Abstract
The current study aimed at examining whether titanium dioxide nanoparticles (TiO2 NPs) can ameliorate the performance of menthol mint/corn mint (Mentha arvensis L.), a medicinally imperative plant. The plants were tested with graded concentrations of TiO2 NPs [0 (control-double distilled water), 50, 100, 150 and 200 mg L−1] 40 days after transplantation (DAT), and effects on the growth, photosynthesis, and activities of vital enzymes were assessed at 150 DAT. TiO2 NPs applied at 100 mg L−1 proved most favourable for most of the parameters studied with an upsurge of 13.2% and 11.6% in the activities of carbonic anhydrase and nitrate reductase, respectively, by this concentration as compared to the control. Moreover, the content and yield of plant’s EO recorded a significant enrichment of 26.3% and 109.8%, respectively, compared to the control. An enhancement in the total content (increased by 26.3%) and yield of EO (109.8%) is endorsed to the TiO2 NPs-stimulated enhancement in the growth as well as photosynthetic efficiency. Furthermore, the quality of the EO was improved as an increment of 12.8% and 136.5% was witnessed in the content and yield of menthol compared to the control plants was reported. To conclude, this study demonstrated that foliar application of TiO2 NPs (100 mg L−1) effectively promoted growth, physiology as well as yield of corn mint.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Application of nanotechnology in agriculture commenced with the mounting realization that conventional agricultural technologies lack the potential to enhance productivity any further. Nanotechnology has gained momentum in the field of agriculture during the past decade as it aims at nutrient loss reduction, increased crop yield and sustainable crop production (Abdelaal et al. 2019; Ahmad et al. 2018, 2020, 2022; Ghasemnezhad et al. 2019; Hu et al. 2020; Pestovsky et al. 2017; Vargas-Hernandez et al. 2020). Novel nanomaterials have been formulated and applied in the agri-sector with the aim to not only enhance the productivity but also has vibrant prospects for improving the nutrient-use efficiency through nano-fertilizers, control and surveillance of diverse pests as well as diseases, new-generation pesticide-development and gaining insights into the host–parasite interactions at the gene level (Abdel-Latef et al. 2018; Rastogi et al. 2019; Singh et al. 2021).
NPs show certain unique properties different from their bulk material because of their minute size, various shapes and larger surface area to volume ratio which extends their reactivity and potential biochemical activity (Dubchak et al. 2010). The unique characteristic is possibly responsible for the elicitor effect of different types of NPs which is being exploited to not only raise the production of valuable plant metabolites of primary as well as secondary origin but also to alleviate the toxicity caused due to diverse environmental constraints in plants (Ahmed et al. 2022; Faizan et al. 2020, 2021a, 2021b; Mukarram et al. 2021, 2022). Different types of nanomaterials have been tested on diverse agricultural crops with the purpose of increasing their productivity (Ahmad et al. 2018, 2019; Batsmanova et al. 2013; Ghasemnezhad et al. 2019; Rastogi et al. 2019; Vargas-Hernandez et al. 2020). Among these, application of Titanium dioxide nanoparticles (TiO2 NPs) is known to regulate different processes in plants notably seed germination, growth of radicle and plumule in canola seedlings (Mahmoodzadeh et al. 2013), growth of wheat plants under drought environments (Jaberzadeh et al. 2013), regulation of various enzymatic activities associated with nitrate and ammonia assimilation (Ahmad et al. 2018, 2020, 2022; Mishra et al. 2014; Shabbir et al. 2019; Sharma et al. 2022; Yang et al. 2007). Increased growth due to the application of TiO2 NPs has been endorsed to the augmented photosynthesis in spinach plants delayed chloroplast senescence, and improved photosynthetic pigments which possibly puts in more photosynthates for growth and storage (Hong et al. 2005a, b; Yang et al. 2007).
