Abstract
Multi-phase shear thickening fluids (STFs) are mixture of single-phase STFs and specific additives that allow the rheological properties of STFs to be modified for use in a specific application. This chapter presents the rheological behavior of multi-phase STFs and their advantages and disadvantages. To understand the effect of different additives on the rheological behavior of multi-phase STFs, the mechanism of interaction of additives with STF is reported. The main factors affecting the rheological behavior of multi-phase STF are also presented. Generally, the additives depending on their shape, aspect ratio, weight fraction, surface chemistry etc., are responsible for the enhanced shear thickening behavior of multi-phase STF. Finally, some directions for new developments and future work are outlined.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Shear thickening fluid (STF) is a type of non-Newtonian fluid whose viscosity increases as shear rate increases. In other words, at high shear rates, the viscosity of STF rises dramatically, and it behaves like a solid. However, once the impact stress is removed, STF reverts to a liquid-like state as soon as shear is removed. The reversible process is considered as the main favorable feature of STF. This phenomenon is of special interest for both scientific and technological issues in developing engineering applications [1, 2]. Notably, this phenomenon was initially defined as a problem in many industrial processes such as coating and mixing. For instance, shear thickening leads to the failure of mixer motors due to overloading. However, the unique characteristics of the STF have been used in developing smart materials and structures more recently. For instance, STF has been combined with high performance fabrics to provide a uniquely thin, flexible, cost-effective material that is comparable to or even better than the present protective materials in terms of ballistic, stab, and puncture protection. The idea of impregnating fabric with STF to increase the impact resistance of textile structures has recently attracted a lot of attention [3,4,5,6,7,8]. The absorption of the shock waves from the earthquake or severe wind conditions and the integration of STF within the damper systems are other applications of STF that have been recently investigated [9]. STF is recommended for structural components to increase the vibratory and damage resistance of systems and to avoid unexpected accelerations; it should also limit the range of motion in the shoulders, knees, elbows, ankles, and hips [10]. Figure 3.1 depicts the rise of STF-related publications over the past ten years based on the Scopus database.
STF is a suspension of nano- or micro-particles like silica or silicon dioxide in a Newtonian fluid-like water or polyethylene glycol (PEG). This combination produces a material with extraordinary properties. Common types of STF are silica suspended in ethylene glycol (EG) [6], polyvinylchloride in dioctyl phthalate [11], kaolin clay in glycerol [12], poly(methyl methacrylate) (PMMA) in PEG [13], fumed silica in propylene glycol [14, 15], and silica suspended in PEG [16]. Among these, the suspension of silica particles in PEG has been extensively investigated and frequently reported in STF literature (Table 3.1).
Multi-phase STF suspensions with particle additives have recently been fabricated to benefit from additives. In this chapter, the literature on the rheological characteristics of multi-phase STF is thoroughly reviewed. We discuss the shear thickening mechanism in concentrated colloidal suspension and the crucial factors affecting the rheological characteristics of multi-phase STF. We also explain how additional particles like carbon nanotubes, graphene, and nanoparticles affect the rheology of multi-phase STF systems. It is persuaded that a thorough analysis of the rheological characteristics of multi-phase STF is a crucial first step in comprehending the function of multi-phase STFs in the creation of engineering structures.
3.2 Shear Thickening Mechanism
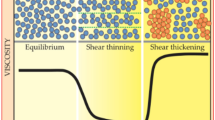
STF, as a non-Newtonian fluid, is usually identified by a drastic increase in viscosity as the applied shear rate reaches a critical value. Figure 3.2 shows a schematic representation of shear thickening behavior. In the equilibrium state, the particles are well dispersed in the medium. STF exhibits reduced viscosities at lower shear rates before the critical point. The chaotic nature of the particles under the applied stress leads to the formation of particle layers as shear rate increases. When shear rate increases to further levels, hydroclusters are formed, thereby causing the particles to agglomerate. After the stress is removed from the medium, the suspension reverts into an easy-flowing state and resumes flowing like any other liquid. The shear thickening phenomenon is fully reversible.
Based on the literature, researchers have brought forth some theories to account for the fundamental reasons behind the shear thickening behavior. The “order-disorder transition” (ODT) [30,31,32,33,34] and the “hydrocluster” mechanism [35, 36] provide a more comprehensive explanation of this behavior than the other ideas. The ODT explains how the application of shear rate causes the particle arrangements to break down in the suspension, increasing the drag forces between the particles. Hoffman [37], one of the pioneers to research shear thickening in depth, created a micromechanical model of shear thickening as a flow-induced ODT based on this information. He suggested that the shear thickening tendency was the result of a change from a layer-ordered easy-flowing state to a disordered state. He claimed that the van der Walls-London, electric double layer, and shear stress forces acting on a collection of particles are responsible for this transformation. Additionally, it was proposed that the particles within the moving layers undergo hydrodynamically induced forces, which will cause the particles to be pushed out of their layer and disturb the orderly flow. When shear rate reaches a critical point, this transition shows a sharp increase in viscosity. Particle interactions in a liquid medium are the generation of the hydrocluster mechanism. Based on this, it can be stated that hydrodynamic lubricating forces between particles are the source of shear thickening behavior in concentrated colloidal suspensions. Hydrodynamic pressure increases when particles suspended in a fluid collide upon applying shear stress. Due to the formation of clusters under shear force, the drag forces between the particles grow stronger. Rheological, rheo-optic, and flow-SANS studies, as well as computer simulations, are used to experimentally support the hydrocluster mechanism [38]. Although the microscopic mechanisms have been proposed to explain the shear thinning phenomenon, the “hydrocluster” mechanism is more accurate and generalized.
