Abstract
Purpose of Review
To highlight recent research about frailty and its role as a predictor of adverse, long-term post-kidney transplant (KT) outcomes.
Recent Findings
Frailty is easily measured using the physical frailty phenotype (PFP) developed by gerontologist Dr. Linda Fried and colleagues. In recent studies, > 50% of KT recipients were frail (20%) or intermediately frail (32%) at KT admission. Frail recipients were at 1.3-times higher risk of immunosuppression intolerance and 2.2-times higher risk of mortality, even after accounting for recipient, donor, and transplant factors; these findings were consistent with those on short-term post-KT outcomes. Pilot data suggests that prehabilitation may be an intervention that increases physiologic reserve in frail KT recipients.
Summary
PFP is an effective tool to measure frailty in ESRD that improves risk stratification for short-term and long-term post-KT outcomes. Interventions to improve physiologic reserve and prevent adverse KT outcomes, particularly among frail KT recipients, are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the population ages and experiences more chronic conditions like diabetes and hypertension, end-stage renal disease (ESRD) is becoming more common among older adults aged 65 years and older, and access to kidney transplantation (KT) has expanded in this population [1]. In 2016, there were nearly 20,000 adult KTs performed in the USA, a substantial rise from 8719 in 1995. This expansion occurred particularly in older adults: currently, 18%–20% of KT recipients are over 65 years of age [1, 2], a steep increase from 3% in 1990 [1]. Moreover, the overall distribution of KT recipients is getting older with demographic shifts witnessed in the USA; patients older than 70 comprised 22.4% of older KT recipients between 1990 and 1993 compared to 41.5% between 2009 and 2011 [1].
Compared to community-dwelling older adults, older KT recipients are at elevated risk of age-related outcomes including early hospital readmission, dementia, Alzheimer’s disease, infections, malignancy, and fractures [2,3,4,5,6]. For example, we recently found that the 10-year risk of post-KT dementia was 17.0% for KT recipients aged 75 years and older; this is a much higher proportion than the estimated 7.5% risk for community-dwelling older adults of the same ages [7].
As the population of KT recipients continues to get older and experience age-related outcomes, it is important to identify novel metrics of aging that can help stratify risk for long-term post-KT outcomes. Frailty, a syndrome of decreased physiologic reserve and resistance to stressors underlain by multisystem dysfunction, has recently immerged as a potentially important geriatric construct among KT recipients because it captures the accelerated aging associated with ESRD and enhances long-term risk prediction in adult KT recipients of all ages.
Summary of Current Understanding of Frailty
Measuring Frailty Using the Physical Frailty Phenotype
Frailty is a syndrome of decreased physiologic reserve with a profound biological basis of vulnerability that becomes more evident during periods of chronic or acute stress, including surgery. Initial studies of frailty among patients with ESRD measured the physical frailty phenotype (PFP) and is sometimes referred to as the Fried frailty phenotype [8,9,10], which was originally designed and validated among community-dwelling older adults by Dr. Linda Fried and colleagues [11, 12]. Importantly, the PFP identifies those with diminished physiologic reserve even without disability and comorbidity [11].
In order to identify community-dwelling older adults at highest risk of adverse health outcomes, and to better understand the biological basis of frailty, Fried and colleagues operationalized a frailty phenotype as an aggregate measure that consists of 5 components [11]. The first component is shrinking or the unintentional weight loss of more than 10 pounds in the past year. The second component is weakness, measured by the grip strength of the dominant hand using a handheld dynamometer and defined based on established cutoffs by gender and body mass index (BMI). The third component is exhaustion and is measured by self-report based on two questions from the Centers for Epidemiologic Studies-Depression (CES-D) [13]. The fourth component is low physical activity in the past 2 weeks based on the Minnesota Leisure Time Physical Activity (MLTA) questionnaire but can also be estimated using other physical activity measurement tools. The fifth and final component is slowness which is directly measured by walking time of 15 ft; speeds below an established cutoff based on recipient sex/gender and height are considered slowed walking speed (Table 1) [11]. Theoretically, each of these criteria represents varying physiologic systems, where the confluence of 3 or more components captures an underlying multisystem dysfunction and pinpoints patients who are considered frail.