Mentha arvensis L. (member of Lamiaceae family), generally known as corn mint or menthol mint, an industrial field crop, holds an important status and cultivated for its essential oil (EO) (Lal 2013). The trade products of this cash crop are menthol and de-mentholized oil which are extremely demanding in pharmaceutical, cosmetic and food industries (Gupta et al. 2017; Prakash et al. 2018). The EO of Mentha is usually obtained by the steam distillation of the vegetative shoot biomass. Mentha oil has a distinctive strong minty aroma and in the total production of mint EO around the world, India controlled 60% share (Lawrence 2007; Prakash et al. 2018).
Though impact of TiO2 NPs on the photosynthetic parameters of some crop plants has been worked out, a detailed study on the economically valuable secondary metabolite-containing plant is inadequate. Therefore, accepting the supreme medicinal significance of corn mint and its EO and keeping the positive effects of TiO2 NPs on various plants in mind, the present investigation was formulated with the objective to inspect the impact of TiO2 NPs in terms of growth, physiological and biochemical attributes, essential oil production and quality of corn mint.
2 Materials and methods
2.1 Experimental setup and titanium nanoparticles (TiO2 NPs) preparation
The plants of menthol mint were grown in plastic pots of 40 cm in diameter and 45 cm in height in the net house under natural environmental conditions at the Department of Botany, Aligarh Muslim University, Aligarh, India [27°52′N latitude, 78°51′E longitude and 187.45 m altitude]. The average temperature was 4.4 °C–22.1 °C between December and February and 12.2 °C–32.3 °C between February and May. Average precipitation ranged between 4.2 and 15.5 mm, and relative humidity was 38–79%. The healthy suckers of Mentha arvensis L., procured from Central Institute of Medicinal and Aromatic Plants (CIMAP), Lucknow, Uttar Pradesh, India, were used for propagation. Prior to transplanting, surface sterilization of the suckers was done by using 0.02% of the HgCl2 solution followed by vigorous washing with de-ionized water. Each plastic pot used for the present experiment contained 8.5 kg homogenous mixture of soil and organic manure. Physicochemical characteristics of the soil were determined at the Soil-Testing Laboratory, Indian Agricultural Research Institute (IARI), New Delhi, India. The soil was sandy loam in texture with pH (1:2) 7.9, E.C. (1:2) 0.50 mmhos cm−1 and 94.6, 8.5 and 137.1 mg available mineral nutrients, viz. N, P and K per kg of soil, respectively. A uniform basal dose of N (urea), P (single superphosphate) and K (muriate of potash) was applied at the rate of 86.2, 137.9 and 32.2 mg kg−1, respectively.
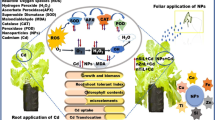
For foliar spray, different aqueous concentrations, i.e., 0 (control), 50, 100, 150 and 200 mg L−1 of TiO2 NPs (obtained from Department of Applied Physics, Aligarh Muslim University, Aligarh, India) were prepared by dissolving in double distilled water (DDW). The different concentrations of TiO2 NPs were applied to the Mentha arvensis L. plants after proper sonication in order to evade the aggregation (if any) of NPs after 40 days after transplantation (DAT). The experimental pots were arranged in simple randomized design and conducted in three replications. Total 5 foliar sprays of different concentrations were applied at 7 days interval. Agronomic practices such as weeding, hoeing and irrigation of the plants were performed accordingly. The plants were sampled at 150 DAT to record different attributes like growth, physio-biochemical properties and yield of Mentha arvensis L.