Even though shear thinning is thought to be more common than shear thickening, it has been proposed that shear thickening can occur in all dense mixtures under certain conditions [15]. However, one of the major challenges is determining why not all dense mixtures show shear thickening properties. One of the more perplexing questions is why mixtures with soft particles, which can easily deform, do not exhibit shear thickening behavior, whereas densely packed mixtures with hard particles do. A recent research has shown that nanoparticle behavior varies significantly for hard spheres due to strong electrostatic, charge-dipole, dipole-dipole, and van der Waals interactions. In particular, the interactions between the nanoparticles as well as those between the nanoparticle and liquid medium in such systems influence the state of the nanoparticles. The literature indicates that lubricating forces and frictional forces play a substantial role in the shear thickening process. To determine the hydrodynamic and contact force contributions to the shear thickening process, Lin et al. [39] investigated the shear thickening behavior in the interaction of micron-sized silica and latex particles. They found that as the suspension gets thickened, increasing the shear rate causes the contact contribution to growing while the hydrodynamic contribution stays the same. This phenomenon shows how contact forces have a significant impact on the shear thickening of dense colloidal suspensions. Based on the contact rheology models [39, 40], hydrodynamic lubrication forces produce a Newtonian behavior or shear thinning at low shear rates (Zone I) as a result of the insignificant interactions between particles. Lubrication breaks down when the shear rate (Zone II) is increased. As a result, frictional forces result in force chains, which cause discontinuous shear thickening [41]. The force network becomes tighter as shear rate increases (Zone III), eventually reaching a fully shear jammed state. Large clusters are produced when shear rearranges the particles into anisotropic formations. While frictionless contact results in a smooth and reversible viscosity increase associated with continuous shear thickening, frictional hydrodynamic interactions between suspended particles produce large jumps while rapid increase in viscosity associated with strong and discontinuous shear thickening [37].
3.3 STF Rheology
Up till now, several investigations on the rheological characteristics of STF under steady-state and dynamic conditions have been conducted [18, 20]. It has been observed that some influencing elements including particles, flow field, suspending medium, additives, and the interaction between particles and additives affect the STF rheology [15, 21, 42]. Table 3.2 summarizes the key factors influencing the rheological properties. Particle properties (shape, solid volume fraction, size distribution, and interaction with other particles), suspending phase properties (viscosity and molecular weight of carrier liquid), and STF properties are the main factors influencing the rheological properties.
Barnes [15] suggested that above the particle volume fraction of 0.5, the fluid’s behavior changes dramatically with shear rate. The critical shear rate (the shear rate at which shear thickening begins) decreases as the volume fraction of the particle increases. Similarly, Kang et al. [20] investigated the effect of silica loading on the shear thickening properties. They stated that critical shear rate develops at lower shear rates as silica particle concentration increases. Moreover, with increasing the silica particle concentration, the shear thickening phenomenon realizes sharply. Wetzel et al. [43] found that when the aspect ratio of the particles grows, lower volume percentages of particles are sufficient to trigger the shear thickening behavior. According to Maranzano et al. [42] particle size significantly affects the transition from reversible shear thickening to dense colloidal suspensions. They also noted that the flow curves systematically change to decrease shear stresses as particle size increases. Lee et al. [22] also observed a similar pattern of behavior. Kang et al. [20] reported the temperature effect on the critical shear rate and STF viscosity. From this work, as temperature increases, hydrodynamic interactions grow stronger due to the increased Brownian motion with the colloidal particles and, thus, critical shear rate slightly moves toward higher shear rates.
Is there a regulating factor, such as a range above or below which the critical shear rates appear to be lower? As was already mentioned, the solid volume percentage affects how colloidal suspensions thicken under shear. However, due to variations in form, size, size distribution, and interaction with other particles, distinct nanoparticles display different shear thickening rheology. The solid particle amount cannot, therefore, be the only determining factor. Another difficulty is knowing how to change STF characteristics. Creating a straightforward and organized way of showing the interrelated effects of elements is another challenge. There has not been any publication on comprehensive quantitative analysis of effective parameters yet in the literature.
3.4 Rheology of Multi-phase STF
Several studies on the rheological behavior of STF have been conducted up to now. Integrating particle additives into STF and studying the rheological behavior of multi-phase STF have recently received much attention. As mixtures of single-phase STF and different additives, multi-phase STF offers the opportunity to tune the rheological behavior of suspension concerning the application field. For example, Hasanzadeh and Mottaghitalab [44] investigated the use of multi-walled carbon nanotubes (MWCNTs) as an additive to tune the rheological properties of a fumed silica/PEG-based STF. Rheological measurements revealed that MWCNTs have a significant impact on the rheology of STF. Even at low concentrations of MWCNTs (0.4 wt.%), the critical viscosity of the STF decreases with the addition. Furthermore, shear thickening appears at higher shear rates in the multi-phase STF system. In other words, the MWCNTs interfere with the shear thickening behavior. The proposed mechanism of MWCNT incorporation and shear thickening behavior of a multi-phase STF system is depicted in Fig. 3.3. According to this mechanism, the increased interactions between the fumed silica nanoparticles, PEG as a carrier fluid, and MWCNTs are responsible for affecting the shear thickening properties. The silanol groups on the surface of fumed silica nanoparticles form hydrogen bonds with the PEG hydroxyl groups and the internal oxygen atoms. When MWCNTs are added to the STF, the hydroxyl/carboxyl groups on the MWCNT surfaces can form hydrogen bonding with silica particles and PEG. However, hydrogen bonds are more likely to be formed with PEG than with silica particles. Fourier transform infrared spectroscopy (FTIR) confirms that the hydrogen bonding in the MWCNT-silica-PEG suspension enhances. As a result of the strong interactions between MWCNTs and PEG, higher shear force is needed to withstand the strong interactions, and the thickening in the mixture is postponed to higher critical shear rates. Finally, the presence of MWCNTs in the STF reduces the thickening ratio so that the shear thickening properties.
