Abstract
This article presents a study on the rheological behavior of shear thickening fluids (STF) produced with various dispersing mediums selected according to their molecular weight, number of hydroxyl groups, branching and chain length. STF is non-Newtonian fluid behavior in which the increase in the viscosity increases with the applied shear rate. Polyethylene glycol 200, polyethylene glycol 400, 1,2-propanediol, tetraethyl orthosilicate, monoethanolamine, glycerin and diethylene glycol were used as the dispersing mediums. The steady-state rheological properties of STFs were investigated. After the rheological test, STF produced with glycerin with a concentration of 27.5% by weight gave better rheological results than STFs produced with other liquids. Although glycerin has a relatively low molecular weight, it appears that the rheological properties of glycerin are better because the STF produced with glycerin has three hydroxyl groups (3 OH−) in its structure. The critical shear rate of STF at 27.5% concentration by weight produced with glycerin is 2.39 1/s, and the peak viscosity is 732.4 Pa·s. Additionally, the weight concentration effect was also investigated. STFs were produced with polyethylene glycol 400 and 1,2-propanediol liquids at concentrations of 20%, 22.5%, 25% and 27.5% by weight. When the rheological properties of these STFs were examined, it was seen that the rheological properties improved as the concentration amount increased. For this reason, it may not be sufficient to consider only the molecular weight when choosing the dispersing medium during STF production. In addition to the molecular weight, it is necessary to consider the number of hydroxyl groups, branching and the chain length.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
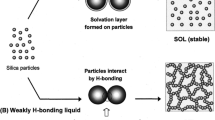
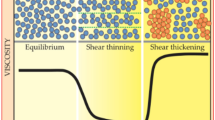
Shear thickening fluids (STFs), or dilatant fluids, are steady non-Newtonian fluids in which viscosity increases with shear rate [1, 2]. When the shear rate reaches a critical value, it exhibits a significant increase in viscosity and can be restored when the shear load is removed. [3, 4]. Due to these fluids reversible properties and excellent energy absorption and impact resistance capacities have been used in various high-tech fields, such as increasing the ballistic and blade impact resistance of flexible body armor and industrial equipment [5,6,7,8]. Recently, many scientific studies have been conducted to understand the rheological behavior of STFs and to use them in engineering applications. Besides scientific articles, numerous patent applications utilize STFs in various fields, such as sports equipment, medical instruments and machine assembly [9, 10].
The rheological behavior of STFs varies systematically according to numerous factors [11]. Among these factors, particle size, particle shape, dispersing medium and ambient temperature come to the fore [12,13,14]. The dispersing medium that makes up the STF significantly influences the rheological properties [15, 16]. When we look at the literature in general, it is seen that polyethylene glycol (PEG) is used as the dispersing medium [17, 18]. There are many varieties of PEG used to produce STF. PEG varieties are named according to their molecular weight. For example, it can be seen as PEG 200, PEG 400 and PEG 600 [19, 20]. The viscosity of the dispersing medium is controlled by its molecular weight, and the higher its molecular weight, the higher the viscosity to be delivered. As the molecular weight of the dispersing medium in shear thickening fluids decreases, the critical shear rate will be higher. This means the shear thickening behavior will occur later [21, 22]. In the study conducted by Qin et al., it was observed that the critical shear deformation rate slightly decreased as the molecular weight of PEG increased. In contrast, the critical viscosity and thickening ratio increased [23]. In many studies, using PEG as a dispersing medium to obtain STF limits the variety of liquids that can be used. In addition, another disadvantage of the PEG preference is that the branching structure and hydroxyl group number are the same in PEG varieties, so the effect of these parameters is not understood. Selection processes were conducted only according to their molecular weights.
In addition to the molecular weights of the dispersing medium, it has been observed that the polymeric chain lengths, branching structure and hydroxyl group number of these fluids will affect the rheological behavior [24,25,26,27]. As seen in the study of Liu et al., the polymer chain length increases as the molecular weight of pure PEG increases. Long polymer chains cause interaction between particles. Therefore, it facilitates the aggregation of particles. Thus, particle clumps can be formed even at lower shear rates, reducing the critical shear rate [15, 16].