2.2 Scanning electron microscopy (SEM), UV–visible (UV–Vis) and Raman spectroscopies of TiO2 NPs
Morphological features and the structure of TiO2 NPs were analysed by SEM, UV–VIS and Raman spectroscopies. SEM (JEOL, JSM-6510 LV, Japan) was done at Ultra Sophisticated Instrumentation Facility, Aligarh Muslim University, Aligarh, India. The TiO2 NP’s surface characteristic was assessed at accelerating voltage of 10 kV (Fig. 1a) magnification of 2,500 × with spot size of 25. For imaging at 10 μm scale, a secondary imaging detector was used. For UV–Visible spectroscopy of TiO2 NPs, Lambda 950 UV–Vis-NIR Spectrophotometer (PerkinElmer Lambda Spectrometer) in the region of 200–800 nm (Fig. 1b) was used. In order to determine the crystalline structure of TiO2 nanoparticles, the Raman spectra of the nanopowders were recorded using a micro-Raman spectrometer (Renishaw plc, United Kingdom) installed in Central Research Facility, Indian Institute of Technology (IIT) Delhi, India. The Raman spectra were excited by 785 nm with 20 MW power at a resolution of 1.0 cm−1.
2.3 Measurement of growth parameters
The growth attributes like height, fresh and dry mass of the plant were measured. Plants of each treatment pot were uprooted, washed carefully with tap water and dried by blotting paper to remove the adhered water. Fresh weight of the plants was recorded by using weighing balance and after that plant samples were kept in a hot-air oven at 80 °C until completely dried and then weighed the dry weight. Plant height was recorded by using a meter scale.
2.4 Determination of physiological activities
2.4.1 Measurements of total chlorophyll and carotenoids content
Chlorophyll and carotenoids contents were assessed in the fresh leaves by using the Lichtenthaler and Buschmann (2001) method. For fresh tissue, the interveinal part of the leaf was taken and ground with 100% acetone by using a mortar and pestle. The optical density (OD) for chlorophyll a and chlorophyll b was recorded at 662 nm and 645 nm, respectively, and total carotenoids were recorded at 470 nm by using a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan). The total chlorophyll content was assessed by adding the chlorophyll a and b contents. The chlorophyll and carotenoids contents were expressed as mg g−1 leaf fresh weight.
2.4.2 Determination of nitrate reductase (NR) and carbonic anhydrase (CA) activities
The NR enzyme activity was determined by the method given by Jaworski (1971). The absorption of the reaction mixture was recorded at 540 nm by using the spectrophotometer. However, the method of Dwivedi and Randhawa (1974) was adopted for the determination of CA enzyme activity.
2.4.3 Determination of chlorophyll fluorescence parameters (PS II activity)
Chlorophyll fluorescence parameters, viz. PSII operating efficiency (ΦPSII), maximum PSII efficiency (Fv/Fm), and electron transport rate (ETR), were examined by using PAM-2000 in the diurnal period. Young, healthy, unifoliate and fully expanded leaves were used for the analysis of fluorescence parameters. Before the analysis, the leaves were dark-adapted for 30 min to calm down the reaction centre. The minimal fluorescence (Fo') and maximal fluorescence (Fm'), under light-adapted state, were determined by using actinic light with intensity of 625 μ mol m−2 s−1.
2.4.4 Quantification of leaf nitrogen content
Fresh leaf samples from all the treatments were dried in oven at 100 °C for 24 h. The dried leaves were grounded into fine powder with the help of mortar-pestle. The powdered material was sieved through a 72 mesh and used for the nitrogen content estimation. After that, 100 mg of this powdered material was digested in H2SO4 at 100 °C for 2 h in temperature controlled Kjeldahl apparatus. After cooling for around 15 min at room temperature, 0.5 mL of 30% hydrogen peroxide (H2O2) was applied. The process of heating and cooling was repeated again until the content in the tube turned colourless. The nitrogen concentration in the leaves was determined on a dry weight basis using the aliquot (acid-peroxide-digested leaf-material).The method of Lindner (1944) with slight modification by Novozamsky et al. 1983 was employed for nitrogen (N) content.