To measure the PFP among KT, similar to what has been done for community-dwelling older adults, all that is needed is a handheld dynamometer, a stopwatch, and two questionnaires: the MLTA [14] and the CES-D [13]. The phenotype takes approximately 10 min to measure in a clinical setting. One ESRD-specific consideration for measuring the shrinking criterion of the PFP among those who are undergoing dialysis is to use of the dry weight (Table 1). We have classified KT recipients with one or fewer components as non-frail, 2 components as intermediately frail, and 3 or more components as frail [15, 16]; these thresholds are specific to the ESRD population.
PFP in Geriatric Populations
The PFP was first described in 5317 older participants in the Cardiovascular Health Study and has been studied in nationally representative studies [17,18,19,20,21,22,23]. In the original study, 7% of community-dwelling older adults aged 65 years and older were frail, and frailty was associated with a 2.2-fold increased mortality risk [11]. Subsequent studies using the PFP provided strong evidence of deep biological change and multisystem dysregulation in older adults [24,25,26]. Predictive criterion validity for frailty was demonstrated based on the high risk of functional disability, institutionalization, and death [12], and there was evidence for strong internal construct validity characterizing frailty as a syndrome [12]. However, recent findings suggest that the operationalization of the PFP does not require all 5 components to capture physiologic reserve without losing syndrome measurement validity [27]; the constituent components depend on the population and setting.
Biological Basis of Frailty
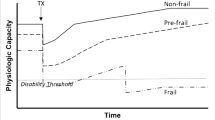
The PFP measure of frailty in older adults has been characterized by multisystem dysregulation [26] that undermines homeostatic mechanisms, and in turn contributes to a host of adverse health outcomes including worsening chronic conditions, mobility limitations, disability, altered cognition, and premature mortality [26, 28,29,30]. Over the past several years, investigators have increasingly honed in on dysregulated stress response systems responsible for the distinct vulnerability to adverse outcomes observed in frailty, including chronic inflammation, altered glucose metabolism, and decreased mitochondrial energy production [24, 26, 28, 31,32,33,34,35,36,37,38,39]. It is also increasingly evident that frailty-related biological changes are multi-factorially triggered by age-related biological changes (such as mitochondrial decline and/or cellular senescence), behavioral factors related to diet and exercise, medications, chronic illnesses/comorbidities, and environmental stressors [30, 40]. These insults lead to a cycle of decline in energy, skeletal muscle, and altered nutritional state, and the biological changes that ensue do not exist in isolation; rather, they persist together in a variety of constellations, which aggregately contribute to a manifestation of distinct biological vulnerability [30] (Fig. 1).
Authors’ Personal Views of Measurement Controversy
The PFP is one of 67 tools that are referred to as frailty [41]; the vast majority are markers of general susceptibility that lack a strong biological foundation, and fail to identify patients prior to onset of comorbidity and disability. Given the strong biological basis of the PFP, and its distinction from comorbidity and disability, we felt that the PFP best captures frailty as a specific construct of reduced physiologic reserve that may eventually be intervened upon, and not one that broadly represents general vulnerability that makes identification of interventions virtually impossible. Furthermore, the PFP has been found to be feasible to measure in the KT setting [42]. Only one study has used a frailty index that summarizes domains of functioning to assess frailty in KT recipients [43]; however, this tool, the Groningen Frailty Indicator, is distinct from the PFP. Therefore, we have measured and studied the PFP in over 1300 KT recipients over the past 10 years.
Summary of Current Data on Frailty and Long-Term Post-KT Outcomes
Frailty at the Time of Admission for KT
Frailty, as measured by the PFP, is common at the time of admission for KT; over 50% of KT recipients aged 18 years and older are frail (20%) or intermediately frail (32%) [44]. In a study of 663 KT recipients, frailty was more common in older recipients (22.7% of recipients aged 75 years and older) but still occurred in 13.6% of KT recipients aged 18–35 years. Independent of other transplant, recipient, and donor factors, KT recipients aged 65 years and older were at a 2-fold increased risk of being frail compared with their younger counterparts. These findings suggest that there is a high prevalence of frailty among KT recipients even among those who are not chronologically “old” [44].
Out of the five PFP components (shrinking, weakness, exhaustion, low physical activity, and slowed walking speed), the two most frequently observed among KT recipients were poor grip strength (50%) and low self-reported physical activity (49%) [44]. Additionally, the most common pattern of these PFP components was poor grip strength, low physical activity, and slowed walk speed, which occurred in 19% of all frail KT recipients [44].