In the present study, we have selected various liquids in addition to PEG while considering other factors that may affect the dispersing medium, to enhance the diversity of the liquid phase. The effects of the selected dispersing medium on rheological properties will be compared.
2 Materials and methods
2.1 Materials
The properties of nano-silica (SiO2) particles used in the study are given in Table 1. Silica was obtained from Nanografi® (Turkey). Using scanning electron microscopy (SEM), Fig. 1 shows that the silicas have a spherical shape. The diameter of nano-silica particles varies between 55 and 75 nm (nm). Four different dispersing mediums were used in this study. Polyethylene glycol 200, polyethylene glycol 400, 1,2-propanediol, tetraethyl orthosilicate, monoethanolamine, glycerin and diethylene glycol with molecular weights varying between 60 and 400 g/mol were used as these medium liquids. The properties and chain structures of these dispensing mediums are given in Table 2.
2.2 Preparation of STFs
Before producing the STF, nano-silica particles from the structures forming the STF were dried in a vacuum oven at 100 °C for approximately 12 h. It was observed that the silicas were mostly agglomerated by bonding among themselves when the production was attempted without drying. The moisture-absorbing property of silica can explain the reason for this. Silicas were added carefully and slowly into the dispersing mediums as a further step to prevent aggregation (the silica bonding among themselves). The STFs in Table 3 were produced by mixing nano-silica and liquids with a mechanical mixer for approximately two hours. Concentration amounts by mass were adjusted with a precision balance. There are 20%, 22.5%, 25% and 27.5% nano-silica in these formed STFs. All produced STFs were heated in a vacuum oven for 20 min at 70 °C to remove air bubbles that occurred during production.
2.3 Characterization of STFs
Rheological tests were carried out with Thermo-Haake Mars III at room temperature. C 354 Ti with a diameter of 35 mm and an angle of 4° was used as the measuring probe, and measurements were taken in a conical plate configuration. Analysis was started by placing an 0.8 ml sample between the cone and plate with an automatic pipette. Before beginning the analysis, pre-shear was applied for 60 s at a low shear rate of 0.1 1/s to prepare the sample for measurement. After 60 s, the shear process started in the range of 0.1–1000 1/s. After the measurements, “viscosity” and “shear rate” values were taken.
Fourier transform infrared spectroscopy (FTIR) was used to characterize the molecular bonding of the produced STFs. PerkinElmer brand 400 FT-IR/FT-FIR spectrometer spotlight was analyzed in the 400–4000 cm−1 wave number range using the 400-imaging system model.
3 Results and discussion
3.1 Rheological properties of STF
In the literature, polyethylene glycol (PEG), generally preferred in forming STF, exhibits a Newtonian behavior. However, when PEG bonds with silica particles, the thickening mechanism becomes active and exhibits non-Newtonian behavior [28,29,30,31]. This study used seven dispersing mediums: polyethylene glycol 400 (PEG 400), polyethylene glycol 200 (PEG 200), 1,2-propanediol, tetraethyl orthosilicate, monoethanolamine, glycerin and diethylene glycol. The logarithmic scale rheological curves of the produced STFs are shown in Figs. 2, 3, 4, 5 and 6. In addition to PEG, six other dispersing mediums were preferred based on chain length, hydroxyl group content, branching structure and molecular weight. The percent by mass of the produced STFs is shown in Table 3. First, STFs containing 20%, 22.5%, 25% and 27.5% by mass silica were produced with PEG 400.