2.4.5 Estimation of leaf carbohydrate content
It was estimated by the method of DuBois et al. 1956. In a test tube, 1 mL extract was taken to which 5% phenol (0.5 mL) was added. The test tubes were placed in chilled water. H2SO4 (2.5 mL) was added, and the OD was recorded using the spectrophotometer at 490 nm.
2.5 Yield and quality attributes
Extraction of essential oil and gas chromatography (GC) analysis
Fresh leaves of the plants were collected from each treatment pot and chopped and used for the determination of the EO. As per method given by Guenther (1972), the EO of menthol mint was collected and measured gravimetrically. The extraction of EO was done by a three-hour distillation process using a Clevenger's apparatus. For GC analysis, the extracted EO was then dried over anhydrous sodium sulphate and stored at 4 °C in sealed glass vials. The active constituents of menthol mint EO were calculated using a GC apparatus [(Agilent) USA, 7890B] fitted with HP5 capillary column (polyimide and fused silica coated) of 30 m × 0.32 mm × 0.00025 mm in size, with nitrogen as the carrier gas, a flame ionization detector and injector. The GC temperature schedule was 270 °C, 260 °C and 250 °C for detector, oven and injector, respectively. 0.2 μL was the sample size. The temperature was started at 40 °C and held for 2 min before being increased to 260 °C at a pace of 5 °C per minute for a final hold of ten minutes. Retention time was used to identify active constituents such as menthol and menthone. It was measured in terms of per cent content by comparing their peaks to the peaks obtained from a published reference norm. (Adams 2007).
Determination of specific gravity and refractive index (RI) of essential oil
The specific gravity of the EO was calculated according to Afaq et al. (1994) at 25 °C with a ‘specific gravity bottle’ by using the formula given below:
The RI of the EO was calculated according to the method of Jenkins et al. (1967). A few drops of EO were applied to the double prism before tightly clamping the prisms together. The instrument was calibrated until the cross hairs of the telescope absolutely coincided with the border line between the light and dark portions of the view-field, and the RI of oil was recorded. The refractive index of essential oil's was calculated using the average of three readings and expressed as N24°D, where N24°D stands for the light refraction index of the ‘D' line (sodium light) recorded at 24°.
2.6 Statistical analysis
SPSS Statistics software for Windows, Version 20.0, was used to conduct the statistical analysis. Means were compared using the Duncan multiple range test (DMRT) at p ≤ 0.05. Standard error (± SE) was also used.
3 Results
3.1 Characterization of TiO2 NPs using SEM, UV–vis and Raman spectroscopies
NPs of TiO2 are pure, non-hazardous, white colour pigment with 80–90%, by weight, content of anatase. Spherical shape of TiO2 NPs was detected by SEM image (Fig. 1a). The aggregates of NP have a size of several hundred nanometers, whereas the primary particles have a mean diameter upto 22 nm. UV–visible spectrum, investigated at the wavelengths of 200–800 nm, reveals that the characteristic light absorption peak was observed at around 300 nm (Fig. 1b). Figure 2 shows Raman spectrum of TiO2 NPs that displayed peaks at 158, 202, 399, 521, and 644 cm−1. The peaks at 158, 202, and 644 cm−1 have been noted due to Eg mode, while the peak at 399 and 521 cm−1 is assigned to B1g mode of TiO2 NPs. The well-crystallized anatase nature of TiO2 NPs was confirmed by 521 cm−1 peak (Sharma et al. 2011). The Raman spectra recorded in our study are in line with the finding of Bharti et al. (2018).
3.2 Foliar-applied TiO2 NPs improve growth attributes
The foliar spray of graded doses of TiO2 NPs increased the growth traits of menthol mint plants. Data in Table 1 display that, as compared to other concentration of TiO2 NPs, 100 mg L−1 proved best for various growth attributes studied. Beyond 100 mg L−1 concentration, a progressive decline was observed in various growth parameters; however, all the treatments of TiO2 beyond 100 mg L−1 registered better performance in comparison with double distilled water sprayed (control) plants. The plant fresh weight, dry weight and height were increased by 56.7%, 49.9%, and 52.1%, respectively, by 100 mg L−1 of TiO2 NPs.