A similar study on the rheological behavior of multi-phase STF with MWCNTs additive was carried out by Wei et al. [45]. They found that the peak viscosity of the multi-phase STF with 1 wt.% MWCNTs increases about 360% and the critical shear rate decreases about 70%. Investigation of the variation of the mass fraction of MWCNT additives shows the irregular change in the viscosity of multi-phase STF. For instance, the multi-phase STF with 0.2 wt.% MWCNTs does not show shear thickening behavior, which is likely due to the hindering of the flow by flocculent structure and the large aspect ratio of MWCNT. Figure 3.4 shows the schematic of the interaction mechanism between silica and MWCNTs. In multi-phase STF with a low mass fraction of MWCNT, a large amount of silica nanoparticles adsorbs on a small amount of MWCNT and forms a larger new particle group containing MWCNT. Due to the no “particle clustering” mechanism, the multi-phase STF (2 wt.% MWCNT) has no obvious shear thickening effect. However, when the mass fraction of MWCNTs increases, the “particle clustering” mechanism develops. The reason is that increasing the mass fraction of MWCNT leads to an increase in the interaction forces between the particles as well as the number of new particles, which decreases the inter-particle distances and thereby hinders their flow. Hence, a significant shear thickening phenomenon occurs. Wei et al. [45] also studied the thermal effect on the rheology of multi-phase STF and found a significant effect of temperature on the shear thickening behavior. The temperature sensitivity of multi-phase STF is similar to that of single-phase STF and dominates by silica nanoparticles. The MWCNT can significantly enhance the shear thickening effect without influencing the temperature sensitivity. At higher temperatures, the shear thickening properties of multi-phase STF weaken due to the poor hydrogen bonds. This phenomenon may be due to the good thermodynamic properties and high thermal conductivity of MWCNTs. Hence, the temperature of each component in the STF is similar and the particle distribution is uniform due to the effective heat transfer at an elevated temperature environment.
The schematic of the mechanism of interaction between silica and MWCNTs in multi-phase STF [45]. Reprinted by permission from Springer
Li et al. [46] studied the shear thickening rheology of a silica and PEG-based STF using oxygen-plasma-modified MWCNTs for improving the quasi-static stab resistance of textiles. They modified the MWCNTs with plasma treatment, due to the van der Waals interactions and the hydrophobicity of MWCNTs, which led to the agglomeration in STF. The rheology of multi-phase STF was investigated in different mass friction of modified-MWCNTs (0.02–0.06 wt.%). The results showed that the multi-phase STF with 0.06 wt.% of modified MWCNTs exhibits an enhanced peak viscosity of about 120%. Similarly, the critical shear rate reduces by 80%. The multi-phase STF displays higher yield stress in comparison with the single-phase STF. The stronger network formed by the modified-MWCNTs enhances the interaction between the M-MWCNTs and silica particles. This is likely due to the presence of oxygen-containing functional groups, such as hydroxyl and carboxyl groups, on the surface of the modified-MWCNTs, which facilitate the formation of hydrogen bonds with silanol groups on the surface of silica. Hence, more interaction between silica particles and modified-MWCNTs leads to the easy formation of “hydrocluster” and consequently shear thickening at a lower shear rate.
In another study by Gürgen et al. [47, 48], various ceramic particles have been used in a silica-based STF. They found that the incorporation of ceramic particles into STF disrupts the thickening behavior. Moreover, the thickening behavior is dependent on the parameters, including the amount and particle size of additives. For example, at higher loadings of ceramic particles, the volume fraction of silica falls below the effective limit and the shear thickening behavior of suspension fades away. They suggested that the incorporation of additive particles to STF may disrupt the thickening behavior in two ways: (i) lowering the silica percentage of particles by adding additives and (ii) shortening the hydrocluster networks along suspensions due to interstitial additive particles. According to the hydrocluster thickening mechanism, the more the extended hydroclusters, the more the powerful shear thickening behavior. The ceramic micro-particles in suspension occupy the large volume in silica nanoparticles medium and thereby hinders the extension of hydroclusters. Hence, in the multi-phase STFs, the contact networks of silica particles are less likely to form than in the single-phase STF. Moreover, the coarser particles have significant effects on the weakening of shear thickening behavior in comparison to finer particles. This is likely attributed to the larger distance between silica particles in the flow induced by the coarser particles. The ceramic particles with smaller sizes have less effect on the hydroclusters and thickening behavior. The results show that the coarser ceramic particles lead to a decrease in the critical shear rate and the thickening properties of the suspension.