Short-Term Improvements for Frail KT Recipients
Post-KT Frailty is likely dynamic [45]; in KT recipients over all ages, frailty initially worsens in the first month post-KT but then improves by 3 months post-KT [45]. Although KT recipients who were frail at KT had higher frailty scores over the long term, they were most likely to show improvements in their PFP score and likely their underlying physiological reserve than their non-frail counterparts, supporting that idea that KT may be beneficial even for frail candidates and that frailty may not be an irreversible state of low physiological reserve for all KT recipients [20]. This improvement in physiologic reserve may then lead to subsequent improvements in physical and kidney disease-specific HRQOL, but not mental HRQOL, among frail KT recipients [46]. However, not all improvements are sustained long term [46]. For example, frail KT recipients experienced improvements in cognitive function within the first 3-months post-KT, reaching levels of their non-frail counterparts with successful restoration of kidney function. Yet in long term, KT recipients who were frail at admission to KT experienced a significant decline in global cognitive function that was not observed in non-frail recipients [47]. More research needs to be done in this area to determine whether the underlying process of multisystem dysfunction is truly being interrupted by KT and whether post-KT issues like wound healing, immunosuppression, and infections may impact the reversal of frailty after KT.
Long-Term Post-KT Outcomes Associated With Frailty Status
KT recipients who were identified as frail using the PFP were more likely to have needed a reduction in mycophenolate mofetil (MMF) immunosuppression dose by 4 years (67% vs. 51%). Those who were frail were at a 1.3-fold risk of MMF immunosuppression intolerance [15] independent of recipient, donor, and transplant factors. Furthermore, this dose reduction placed KT recipients at a 5.2-fold higher risk of death-censored graft loss [15].
The 5-year risk of post-KT mortality was 91.5% for non-frail recipients, 86.0% for intermediately frail recipients, and 77.5% for frail recipients. Additionally, KT recipients who were frail by the PFP were at a 2.2-fold higher risk of mortality and those who were intermediately frail were at a 1.5-fold higher risk of mortality even after accounting for recipient, donor, and transplant factors [16]. In a subsequent study, we found that there were two groups of KT recipients who were at elevated risk of mortality: those who were frail and those who were intermediately frail. Recipients who had either exhaustion and slowed walking speed or poor grip strength, exhaustion, and slowed walking speed were at a 2-fold increased risk of post-KT mortality [44]. These associations with long-term outcomes may be mediated in part by limited health literacy [48] and the increased risk of KT complications including delirium [49••].
These two studies of frailty and long-term post-KT outcomes were consistent with previous findings that suggest that frail KT recipients were at risk of short-term outcomes including: depressive symptoms [50], longer length of stay [51], delirium [52], delayed graft function [53], and early hospital readmission [54]. These short-term outcomes may be full or partial mediators of the associations between frailty and long-term outcomes, although this is still an area of active research.
Alternative Measures of Vulnerability and Long-Term Post-KT Outcomes
Additionally, alternative measures of vulnerability that may reflect some of the components of the PFP have been studied in KT recipients and found to be associated with post-KT mortality. Self-reported functional status, for example, has been found to be associated with post-KT mortality [25]. However, other objective measures like the timed-up-and-go test was not associated with post-KT length of stay, or early hospital readmission [55]. Additionally, lower extremity impairment, objectively measured by the short physical performance battery (SPPB), is present in 48% of KT recipients [56••]. This measure of vulnerability identifies a different group of KT recipients; only 11% of the KT population was both SPPB-impaired and frail based on the PFP. Among KT recipients with lower extremity impairment, it took 13% longer to be discharged from KT than their non-impaired counterparts [57]. Additionally, SPPB impairment was associated with a 2.3-fold increased mortality risk and an absolute increase in 5-year mortality of 16.1% (20.6% vs. 4.5%) [56••].