Shear thickening behavior with increasing shear rate is shown in Fig. 3. With the increase in shear rate, STFs produced with PEG 400 fluid first exhibited shear thinning behavior (decrease in viscosity). The release of hydroclusters can explain the reason for this formation by bonding the dispensing medium and particle groups under a critical shear rate [32, 33]. In addition, it is known that the change in the concentration by mass of the nanoparticle forming the STF affects the rheological properties [34,35,36]. Consistent with the literature in the study, as the concentration of STFs formed with PEG 400 increases (from 20% to 27.5%), the critical shear rate decreases from 3.06 1/s to 1.78 1/s. This decrease shows that the shear thickening feature appears earlier. In addition, the peak viscosity value increases from 31.9 Pa·s to 52.9.
The rheological properties of STF prepared with 1,2-propanediol at 20%, 22.5% and 27.5% by mass are given in the graph in Fig. 3. As in the STF made with PEG 400, a decrease in the critical shear rate and an increase in the peak viscosity was observed with the increase in the concentration amount in this fluid. As the concentration by mass increased, the critical shear rate decreased from 474.9 1/s to 11.1 1/s. Peak viscosity increased from 3.93 Pa·s to 914.9 Pa·s.
When the STF produced with 1,2-propanediol has a concentration of 20% by mass and the STF produced with PEG 400 with a concentration of 20% by mass, it is seen that the rheological properties of the STF produced with PEG 400 are better. This can be explained by the fact that the molecular weight of PEG 400 (400 g/mol) is higher than that of 1,2-propanediol (76.09 g/mol). As the mass concentration increased, the rheological properties of STF produced with both liquids improved, as shown in Fig. 4. When the concentration value was increased from 20% to 27.5% by mass, the critical viscosity value of the STFs produced with both liquids was compared. While the increase was 171.9% for PEG 400, it was 1287.2% for STF produced with 1,2-propanediol.
Although the molecular weight of PEG 400 is 525.6% higher than 1,2-propanediol, the rheological properties of STF produced with 1,2-propanediol at a concentration of 27.5% by mass are better. It is clearly seen that as the concentration amount increases, the shear thickening behavior in the STF created with 1,2-propanediol liquid becomes more pronounced. In Fig. 5, the rheological properties of STFs produced with PEG 200, PEG 400, glycerin, diethylene glycol and 1,2-propanediol media liquids with 27.5% concentration are compared. Among these liquids, glycerin has the lowest critical shear rate (2,39 1/s). In other words, the shear thickening feature appeared earlier than other liquids.
The rheological properties of PEG 400 and diethylene glycol, which have the same number of hydroxyl groups, are compared in Fig. 5. It is seen that the peak viscosity of PEG 400 (52.9 Pa·s) is higher than that of diethylene glycol (15.3 Pa·s) due to the higher molecular weight of PEG 400 than diethylene glycol (approximately 4 times).
The rheological properties of STFs produced with 20% by the mass concentration of PEG 400, 1,2-propanediol, tetraethoxysilane (ethyl silicate) and monoethanolamine liquids are compared in Fig. 6. STF produced with monoethanolamine liquid could not exhibit shear thickening behavior. According to the graph, the viscosity of the liquid decreased as the shear rate increased. It showed shear thinning behavior. Initial viscosity is also seen as the highest viscosity (537.5 Pa·s). STFs formed with the other three fluids showed shear thickening behavior. STF formed with tetraethoxysilane (0.61 1/s) reached a critical value at a lower shear rate than STF formed with PEG 400 (184.1 1/s) and 1,2-propanediol (474.9 1/s). However, it did not show a sudden increase in viscosity as in PEG 400 and 1,2-propanediol liquids. This is because the silica-formed STF in the tetraethoxysilane structure also activates the particle–particle and the particle-–liquid interactions. In other words, since the solution layer on the surface of the silica particles disappeared, a sudden increase in viscosity could not be observed because a complete hydrocluster was not formed [16].