The rheological properties of multi-phase STF were also investigated by using graphene oxide (GO) as an additive. Huang et al. [49] prepared a multi-phase STF using fumed silica (15 wt.%) and PEG-based suspension. The multi-phase STFs with various amounts (from 0 to 0.3 wt.%) of GO additives were rheologically measured and compared with the single-phase STF. They found that the addition of GO particles leads to a remarkable increase in the viscosity and a decrease in the critical shear rate. Moreover, the thickening ratio gradually decreases with increasing GO content. These results are similar to the output of ceramic particles and MWCNTs investigated by Gürgen et al. [47, 48] and Hasanzadeh and Mottaghitalab [44] respectively. Due to the sheet-like structure of GO, they have a stronger hydrodynamic field effect than that of the silica particles. Hence, GO additives can cause a stronger congestion effect and, therefore, hydro-clusters can be formed at lower shear rates. Moreover, the strong interaction between the GO additives and silica particles leads to more aggregation of silica nanoparticles on the GO surfaces. However, the incorporation of GO into the STF prevents the hydrocluster elongations and consequently lowers the viscosity increase in the multi-phase STF.
Halloysite nanotubes as an additive (1 to 5 wt.%) in multi-phase STFs have been investigated by Passey [50]. Fumed silica (7 nm) as a solid phase and PEG200 as a liquid medium were utilized for the preparation of the STF. The rheological studies revealed that the halloysite nanotubes disrupt the thickening mechanism of STF and reduce the viscosity of the suspension. They suggested the formation of strong hydrogen bonding between the fumed silica and halloysite nanotubes. The hydrodynamic forces progressively take control of the suspension as shear rate increases, and the nanotubes align themselves in a layered and parallel orientation. The extension of the clusters in the suspension is suppressed by the formation of hydroclusters around the halloysite nanotubes as shear rate is further increased. The inclusion of more halloysite nanotube additives leads to an increase in the critical shear rates. The addition of halloysite nanotubes enhances the surface area of the particles due to the cylindrical morphology and, consequently, more hydrodynamic forces are required for cluster formation. Similarly, Laha et al. [51] studied the multi-phase STF system using halloysite nanotubes in a spherical silica and PEG200-based STF. However, they discovered that adding more halloysite nanotubes to suspensions causes the critical shear rate to drop. The results demonstrated that the halloysite nanotubes enhance the shear thickening behavior. Although Passey [50] and Laha et al. [51] used the same type of additives, the rheology of the suspensions is completely different. Laha et al. [51] discovered that halloysite nanotubes act as bases for the hydroclusters in which the spherical silica particles are collected around the halloysite nanotubes. In the study by Passey [50], the halloysite nanotubes do not attract fumed silica while the additives prevent the hydrocluster formation by intercepting the growing of the silica chains. The interaction between the halloysite nanotubes and silica may differ depending on the shape of the silica particles as the spherical silica may be attractive while the fumed silica may be repulsive to the halloysite nanotubes. However, the particle morphology of silica may not be the only factor influencing the interactions between the silica and additives. Fumed silica, for example, can exist in both hydrophobic and hydrophilic forms, which have a direct impact on the suspension rheology. Hydrophilic silica contains silanol groups, which exhibit enhanced thickening in low polarity media due to strong hydrogen bonds, whereas hydrophobic silica exhibits thickening in high polarity media due to weak hydrogen bonding [52].
Sha et al. [53] studied the rheology of a multi-phase STF using graphene nanoplatelets (GNs) and carbon nanotubes (CNTs). The rheology of the STF with spherical silica in a PEG was investigated as well as the effects of GNs and CNTs as additives. They discovered that the suspension viscosity increases as the additive concentration in the STF rises. Additionally, the viscosity of the multi-phase STF can be increased more effectively by CNTs in comparison to GNs. Because CNTs are shaped like long tubes and PEG has long chemical chains, their relative lubricating forces are stronger than those between GNs and PEG. The GNs play as bridges between the silica particles in a liquid, forming hydrogen bonds and thus connecting the aggregated silica groups. Consequently, shear thinning occurs at lower shear rates. PEG-CNTs have a much weaker internal force than PEG-GNs because GNs are sheet-like additives. As a result, phase separation occurs more easily in the CNT suspensions than in the GN suspensions. Furthermore, CNTs having rigid rod shapes in STF can withstand contact forces and, therefore, provide more advances for shear thickening, whereas GNs with two-dimensional soft structures can be easily deformed. Therefore, CNTs are better for shear thickening behavior than GNs. The coexistence of CNTs and GNs on STF further demonstrates that CNTs predominate over GNs concerning suspensions. The viscosity of suspension increases with an increase in the CNTs/GNs ratio in a multi-phase STF. The results demonstrated the enhanced shear thickening behavior by additive incorporation.
The rheological behavior of a multi-phase STF with the incorporation of cellulose nanofibers (CNFs) was investigated by Ghosh et al. [54]. Different concentrations (0.1–0.3 wt.%) of CNFs were used for the preparation of the multi-phase STFs. The rheological measurements showed that the addition of CNFs (0.3 wt.%) to the STF leads to a considerable increase in the viscosity. Moreover, the stronger shear thickening behavior occurs at a lower critical shear rate. This is likely attributed to the large number of hydroxyl groups on the CNFs, which contribute to the formation of hydrogen bonding between the CNFs and silica particles. Increasing the amount of CNFs in the multi-phase STF leads to a reduction in the critical shear rate due to the more interaction between the CNFs with high aspect ratio and silica particles, which induced shear thickening at a lower shear rate. Indeed, the number of available hydroxyl groups increases by increasing the CNFs content, implying stronger interaction and entanglement between the silica particles and CNFs through the formation of a greater number of hydrogen bonds. Figure 3.5 shows the schematic representation of shear thickening behavior in the multi-phase STF using CNFs as additives.