Unanswered Questions
There is one main unanswered question remaining: how can we reduce adverse long-term outcomes among KT recipients who are frail? In other words, is frailty modifiable? Based on the recently published commentaries [58, 59] and a pilot study [60•], one particular approach appears to hold the most promise: prehabilitation, or intensive exercise therapy, prior to an elective surgical intervention. Exercise has been shown to improve and reverse frailty status (as measured by the PFP) in community-dwelling older adults [61]. We have recently completed a pilot involving intradialytic prehabilitation in KT [60•], which has been proposed as an appealing strategy for potentially overcoming the high drop-out observed in post-KT interventions [59]; it shifts the focus to optimization prior to surgery, rather than rehabilitation after surgery. The most common form of prehabilitation includes exercise components with strength, aerobics, and stretching [62], although nutritional components [63,64,65] and psychological components [66] have been included. In a recent survey, both clinicians (97%) and patients (94%) agreed that pre-KT prehabilitation would help patients undergoing KT and that prehabilitation would make ESRD patients less frail (clinicians: 100% and patients: 84%) [67]. Additionally, 97% of clinicians and 80% of patients agreed that patients would be interested in pre-KT prehabilitation [67]. However, a multi-center randomized controlled trial of prehabilitation among frail KT recipients is needed to confirm the efficacy of this intervention in preventing long-term outcomes and associated costs.
Conclusions
As the number of older KT recipients continues to grow each year, there is a pressing urge to improve risk stratification for long-term post-KT adverse outcomes. Frailty as measured by the PFP has recently emerged as a promising tool to help clinicians and transplant centers identify those with decreased physiologic reserve that may be more vulnerable to stressors. Importantly, this tool has a well-established biological basis, and is not solely driven by a KT recipients’ chronologic age. The next crucial step is to identify an efficacious and effective prehabilitation program to help build physiologic reserve prior to undergoing the surgical stressors introduced by transplantation, and hopefully prevent long-term adverse outcomes and related costs.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
McAdams-DeMarco MA, James N, Salter ML, Walston J, Segev DL. Trends in kidney transplant outcomes in older adults. J Am Geriatr Soc. 2014;62(12):2235.
McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL. Early hospital readmission after kidney transplantation: patient and center-level associations. Am J Transplant. 2012;12(12):3283–8.
McAdams-DeMarco MA, Bae S, Chu N, et al. Dementia and Alzheimer’s disease among older kidney transplant recipients. J Am Soc Nephrol. 2016.
Salter M, Liu J, Bae S, et al. Fractures and associated graft loss and mortality among older kidney transplant recipients. J Am Geriatr Soc, under revision.
Meier-Kriesche HU, Ojo AO, Hanson JA, Kaplan B. Exponentially increased risk of infectious death in older renal transplant recipients. Kidney Int. 2001;59(4):1539–43.
Sprangers B, Nair V, Launay-Vacher V, Riella LV, Jhaveri KD. Risk factors associated with post-kidney transplant malignancies: an article from the Cancer-Kidney International Network. Clin Kidney J. 2018;11(3):315–29.
Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, et al. The lifetime risk of stroke: estimates from the Framingham study. Stroke. 2006;37(2):345–50.
Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease frailty, dialysis initiation, & mortality in ESRD. Arch Intern Med. 2012;1.
McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901.
Painter P, Kuskowski M. A closer look at frailty in ESRD: getting the measure right. Hemodial Int. 2013;17(1):41–9.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–57.
Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, et al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–6.
Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401.
Taylor HL, Jacobs DR, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–55.
McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99:(4):805–10.
McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15(1):149–54.
Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427–34.
Brown JC, Harhay MO, Harhay MN. Appendicular lean mass and mortality among prefrail and frail older adults. J Nutr Health Aging. 2017;21(3):342–5.
Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60(3):464–70.
Eichholzer M, Richard A, Walser-Domjan E, Linseisen J, Rohrmann S. Urinary phytoestrogen levels and frailty in older American women of the National Health and Nutrition Examination Survey (NHANES) 1999-2002: a cross-sectional study. Ann Nutr Metab. 2013;63(4):269–76.
Park SK, Richardson CR, Holleman RG, Larson JL. Frailty in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003-2006). Heart Lung. 2013;42(3):163–70.
Eichholzer M, Barbir A, Basaria S, Dobs AS, Feinleib M, Guallar E, et al. Serum sex steroid hormones and frailty in older American men of the third National Health and nutrition examination survey (NHANES III). Aging Male. 2012;15(4):208–15.
Wilhelm-Leen ER, Hall YN, M KT, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122(7):664.
Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clin Geriatr Med. 2011;27(1):79–87.
Walston J, Fried LP. Frailty and the older man. Med Clin N Am. 1999;83(5):1173–94.
Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64(10):1049.
Xue QL, Tian J, Fried LP, Kalyani RR, Varadhan R, Walston JD, et al. Physical frailty assessment in older women: can simplification be achieved without loss of syndrome measurement validity? Am J Epidemiol. 2016;183(11):1037–44.
Walston JD. Connecting age-related biological decline to frailty and late-life vulnerability. Nestle Nutr Inst Workshop Ser. 2015;83:1.
Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al. Frailty: an emerging research and clinical paradigm–issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62(7):731–7.
Ferrucci L, Windham BG, Fried LP. Frailty in older persons. Genus. 2005;61(1):39–53.
Varadhan R, Chaves PH, Lipsitz LA, et al. Frailty and impaired cardiac autonomic control: new insights from principal components aggregation of traditional heart rate variability indices. J Gerontol A Biol Sci Med Sci. 2009;64(6):682.
Chaves PH, Varadhan R, Lipsitz LA, et al. Physiological complexity underlying heart rate dynamics and frailty status in community-dwelling older women. J Am Geriatr Soc. 2008;56(9):1698–703.
Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci. 2008;63(2):190–5.
Leng SX, Tian X, Matteini A, Li H, Hughes J, Jain A, et al. IL-6-independent association of elevated serum neopterin levels with prevalent frailty in community-dwelling older adults. Age Ageing. 2011;40(4):475–81.
Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–71.
Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered dynamics of circulating energy metabolism hormones after oral glucose in older women. J Nutr Health Aging. 2012;16(8):679–86.
Weiss CO, Cappola AR, Varadhan R, Fried LP. Resting metabolic rate in old-old women with and without frailty: variability and estimation of energy requirements. J Am Geriatr Soc. 2012;60(9):1695–700.
Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci. 2012;67(12):1300–6.
Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50(7):1268–71.
Leng SX, Yang H, Walston JD. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin Exp Res. 2004;16(3):249–52.
Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61.
Adlam T, Ulrich E, Kent M, Malinzak L. Frailty testing pilot study: pros and pitfalls. J Clin Med Res. 2018;10(2):82–7.
Schopmeyer L, El Moumni M, Nieuwenhuijs-Moeke GJ, Berger SP, Bakker SJL, Pol RA. Frailty has a significant influence on postoperative complications after kidney transplantation-a prospective study on short-term outcomes. Transpl Int. 2019;32(1):66–74.
McAdams-DeMarco MA, Ying H, Olorundare I, et al. Individual frailty components and mortality in kidney transplant recipients. Transplantation. 2017;101(9):2216–32.
McAdams-DeMarco MA, Isaacs K, Darko L, et al. Changes in frailty after kidney transplantation. J Am Geriatr Soc. 2015;63(10):2152–7.
McAdams-DeMarco MA, Olorundare IO, Ying H, et al. Frailty and post kidney transplant health-related quality of life. Transplantation. 2017.
Chu NM, Gross AL, Shaffer A, et al. Frailty and changes in cognitive function after kidney transplantation—failure to recover to baseline levels. Under Review in J Am Soc Nephrol. 2019.
Warsame F, Haugen CE, Ying H, Garonzik-Wang JM, Desai NM, Hall RK, et al. Limited health literacy and adverse outcomes among kidney transplant candidates. Am J Transplant. 2018.
•• Haugen CE, Mountford A, Warsame F, et al. Incidence, risk factors, and sequelae of post-kidney transplant delirium. J Am Soc Nephrol. 2018;29(6):1752 Frail kidney transplant (KT) recipients are particularly vulnerable to surgical stressors resulting in delirium, an acute decline and fluctuation in cognitive function; 20.0% of frail recipients aged ≥ 75 experienced delirium. These frail recipients with delirium had an increased risk of ≥2 week length of stay, institutional discharge (discharge to skilled nursing facility or rehabilitation center), mortality, and graft loss.
Konel JM, Warsame F, Ying H, et al. Depressive symptoms, frailty, and adverse outcomes among kidney transplant recipients. Clin Transpl. 2018;32(10):e13391.
McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, length of stay, and mortality in kidney transplant recipients: a national registry and prospective cohort study. Ann Surg. 2017;266(6):1084–90.
Haugen CE, Mountford A, Warsame F, et al. Incidence, risk factors, and sequelae of post-kidney transplant delirium. J Am Soc Nephrol. 2018;29(6):1752–9.
Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147(2):190–3.
McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13(8):2091–5.
Michelson AT, Tsapepas DS, Husain SA, Brennan C, Chiles MC, Runge B, et al. Association between the “timed up and go test” at transplant evaluation and outcomes after kidney transplantation. Clin Transpl. 2018;32:e13410.
•• Nastasi AJ, McAdams-DeMarco MA, Schrack J, et al. Pre-Kidney Transplant Lower Extremity Impairment and Post-Kidney Transplant Mortality 2018;18(1):189–196. This study suggests that frailty is distinct from lower extremity impairment. Using the Short Physical Performance Battery identifies a different group of vulnerable KT recipients than the PFP.
Nastasi AJ, Bryant TS, Le JT, et al. Pre-kidney transplant lower extremity impairment and transplant length of stay: a time-to-discharge analysis of a prospective cohort study. BMC Geriatr. 2018;18(1):246.
Rumer KK, Saraswathula A, Melcher ML. Prehabilitation in our most frail surgical patients: are wearable fitness devices the next frontier? Curr Opin Organ Transplant. 2016;21(2):188–93.
Cheng XS, Myers JN, Chertow GM, et al. Prehabilitation for kidney transplant candidates: is it time? Clin Transplant, 2017. 31(8).
• McAdams-DeMarco MA, Ying H, Van Pilsum Rasmussen S, et al. Prehabilitation prior to kidney transplantation: preliminary results from a pilot study. Clin Transpl. 2019. This is the first study of prehabilitation in KT recipients. Frail recipients were able to participate in prehabilitation and prehabilitation was associated with increased physical activity. There was initial evidence that prehabilitation was associated with a decreased length of stay post-KT.
Puts MT, Toubasi S, Andrew MK, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017;46(3):383–92.
Myers JN, Fonda H. The impact of fitness on surgical outcomes: the case for prehabilitation. Curr Sports Med Rep. 2016;15(4):282–9.
Gillis C, Carli F. Promoting perioperative metabolic and nutritional care. Anesthesiology. 2015;123(6):1455–72.
Gillis C, Loiselle SE, Fiore JF, Jr., et al. Prehabilitation with whey protein supplementation on perioperative functional exercise capacity in patients undergoing colorectal resection for cancer: a pilot double-blinded randomized placebo-controlled trial. J Acad Nutr Diet. 2016;116(5):802–12.
Gillis C, Loiselle SE, Fiore JF Jr, Awasthi R, Wykes L, Liberman AS, et al. Prehabilitation with whey protein supplementation on perioperative functional exercise capacity in patients undergoing colorectal resection for cancer: a pilot double-blinded randomized placebo-controlled trial. J Acad Nutr Diet. 2016;116(5):802–12.
Tsimopoulou I, Pasquali S, Howard R, et al. Psychological prehabilitation before cancer surgery: a systematic review. Ann Surg Oncol. 2015;22(13):4117–23.
Van Pilsum RS, Konel J, Warsame F, et al. Engaging clinicians and patients to assess and improve frailty measurement in adults with end stage renal disease. BMC Nephrol. 2018;19(1):8.
Funding
This study was supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) grant numbers: R01DK114074 (PI: McAdams-DeMarco), K24DK101828 (PI: Segev), and the National Institute on Aging (NIA) grant numbers: R01AG042504 (PI: Segev), K01AG043501 (PI: McAdams-DeMarco), and R01AG055781 (PI: McAdams-DeMarco). Mara A. McAdams-DeMarco was also supported by the Johns Hopkins University Claude D. Pepper Older Americans Independence Center (P30AG021334).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mara McAdams-DeMarco, Nadia Chu, and Dorry Segev declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Frailty and Gerontology
Rights and permissions
About this article
Cite this article
McAdams-DeMarco, M.A., Chu, N.M. & Segev, D.L. Frailty and Long-Term Post-Kidney Transplant Outcomes. Curr Transpl Rep 6, 45–51 (2019). https://doi.org/10.1007/s40472-019-0231-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-019-0231-3