3.2 FTIR analysis results
The bonds formed by the STFs produced with a mass concentration of 20% using seven different dispersing mediums in the wave number range of 400–4000 cm-1 are supported by FTIR analyses in Fig. 7. These seven liquids are polyethylene glycol 400 (A), 1,2-propanediol (B), tetraethoxysilane (ethyl silicate) (C), monoethanolamine (D), polyethylene glycol 200 (E), glycerin (F) and diethylene glycol (G). Characteristic peaks of STF produced with liquid A were observed at 948, 1110, 2871 and 3357 cm−1. Of these, the stretching vibrations at 1106 cm−1 belong to the C–O functional groups, while those at 3357 cm−1 belong to the O–H functional groups. The stretching vibrations at 2871 and 950 cm−1 correspond to the –CH2 groups of liquid A. The bands observed around 792 cm−1 are attributed to a Si–O–Si bending vibration [40, 41]. In the IR spectrum of liquid B, the absorption of the carbon–hydrogen (CH) sp3 group can also be seen in the range of 2960 to 2850 cm−1. The peak of O–H functional groups was observed in 3348 cm−1. The common point of STFs formed with A, B, D, E, F and G fluids is that they peak around 3400 cm−1 in dispersing mediums. This proved that the hydroxyl group was present. [37, 42].
There is no hydroxyl group in the STF produced with liquid C. Typical leading peaks between 2975 and 2880 cm-1 represent C–H stretching in ester groups. The asymmetrical bending of the C–H bonds explains the peaks between 1443 and 1296 cm-1. The peaks at 1168 and 968 cm−1 belong to the C–H swing. The ethoxy group attached to the silicon atom (Si–OCH2CH3) is characterized by peaks around 1100 cm−1 [43]. In the spectrum of STF produced with liquid D, the band at 1610 cm−1 is attributed to the amino group, and the band at 3175 cm−1 is attributed to the hydroxyl group [44].
3.3 Influence of chain branching, chain length and hydroxyl group of dispersing media on the rheological property of STFs
The study shows that the rheological properties of STFs produced with polyethylene glycol 400 liquid at 20% concentration by mass are better than STFs produced with 1,2-propanediol liquid at 20% concentration by mass. Although the molecular weight of 1,2-propanediol (76.09 g/mol) is lower than that of PEG 400 liquid (400 g/mol), it has been observed that the rheological properties of the STFs produced with 1,2-propanediol are better when looking at the STFs at concentrations of 22.5%, 25% and 27.5% by mass. The reason for this may be that the 1,2-propanediol structure is in a branched form. It has been observed that the branched form blocks the movement of particles in the dispersing medium, enabling shear thickening behavior to occur earlier [42].
Another factor affecting rheological properties is the excess number of hydroxyl groups. When Fig. 5 is examined, it can be seen that the rheological properties of STF produced with Glycerin are better than other liquids. The main reason for this is that glycerin liquid contains more hydroxyl groups (3–OH) in its chemical chain, allowing it to form more bonds with silica. As shown in Fig. 8, silica nanoparticles can form bonds with hydroxyl groups present in the liquid. The chemical structure of glycerin contains more hydroxyl groups compared to PEG 200, PEG 400, 1,2-propanediol and diethylene glycol, which have two hydroxyl groups (2–OH). This indicates that there are more opportunities for these groups to combine with nano-silica particles and form a network structure (hydroclusters) [37,38,39].
Tetraethoxysilane liquid does not contain hydroxyl groups, but its molecular weight (208.33 g/mol) is relatively high. This liquid reacts with silica dioxide (SiO2) to form orthosilicate esters. This reaction can occur under physical conditions such as temperature or pressure without adding catalysts. This reaction causes the silica dioxide to be broken down into monomer components, which combine with tetraethoxysilane liquid to form silicate polymer structures. It is the liquid with the highest molecular weight after PEG 400 and has a relatively long chain length. As a rheological property, it showed shear thickening behavior earlier than other liquids. However, there was no usual sudden increase in viscosity.