Sun et al. [55] investigated the rheological properties of a multi-phase STF based on zirconia nanoparticle additives. They found that the incorporation of zirconia nanoparticles into the STF (20% silica nanoparticles) leads to a growth in the shear thickening behavior while increasing the apparent viscosity level. The formation of a cluster of zirconia particles around the functional groups induces the shear thickening behavior and consequently increases the viscosity of the suspension. No regular changes are observed with increasing additive contents. In the multi-phase STF with zirconia content more than the cut-off point (12%), the initial viscosity and critical shear rate increase while the peak viscosity decreases. This is likely due to the hindering of the interaction of the silica nanoparticles by the excess amount of zirconia. Interestingly, the effect of temperature on the viscosity of the multi-phase STF becomes less pronounced when the content of zirconia increases. However, it still significantly influences the critical shear rate and shear thickening behavior.
The rheological behavior of a multi-phase STF was investigated by Sun et al. [56]. Neodymium oxide (Nd2O3) nanoparticles were used as an additive in the suspensions. They prepared various suspensions with different concentrations (9–15 wt.%) and found that an appropriate amount of Nd2O3 (12 wt.%) results in a significant increase in the peak viscosity by about 320%. The critical shear rate decreases by 75%. The investigation on the effect of temperature reveals a reduction in the peak viscosity with increasing temperature. Applying shear force to the multi-phase STF containing Nd2O3 particles with more mass fraction and larger size than silica particles results in the movement of Nd2O3 particles and adherence of silica particles to the Nd2O3 particles. By increasing shear force, the Nd2O3 particles are well organized and distributed uniformly, which leads to the shear thinning behavior. Further increase in shear force leads to a destroying effect in the layered structure of Nd2O3 particles. The silica particles form particle clusters by adhering to the Nd2O3 particles, which leads to a sharp increase in the viscosity of the Nd2O3-based multi-phase STF. Figure 3.6 shows the schematic illustration of the shear thickening mechanism. The results show that the incorporation of Nd2O3 particles significantly enhances the shear thickening effect especially at higher weight fractions and lower temperatures.
Schematic representation of shear thickening behavior of Nd2O3-based multi-phase STF [56]. Reprinted by permission from Springer
Although several studies have been carried out on the rheology of different multi-phase STF systems, further investigations are needed using various types of additives for a comprehensive understanding of the role of additives in the multi-phase STF. Table 3.3 summarizes the most relevant research works on the rheology of multi-phase STF. The effect of additives on the shear thickening behavior does not have the same trend each other as the literature provides only case-dependent studies. Some additives disrupt the shear thickening mechanism and some improve this behavior. However, it can be concluded that the rheology of multi-phase STF is dependent on various factors such as chemistry, material, geometry, weight fraction, aspect ratio, temperature, and mechanical properties.
3.5 Conclusions
The unique rheological behavior of STF provides the opportunity for different various applications such as protective fabrics and mechanical dampers. Several attempts have been carried out to design multi-phase STF by adding particular additives. A detailed review of the rheological behavior of multi-phase STF is presented in this chapter. This chapter shows that there are many significant parameters influencing the shear thickening properties of multi-phase STF such as the shape, size, and weight fraction of additives as well as their interactions with the base particles and carrier liquid in STF. There is an increasing attention and necessity to understand the rheological characteristic of multi-phase STF to figure out and tune it according to their special applications. Several micro and nano additives have been utilized for tuning the STF rheology including carbon based structures, metal oxides, and inorganic materials. Although the effect of additives on the shear thickening behavior of multi-phase STF does not have the same trend, they often lead to enhanced rheological properties in the optimum conditions. Hence, the fabrication of multi-phase STF with unique structural and rheological characteristics provides new possibilities for the design and development of efficient STF-based systems for various applications.
