R, Yilmaz MH, Tahan V et al (2003) Spontaneous hematoma of the rectus abdominis muscle: a rare cause of acute abdominal pain in the elderly. Acta Chir Belg 103:332–333
Lee JC, Mitchell AW, Healy JC (2012) Imaging of muscle injury in the elite athlete. Br J Radiol 85:1173–1185
Draghi F, Cocco G, Richelmi FM et al (2020) Abdominal wall sonography: a pictorial review. J Ultrasound https://doi.org/10.1007/s40477-020-00435-0(published online ahead of print, 2020 Mar 3)
Grevious MA, Cohen M, Shah SR et al (2006) Structural and functional anatomy of the abdominal wall. Clin Plast Surg 33:169–v
Parikh KR, Al-Hawary M, Millet JD et al (2017) Incisional hernia repair: what the radiologist needs to know. AJR Am J Roentgenol 209:1239–46
Hellinger A, Roth I, Biber FC, Frenken M et al (2016) Chirurgische anatomie der bauchdecke [Surgical anatomy of the abdominal wall] [published correction appears in Chirurg. 2017 Jun; 88(6):536]. Chirurg 87:724–30
Punekar IRA, Khouri JS, Catanzaro M et al (2018) Redefining the rectus sheath: implications for abdominal wall repair. Plast Reconstr Surg 141:473–9
Naraynsingh V, Maharaj R, Dan D et al (2012) Stronglinea alba: myth or reality? Med Hypotheses 78:291–2
Bianchi S, Martinoli C (2007) Ultrasound of the musculoskeletal system. Springer, Berlin, pp 198–332
Hatjipetrou A, Anyfantakis D, Kastanakis M (2015) Rectus sheath hematoma: a review in literature. Int J Surg 13:267–71
Setzen S, Platt M (2019) The dangers of sneezing: a review of injuries. Am J Rhinol Allergy 33:331–7
Hasegawa T, Katsuhira J, Matsudaira K et al (2014) Biomechanical analysis of low back load when sneezing. Gait Posture 40:670–5
Salemis NS (2009) Spontaneous rectus sheath hematoma presenting as acute surgical abdomen: an important differential in elderly coagulopathic patients. Geriatr Gerontol Int 9:200–2
Cherry WB, Mueller PS (2006) Rectus sheath hematoma: review of 126 cases at a single institution. Med (Baltimore) 85:105–10
Cocco G, Ricci V, Boccatonda A et al (2020) Focused ultrasound for the diagnosis of non-palpable endometriotic lesions of the abdominal wall: a not-uncommon surgical complication. J Ultrasound https://doi.org/10.1007/s40477-019-00425-x
Ruff AN, Cornelson SM, Panter AS et al (2019) Rectus abdominis muscle tear diagnosed with sonography and its conservative management. J Ultrasound https://doi.org/10.1007/s40477-019-00416-y(published online ahead of print, 2019 Nov 12)
Draghi F, Zacchino M, Canepari M et al (2013) Muscle injuries: ultrasound evaluation in the acute phase. J Ultrasound 16:209–14
Lehto M, Alanen A (1987) Healing of a muscle trauma: correlation of sonographic and histological findings in an experimental study in rats. J Ultrasound Med 6:425–9
Lee JC, Healy JC (2004) Sonography of lower limb muscle injury. AJR Am J Roentgenol 182:341–51
Pieri S, Agresti P, Buquicchio GL et al (2015) Endovascular management of the rectus muscle hematoma. Radiol Med 120:951–8
Stensby JD, Baker JC, Fox MG (2016) Athletic injuries of the lateral abdominal wall: review of anatomy and MR imaging appearance. Skeletal Radiol 45:155–62
Karapolat B, Tasdelen HA, Korkmaz HAA (2019) Conservative treatment of spontaneous rectus sheath hematomas: single center experience and literature review. Emerg Med Int 2019:2406873
Dhaliwal JK, Garmel GM (2012) Image diagnosis: abdominal wall hematoma. Perm J 16:58–59
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Human or animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cocco, G., Ricci, V., Boccatonda, A. et al. Sonographic demonstration of a spontaneous rectus sheath hematoma following a sneeze: a case report and review of the literature. J Ultrasound 24, 125–130 (2021). https://doi.org/10.1007/s40477-020-00493-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-020-00493-4