Monoethanolamine liquid has the lowest molecular weight (61.08 g/mol) among the liquids used. It contains a hydroxyl and amine group in its structure. The hydroxyl group in the monoethanolamine liquid is potentially closed to bond with the nano-silica particle. Silica is a silicate compound and contains hydroxyl groups, so it is possible to bond with silica. However, suitable conditions must be created for the monoethanolamine liquid to form a bond with the silica. The most important among these is the appropriateness of factors such as pH level, silica concentration, temperature and reaction time. If these conditions are not met, the bond will not be established. For this reason, STF produced with monoethanolamine liquid could not show shear thickening behavior. On the contrary, it exhibited shear thinning behavior as the shear rate increased.
It is known that the dispersing medium affects the rheological properties as a result of changing the polymeric chain length [27]. In order to make a comparison, the rheological properties of STFs produced with PEG 200 and PEG 400 liquids at 27.5% concentration by weight were examined, as shown in Fig. 5. STF produced with PEG 400, which has a longer polymeric chain, showed better rheological results than STF produced with PEG 200. When the results were examined, the peak viscosity of STF produced with PEG 400 was 52.9 Pa·s, while the peak viscosity of STF produced with PEG 200 was 9.62. This means that the peak viscosity of STF produced with PEG 400 increased by 549.8% compared to STF produced with PEG 200.
4 Conclusion
In this study, various dispersing mediums were selected to increase the fluids' diversity while producing STF. These dispersing mediums were chosen by looking at the molecular weight and considering the chain branching, chain length and hydroxyl group numbers. The contribution of STFs formed with these liquids to the rheological properties was investigated. The rheological results examined critical shear rate, critical viscosity and the highest viscosity value. According to these results, despite having a relatively low molecular weight, the rheological properties of STFs produced with glycerin liquid at concentrations of 27.5% (critical shear rate of 2.39 1/s, max. viscosity of 732.4 Pa·s), which have a higher number of hydroxyl groups, are better than STFs produced with other dispersing mediums.
STFs were produced with 1,2-propanediol liquid at concentrations of 20%, 22.5%, 25% and 27.5% by weight. It was observed that rheological properties improved when all parameters remained constant and the concentration by weight was increased 22.5% (critical shear rate of 22.8 1/s, max. viscosity of 63.3 Pa·s), 25% (critical shear rate of 15.6 1/s, max. viscosity of 134.5 Pa·s), and compared to other concentrations, 1,2-propanediol liquid and 20% (critical shear rate 474.9 1/s, max. viscosity 3.93 Pa·s) concentration of STF showed shear thickening later. This can be attributed to the fact that the hydroclustering mechanism is not fully formed in the entire structure since the initial viscosity is low. However, as its concentration increases, it forms more bonds due to the presence of hydroxyl groups in its structure and its branched structure. This situation revealed the hydrocluster mechanism more prominently.
STF produced with liquid C has a relatively high molecular weight (208.33 g/mol), but does not contain hydroxyl groups. Compared to other liquids, STF produced with liquid C showed the thickening property at the lowest shear rate (0.61 1/s). Here, long silicate polymer structures have been the highlight of the C fluid. However, liquid C did not show a sudden increase in viscosity at the critical shear rate. If only the molecular weight of the dispersing medium was considered, it was expected that the improvement in the rheological properties of the STFs produced with A liquid would be higher as the mass concentration increased. However, it has been observed that the improvement in the STFs produced with B liquid is higher. Considering the critical viscosity value, the increase was 171.9% for STF produced with liquid A, while 1287.2% for STF produced with liquid B.
It has been observed that the dispersing medium with longer polymeric chain length improves the rheological properties. It was determined that PEG 400 increased the peak viscosity by 549.8% compared to PEG 200.