Abbreviations
- STF:
-
Shear thickening fluid
- EG:
-
Ethylene glycol
- PMMA:
-
Poly(methyl methacrylate)
- PEO:
-
Poly(ethylene oxide)
- PEG:
-
Polyethylene glycol
- AF:
-
Aramid fiber
- CNTs:
-
Carbon nanotubes
- GO:
-
Graphene oxide
- GNs:
-
Graphene nanoplatelets
- UHMWPE:
-
Ultra-high molecular weight polyethylene
- MWCNTs:
-
Multi-walled carbon nanotubes
- FTIR:
-
Fourier transform infrared spectroscopy
- CNFs:
-
Cellulose nanofibers
- ODT:
-
Order-disorder transition
- SiC:
-
Silicon carbide
- PDA:
-
Polydopamine
- PS–AA:
-
Poly(styrene–acrylic acid)
- ZIF-8:
-
Zeolitic imidazolate framework-8
- Al2O3:
-
Aluminum oxide
- ZrO2:
-
Zirconium dioxide
- Nd2O3:
-
Neodymium oxide
References
M. Hasanzadeh, V. Mottaghitalab, The role of shear-thickening fluids (STFs) in ballistic and stab-resistance improvement of flexible armor. J. Mater. Eng. Perform. 23(4), 1182–1196 (2014)
M. Hasanzadeh, V. Mottaghitalab, M. Rezaei, Rheological and viscoelastic behavior of concentrated colloidal suspensions of silica nanoparticles: A response surface methodology approach. Adv. Powder Technol. 26(6), 1570–1577 (2015)
K. Yu, H. Cao, K. Qian, L. Jiang, H. Li, Synthesis and stab resistance of shear thickening fluid (STF) impregnated glass fabric composites. Fibres Text East Eur. 95(6), 126–128 (2012)
A. Srivastava, A. Majumdar, B.S. Butola, Improving the impact resistance performance of Kevlar fabrics using silica based shear thickening fluid. Mater. Sci. Eng. A 529(1), 224–229 (2011)
E.V. Lomakin, P.A. Mossakovsky, A.M. Bragov, A.K. Lomunov, A.Y. Konstantinov, M.E. Kolotnikov, et al., Investigation of impact resistance of multilayered woven composite barrier impregnated with the shear thickening fluid. Arch. Appl. Mech. 81(12), 2007–2020 (2011. [Internet]. 2011 Apr 11 [cited 2022 Aug 9]). https://doi.org/10.1007/s00419-011-0533-0
Y.S. Lee, E.D. Wetzel, N.J. Wagner, The ballistic impact characteristics of Kevlar® woven fabrics impregnated with a colloidal shear thickening fluid. J. Mater. Sci. 38(13), 2825–2833 (2003. 3813 [Internet]. 2003 Jul 1 [cited 2022 Aug 9]). https://doi.org/10.1023/A:1024424200221
T.A. Hassan, V.K. Rangari, S. Jeelani, Synthesis, processing and characterization of shear thickening fluid (STF) impregnated fabric composites. Mater. Sci. Eng. A 527(12), 2892–2899 (2010)
L.L. Sun, D.S. Xiong, C.Y. Xu, Application of shear thickening fluid in ultra high molecular weight polyethylene fabric. J. Appl. Polym. Sci. 129(4), 1922–1928 (2013. [Internet] [cited 2022 Aug 9]). https://doi.org/10.1002/app.38844
S. Gürgen, M.A. Sofuoǧlu, M.C. Kuşhan, Rheological compatibility of multi-phase shear thickening fluid with a phenomenological model. Smart Mater. Struct. 28(3), 035027 (2019)
S. Gürgen, M.C. Kuşhan, W. Li, Shear thickening fluids in protective applications: A review. Prog. Polym. Sci. 75, 48–72. [Internet] (2017). https://doi.org/10.1016/j.progpolymsci.2017.07.003
W.H. Boersma, P.J.M. Baets, J. Laven, H.N. Stein, Time-dependent behavior and wall slip in concentrated shear thickening dispersions. J. Rheol. (N Y N Y) 35(6), 1093 (1998. [cited 2022 Aug 9], [Internet]). https://doi.org/10.1122/1.550167
M.J. Decker, C.J. Halbach, C.H. Nam, N.J. Wagner, E.D. Wetzel, Stab resistance of shear thickening fluid (STF)-treated fabrics. Compos. Sci. Technol. 67(3–4), 565–578 (2007)
D.P. Kalman, J.B. Schein, J.M. Houghton, C.H.N. Laufer, E.D. Wetzel, N.J. Wagner, Polymer dispersion based shear thickening fluid-fabrics for protective applications. Int. SAMPE Symp. Exhib., 52 (2007)
S.R. Raghavan, S.A. Khan, Shear-thickening response of Fumed silica suspensions under steady and oscillatory shear. J. Colloid Interface Sci. 185(1), 57–67 (1997)
H.A. Barnes, Shear-thickening (“dilatancy”) in suspensions of nonaggregating solid particles dispersed in Newtonian liquids. J. Rheol. (N Y N Y) 33(2), 329 (2000. [Internet, cited 2022 Aug 9]). https://doi.org/10.1122/1.550017
T.A. Hassan, V.K. Rangari, S. Jeelani, Sonochemical synthesis and rheological properties of shear thickening silica dispersions. Ultrason. Sonochem. 17(5), 947–952. [Internet] (2010). https://doi.org/10.1016/j.ultsonch.2010.02.001
K. Yu, H. Cao, K. Qian, X. Sha, Y. Chen, Shear-thickening behavior of modified silica nanoparticles in polyethylene glycol. J. Nanopart. Res. 14(3), 1–9 (2012. [Internet, cited 2022 Aug 9]). https://doi.org/10.1007/s11051-012-0747-2
Q.M. Wu, J.M. Ruan, B.Y. Huang, Z.C. Zhou, J.P. Zou, Rheological behavior of fumed silica suspension in polyethylene glycol. J. Cent. S. Univ. Technol. 13(1), 1–5 (2006. [Internet, cited 2022 Aug 9]). https://doi.org/10.1007/s11771-006-0096-3
X.Z. Zhang, W.H. Li, X.L. Gong, The rheology of shear thickening fluid (STF) and the dynamic performance of an STF-filleddamper. Smart Mater. Struct. 17(3), 035027 (2008., [Internet, cited 2022 Aug 9]). https://doi.org/10.1088/0964-1726/17/3/035027
T.J. Kang, C.Y. Kim, K.H. Hong, Rheological behavior of concentrated silica suspension and its application to soft armor. J. Appl. Polym. Sci. 124(2), 1534–1541 (2012. [Internet, cited 2022 Aug 9]). https://doi.org/10.1002/app.34843
D.P. Kalman, R.L. Merrill, N.J. Wagner, E.D. Wetzel, Effect of particle hardness on the penetration behavior of fabrics intercalated with dry particles and concentrated particle-fluid suspensions. ACS Appl. Mater. Interfaces 1(11), 2602–2612 (2009. [Internet, cited 2022 Aug 9]). https://doi.org/10.1021/am900516w
B.W. Lee, I.J. Kim, C.G. Kim, The influence of the particle size of silica on the ballistic performance of fabrics impregnated with silica colloidal suspension. J. Compos. Mater. 43(23), 2679–2698 (2009. [Internet, cited 2022 Aug 9]). https://doi.org/10.1177/0021998309345292
A. Majumdar, B.S. Butola, A. Srivastava, Optimal designing of soft body armour materials using shear thickening fluid. Mater. Des. 1(46), 191–198 (2013)
Houghton JM, Schiffman BA, Kalman DP, Wetzel ED, Wagner NJ. Hypodermic needle puncture of shear thickening fluid (STF)-treated fabrics. Int SAMPE Symp Exhib. 2007; 52 (October)
Y.S. Lee, N.J. Wagner, Rheological properties and small-angle neutron scattering of a shear thickening, nanoparticle dispersion at high shear rates. Ind. Eng. Chem. Res. 45(21), 7015–7024 (2006. [Internet, cited 2022 Aug 9]). https://doi.org/10.1021/ie0512690
M. Takeda, T. Matsunaga, T. Nishida, H. Endo, T. Takahashi, M. Shibayama, Rheo-SANS studies on shear thickening in clay-poly(ethylene oxide) mixed solutions. Macromolecules 43(18), 7793–7799 (2010. [Internet, cited 2022 Aug 9]). https://doi.org/10.1021/ma101319j
Y. Wu, S. Cao, S. Xuan, M. Sang, L. Bai, S. Wang, et al., High performance zeolitic imidazolate framework-8 (ZIF-8) based suspension: Improving the shear thickening effect by controlling the morphological particle-particle interaction. Adv. Powder Technol. 31(1), 70–77 (2020)
M. Liu, W. Jiang, Q. Chen, S. Wang, Y. Mao, X. Gong, et al., A facile one-step method to synthesize SiO2@polydopamine core–shell nanospheres for shear thickening fluid. RSC Adv. 6(35), 29279–29287 (2016)
W. Jiang, F. Ye, Q. He, X. Gong, J. Feng, L. Lu, et al., Study of the particles’ structure dependent rheological behavior for polymer nanospheres based shear thickening fluid. J. Colloid Interface Sci. 413, 8–16 (2014)
R.L. Hoffman, Explanations for the cause of shear thickening in concentrated colloidal suspensions. J. Rheol. (N Y N Y) 42(1), 111 (1998. [Internet, cited 2022 Aug 15]). https://doi.org/10.1122/1.550884
W.H. Boersma, J. Laven, H.N. Stein, Viscoelastic properties of concentrated shear-thickening dispersions. J. Colloid Interface Sci. 149(1), 10–22 (1992)
H.M. Laun, R. Bung, S. Hess, W. Loose, O. Hess, K. Hahn, et al., Rheological and small angle neutron scattering investigation of shear-induced particle structures of concentrated polymer dispersions submitted to plane Poiseuille and Couette flowa. J. Rheol. (N Y N Y) 36(4), 743 (1998. [Internet, cited 2022 Aug 15]). https://doi.org/10.1122/1.550314
R.L. Hoffman, Discontinuous and dilatant viscosity behavior in concentrated suspensions. II. Theory and experimental tests. J. Colloid Interface Sci. 46(3), 491–506 (1974)
R.L. Hoffman, Discontinuous and dilatant viscosity behavior in concentrated suspensions. I. Observation of a flow instability. Trans. Soc. Rheol. 16(1), 155 (2000. [Internet, cited 2022 Aug 15]). https://doi.org/10.1122/1.549250
J.W. Bender, N.J. Wagner, Optical measurement of the contributions of colloidal forces to the rheology of concentrated suspensions. J. Colloid Interface Sci. 172(1), 171–184 (1995)
J. Bender, N.J. Wagner, Reversible shear thickening in monodisperse and bidisperse colloidal dispersions. J. Rheol. (N Y N Y) 40(5), 899 (1998. [Internet, cited 2022 Aug 15]). https://doi.org/10.1122/1.550767
M. Zarei, J. Aalaie, Application of shear thickening fluids in material development. J. Mater. Res. Technol. 9(5), 10411–10433 (2020)
B.J. Maranzano, N.J. Wagner, Flow-small angle neutron scattering measurements of colloidal dispersion microstructure evolution through the shear thickening transition. J. Chem. Phys. 117(22), 10291 (2002. [Internet, cited 2022 Aug 15]). https://doi.org/10.1063/1.1519253