References
Barnes HA (1989) Shear-thickening (“Dilatancy”) in suspensions of nonaggregating solid particles dispersed in Newtonian liquids. J Rheol 33(2):329–366. https://doi.org/10.1122/1.550017
Bossis G, Brady JF (1989) The rheology of Brownian suspensions. J Chem Phys 91(3):1866–1874. https://doi.org/10.1063/1.457091
Wagner NJ, Brady JF (2009) Shear thickening in colloidal dispersions. Phys Today 62(10):27–32. https://doi.org/10.1063/1.3248476
Lee YS, Wagner NJ (2006) Rheological properties and small-angle neutron scattering of a shear thickening, nanoparticle dispersion at high shear rates. Ind Eng Chem Res 45(21):7015–7024. https://doi.org/10.1021/ie0512690
Lu Z, Yuan Z, Chen X, Qiu J (2019) Evaluation of ballistic performance of STF impregnated fabrics under high velocity impact. Compos Struct 227:111208. https://doi.org/10.1016/j.compstruct.2019.111208
Majumdar A, Butola BS, Srivastava A (2014) Development of soft composite materials with improved impact resistance using Kevlar fabric and nano-silica based shear thickening fluid. Mater Des 1980–2015(54):295–300. https://doi.org/10.1016/j.matdes.2013.07.086
Lin K, Liu H, Wei M, Zhou A, Bu F (2018) Dynamic performance of shear-thickening fluid damper under long-term cyclic loads. Smart Mater Struct 28(2):025007. https://doi.org/10.1088/1361-665X/aaf079
Bajya M, Majumdar A, Butola BS, Verma SK, Bhattacharjee D (2020) Design strategy for optimising weight and ballistic performance of soft body armour reinforced with shear thickening fluid. Compos B Eng 183:107721. https://doi.org/10.1016/j.compositesb.2019.107721
Khodadadi A, Liaghat G, Vahid S, Sabet AR, Hadavinia H (2019) Ballistic performance of Kevlar fabric impregnated with nanosilica/PEG shear thickening fluid. Compos B Eng 162:643–652. https://doi.org/10.1016/j.compositesb.2018.12.121
Ding J, Tracey P, Li W, Peng G, Whitten PG, Wallace GG (2013) Review on shear thickening fluids and applications. Text Light Ind Sci Technol 2(4):161–173
Gürgen S, Kuşhan MC, Li W (2017) Shear thickening fluids in protective applications: a review. Prog Polym Sci 75:48–72. https://doi.org/10.1016/j.progpolymsci.2017.07.003
Zarei M, Aalaie J (2020) Application of shear thickening fluids in material development. J Mater Res Technol 9(5):10411–10433. https://doi.org/10.1016/j.jmrt.2020.07.049
Srivastava A, Majumdar A, Butola BS (2012) Improving the impact resistance of textile structures by using shear thickening fluids: a review. Crit Rev Solid State Mater Sci 37(2):115–129. https://doi.org/10.1080/10408436.2011.613493
Hasanzadeh M, Mottaghitalab V (2014) The role of shear-thickening fluids (STFs) in ballistic and stab-resistance improvement of flexible armor. J Mater Eng Perform 23(4):1182–1196. https://doi.org/10.1007/s11665-014-0870-6
Baharvandi HR, Alebooyeh M, Alizadeh M, Heydari MS, Kordani N, Khaksari P (2016) The influences of particle–particle interaction and viscosity of carrier fluid on characteristics of silica and calcium carbonate suspensions-coated Twaron® composite. J Exp Nanosci 11(7):550–563. https://doi.org/10.1080/17458080.2015.1094190
Liu XQ, Bao RY, Wu XJ, Yang W, Xie BH, Yang MB (2015) Temperature induced gelation transition of a fumed silica/PEG shear thickening fluid. RSC Adv 5(24):18367–18374. https://doi.org/10.1039/C4RA16261G
Lee BW, Kim IJ, Kim CG (2009) The influence of the particle size of silica on the ballistic performance of fabrics impregnated with silica colloidal suspension. J compos mater. https://doi.org/10.1177/0021998309345292