N.Y.C. Lin, B.M. Guy, M. Hermes, C. Ness, J. Sun, W.C.K. Poon, et al., Hydrodynamic and contact contributions to continuous shear thickening in colloidal suspensions. Phys. Rev. Lett. 115(22), 228304 (2015. [Internet, cited 2022 Aug 14]). https://doi.org/10.1103/PhysRevLett.115.228304
I.R. Peters, S. Majumdar, H.M. Jaeger, Direct observation of dynamic shear jamming in dense suspensions. Nature 532(7598), 214–217 (2016) [Internet, cited 2022 Aug 14]. Available from: https://www.nature.com/articles/nature17167
M. Wyart, M.E. Cates, Discontinuous shear thickening without inertia in dense non-brownian suspensions. Phys. Rev. Lett. 112(9), 098302 (2014. [Internet, cited 2022 Aug 14]). https://doi.org/10.1103/PhysRevLett.112.098302
B.J. Maranzano, N.J. Wagner, The effects of particle size on reversible shear thickening of concentrated colloidal dispersions. J. Chem. Phys. 114(23), 10514 (2001)
E.D. Wetzel, Y.S. Lee, R.G. Egres, K.M. Kirkwood, J.E. Kirkwood, N.J. Wagner, The effect of rheological parameters on the ballistic properties of shear thickening fluid (STF)-Kevlar composites. AIP Conf. Proc. 712(1), 288 (2004. [Internet, cited 2022 Aug 9]). https://doi.org/10.1063/1.1766538
M. Hasanzadeh, V. Mottaghitalab, Tuning of the rheological properties of concentrated silica suspensions using carbon nanotubes. Rheol. Acta 55(9), 759–766 (2016)
M. Wei, Y. Lv, L. Sun, H. Sun, Rheological properties of multi-walled carbon nanotubes/silica shear thickening fluid suspensions. Colloid Polym. Sci. 298(3), 243–250 (2020)
D. Li, R. Wang, X. Liu, S. Fang, Y. Sun, Shear-thickening fluid using oxygen-plasma-modified multi-walled carbon nanotubes to improve the quasi-static stab resistance of Kevlar fabrics. Polymers (Basel). 10(12), 1356 (2018)
S. Gürgen, M.C. Kuşhan, W. Li, The effect of carbide particle additives on rheology of shear thickening fluids. Korea Aust. Rheol. J. 28(2), 121–128 (2016)
S. Gürgen, W. Li, M.C. Kuşhan, The rheology of shear thickening fluids with various ceramic particle additives. Mater. Des. 104, 312–319 (2016)
W. Huang, Y. Wu, L. Qiu, C. Dong, J. Ding, D. Li, Tuning rheological performance of silica concentrated shear thickening fluid by using graphene oxide. Adv. Condens Matter Phys. 2015, 734250 (2015)
P. Passey, M. Singh, S.K. Verma, D. Bhattacharya, R. Mehta, Steady shear and dynamic strain thickening of halloysite nanotubes and fumed silica shear thickening composite. J. Polym. Eng. 38(10), 915–923 (2018. [Internet, cited 2022 Aug 9]). https://doi.org/10.1515/polyeng-2018-0043/html
A. Laha, A. Majumdar, Shear thickening fluids using silica-halloysite nanotubes to improve the impact resistance of p-aramid fabrics. Appl. Clay Sci. 132–133, 468–474 (2016)
H. Barthel, Surface interactions of dimethylsiloxy group-modified fumed silica. Colloids Surf. A Physicochem. Eng. Asp. 101(2–3), 217–226 (1995)
X. Sha, K. Yu, H. Cao, K. Qian, Shear thickening behavior of nanoparticle suspensions with carbon nanofillers. J. Nanopart. Res. 15(7), 1–11 (2013. [Internet, cited 2022 Jul 29]). https://doi.org/10.1007/s11051-013-1816-x
A. Ghosh, I. Chauhan, A. Majumdar, B.S. Butola, Influence of cellulose nanofibers on the rheological behavior of silica-based shear-thickening fluid. Cellulose 24(10), 4163–4171 (2017)
L. Sun, J. Zhu, M. Wei, C. Zhang, Y. Song, P. Qi, Effect of zirconia nanoparticles on the rheological properties of silica-based shear thickening fluid. Mater Res. Express. 5(5), 055705 (2018)
L. Sun, Y. Lv, M. Wei, H. Sun, J. Zhu, Shear thickening fluid based on silica with neodymium oxide nanoparticles. Bull. Mater. Sci. 43(1), 1–6., [Internet] (2020). https://doi.org/10.1007/s12034-020-02134-2
M. Zabet, K. Trinh, H. Toghiani, T.E. Lacy, C.U. Pittman, S. Kundu, Anisotropic nanoparticles contributing to shear-thickening behavior of Fumed silica suspensions. ACS Omega 2(12), 8877–8887 (2017. [Internet, cited 2022 Aug 15]). https://doi.org/10.1021/acsomega.7b01484
J. Ge, Z. Tan, W. Li, H. Zhang, The rheological properties of shear thickening fluid reinforced with SiC nanowires. Results Phys 7, 3369–3372 (2017). https://doi.org/10.1016/j.rinp.2017.08.065. [Internet]
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sheikhi, M.R., Hasanzadeh, M. (2023). Multi-Phase Shear Thickening Fluid. In: Gürgen, S. (eds) Shear Thickening Fluid. Springer, Cham. https://doi.org/10.1007/978-3-031-25717-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-25717-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25716-2
Online ISBN: 978-3-031-25717-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)