Mawkhlieng U, Majumdar A (2019) Deconstructing the role of shear thickening fluid in enhancing the impact resistance of high-performance fabrics. Compos B Eng 175:107167. https://doi.org/10.1016/j.compositesb.2019.107167
Antosik A, Gluszek M, Zurowski R, Szafran M (2017) Influence of carrier fluid on the electrokinetic and rheological properties of shear thickening fluids. Ceram Int 43(15):12293–12301. https://doi.org/10.1016/j.ceramint.2017.06.092
Gürgen S, Sofuoğlu MA, Kuşhan MC (2020) Rheological modeling of multi-phase shear thickening fluid using an intelligent methodology. J Braz Soc Mech Sci Eng 42(11):1–7. https://doi.org/10.1007/s40430-020-02681-z
Shenoy SS, Wagner NJ (2005) Influence of medium viscosity and adsorbed polymer on the reversible shear thickening transition in concentrated colloidal dispersions. Rheol acta. https://doi.org/10.1007/s00397-004-0418-z
Moriana AD, Tian T, Sencadas V, Li W (2016) Comparison of rheological behaviors with fumed silica-based shear thickening fluids. Korea-Aust Rheol J 28(3):197–205. https://doi.org/10.1007/s13367-016-0020-9
Qin J, Zhang G, Shi X (2017) Study of a shear thickening fluid: the suspensions of monodisperse polystyrene microspheres in polyethylene glycol. J Dispers Sci Technol 38(7):935–942. https://doi.org/10.1080/01932691.2016.1216435
Khandavalli S, Rothstein JP (2014) Extensional rheology of shear-thickening fumed silica nanoparticles dispersed in an aqueous polyethylene oxide solution. J Rheol 58(2):411–431. https://doi.org/10.1122/1.4864620
Li W, Xiong D, Zhao X, Sun L, Liu J (2016) Dynamic stab resistance of ultra-high molecular weight polyethylene fabric impregnated with shear thickening fluid. Mater Des 102:162–167. https://doi.org/10.1016/j.matdes.2016.04.006
Gong X, Xu Y, Zhu W, Xuan S, Jiang W, Jiang W (2013) Study of the knife stab and puncture-resistant performance for shear thickening fluid enhanced fabric. J Compos Mater 48(6):641–657. https://doi.org/10.1177/0021998313476525
Li D, Wang R, Liu X, Zhang S, Fang S, Yan R (2020) Effect of dispersing media and temperature on inter-yarn frictional properties of Kevlar fabrics impregnated with shear thickening fluid. Compos Struct 249:112557. https://doi.org/10.1016/j.compstruct.2020.112557
Manukonda BH, Chatterjee VA, Verma SK, Bhattacharjee D, Biswas I, Neogi S (2020) Rheology based design of shear thickening fluid for soft body armor applications. Period Polytech Chem Eng 64(1):75–84. https://doi.org/10.3311/PPch.13626
Liu L, Yang Z, Zhao Z, Liu X, Chen W (2020) The influences of rheological property on the impact performance of kevlar fabrics impregnated with SiO2/PEG shear thickening fluid. Thin-Walled Struct 151:106717. https://doi.org/10.1016/j.tws.2020.106717
Baharvandi HR, Saeedi Heydari M, Kordani N, Alebooyeh M, Alizadeh M, Khaksari P (2017) Characterization of the rheological and mechanical properties of shear thickening fluid-coated Twaron® composite. J Text Inst 108(3):397–407. https://doi.org/10.1080/00405000.2016.1168091
Hassan TA, Rangari VK, Jeelani S (2010) Synthesis, processing and characterization of shear thickening fluid (STF) impregnated fabric composites. Mater Sci Eng A 527(12):2892–2899. https://doi.org/10.1016/j.msea.2010.01.018
He Z, Xuan H, Bai C, Hu Y, Cong P, Bai H, Hong W (2017) Containment tests and analysis of soft wall casing fabricated by wrapping Kevlar fabric around thin metal ring. Aerosp Sci Technol 61:35–44. https://doi.org/10.1016/j.ast.2016.11.018
McMillan A. (2008). Material development for fan blade containment casing. In: Journal of physics: conference series (Vol. 105, No. 1, p. 012011). IOP Publishing. https://doi.org/10.1088/1742-6596/105/1/012011
Baharvandi HR, Alebooyeh M, Alizadeh M, Khaksari P, Kordani N (2016) Effect of silica weight fraction on rheological and quasi-static puncture characteristics of shear thickening fluid-treated Twaron® composite. J Ind Text 46(2):473–494. https://doi.org/10.1177/1528083715589750
Zhang Q, Qin Z, Yan R, Wei S, Zhang W, Lu S, Jia L (2021) Processing technology and ballistic-resistant mechanism of shear thickening fluid/high-performance fiber-reinforced composites: a review. Compos Struct 266:113806. https://doi.org/10.1016/j.compstruct.2021.113806
Majumdar A, Butola BS, Srivastava A (2013) Optimal designing of soft body armour materials using shear thickening fluid. Mater Des 46:191–198. https://doi.org/10.1016/j.matdes.2012.10.018
Ghosh A, Chauhan I, Majumdar A, Butola BS (2017) Influence of cellulose nanofibers on the rheological behavior of silica-based shear-thickening fluid. Cellulose 24(10):4163–4171. https://doi.org/10.1007/s10570-017-1440-5
Jaishankar A, Wee M, Matia-Merino L, Goh KK, McKinley GH (2015) Probing hydrogen bond interactions in a shear thickening polysaccharide using nonlinear shear and extensional rheology. Carbohydr Polym 123:136–145. https://doi.org/10.1016/j.carbpol.2015.01.006
Ueno K, Imaizumi S, Hata K, Watanabe M (2009) Colloidal interaction in ionic liquids: effects of ionic structures and surface chemistry on rheology of silica colloidal dispersions. Langmuir 25(2):825–831. https://doi.org/10.1021/la803124m
Biçer A, Ahmet SARI (2017) Isıl enerji depolama amaçlı yapıca kararlı yeni bir faz değişim malzemesi olarak silikafume/polietilen glikol (peg) kompoziti. Afyon Kocatepe Üniversitesi Fen Ve Mühendislik Bilimleri Dergisi 17(2):683–690. https://doi.org/10.5578/fmbd.57516
Chakraborty S, Biswas S, Sa B, Das S, Dey R (2014) In vitro & in vivo correlation of release behavior of andrographolide from silica and PEG assisted silica gel matrix. Colloids Surf A 455:111–121. https://doi.org/10.1016/j.colsurfa.2014.04.046
MuliaK KE, Terahadi F, Putri S (2015) Selected natural deep eutectic solvents for the extraction of α-mangostin from mangosteen (Garcinia mangostana L.) pericarp. Int J Technol 6(7):1211–1220
Hadela A, Lakić M, Potočnik M, Košak A, Gutmaher A, Lobnik A (2020) Novel reusable functionalized magnetic cobalt ferrite nanoparticles as oil adsorbents. Adsorpt Sci Technol 38(5–6):168–190. https://doi.org/10.1177/0263617420922014
Corobea MS, Stoenescu M, Miculescu M, Raditoiu V, Fierascu RC, Sirbu I, Voicu SI (2014) Titanium functionalizing and derivatizing for implantable materials osseointegration properties enhancing. Dig J Nanomater Biostruct 9(4):1339–1347
Funding
The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The authors would like to acknowledge funding from the Erciyes University Scientific Research Projects Coordination Unit (BAP) under research Grant No. FDK-2021–11055.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest.
Additional information
Technical Editor: Edson José Soares.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ceylan, A., Ercümen, K.M., Aydin, M. et al. The effect of STFs formed with different dispersing mediums on rheological properties. J Braz. Soc. Mech. Sci. Eng. 46, 142 (2024). https://doi.org/10.1007/s40430-024-04725-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40430-024-04725-0