3.3 Foliar feeding of TiO2 NPs enhances the contents of total chlorophyll and carotenoids
Treatment TiO2-100 maximally improved the content of major photosynthetic pigments, viz. chlorophyll and carotenoid in the current investigation. All the remaining concentrations of TiO2 beyond 100 mg L−1 showed a decline, but produced better results as compared to control. An increase of 15.5% for content of total chlorophyll and 10.1% for content of carotenoids, over the control, was reported by TiO2-100 (Fig. 3a, b).
Effect of five concentrations of foliar sprays of TiO2 NPs [0 (control), 50, 100, 150 and 200 mg L−1] on a total chlorophyll content, b carotenoids content, c nitrate reductase activity d carbonic anhydrase activity, e chlorophyll fluorescence, f PS II operating efficiency, g electron transport rate and h leaf nitrogen content of M. arvensis after 150 DAP. Bars showing the same letter s are not significantly different (p ≤ 0.05). Error bars (┬) show SE
3.4 TiO2 NPs-mediated improvement in enzyme activities
Outcomes of the present investigation revealed that foliar application of various concentrations of TiO2 NPs had a positive effect on the CA and NR enzymes activities. A significant augmentation of 13.2% and 11.6% was observed in CA and NR activities, respectively, due to the foliar application of TiO2-100 in comparison with the control plants (Fig. 3 c and d) followed by 150 mg L−1.
3.5 Exogenous application of TiO2 NPs augments PS II activity
All the applied TiO2 NPs doses outshined the control treatment with regard to various parameters of chlorophyll fluorescence (PSII activity). TiO2-100 resulted in maximal upsurge in the values of maximum PSII efficiency (Fv/Fm), PSII operating efficiency (ΦPSII), and electron transport rate (ETR) enhancing their values by 8.5%, 15.0% and 15.0%, respectively, in comparison with control (Fig. 3e, f, g). This was followed by TiO2-150; however, both TiO2-100 as well as TiO2-150 were equally significant for this parameter.
3.6 Foliar application of TiO2 NPs improve the leaf nitrogen (N) and carbohydrate contents
Leaf N content in corn mint was improved significantly by the foliar feeding of TiO2 NPs with TiO2 -100 proved superior among the various doses. A maximum improvement of 8.1% was observed in N content of corn mint by TiO2 -100 as compared to control (Fig. 3 h). TiO2 at 150 mg L−1 concentration (TiO2-150) produced the second-best results.
Leaf carbohydrate content was also augmented by the foliar feeding of TiO2 NPs. TiO2-100 generated the maximum plant response for leaf carbohydrate content (Table 2) by 22.72% in comparison with the control, at 150 DAT.
3.7 TiO2 NPs augment the content and yield of EO and its active constituents
Application of TiO2 NPs increased the content and yield of EO in corn mint. A maximum augmentation of 26.3% in the EO content was recorded by TiO2-100 followed by TiO2-150 as compared to control. The maximum increase of 109.8% in EO yield of corn mint was reported by TiO2-100 which was followed by TiO2-150 in comparison with control (Table 2). In the present study, active constituents of EO were also analysed in control and treated plants. Results revealed that foliar-applied TiO2 NPs increased the content and yield (per plant) of menthol by 12.8% and 136.5%, respectively, by TiO2-100 over control (Table 2 and Fig. 4a, b). However, menthone content of corn mint EO was decreased by 14.7%. But, maximum augmentation of 80.1% was reported in menthone yield per plant by TiO2-100 in comparison with control treated plants (Table 3 and Fig. 4a, b).
3.8 Influence of TiO2 NPs on physical properties of EO
The RI and specific gravity of the EO were not significantly enhanced by the foliar-applied TiO2 NPs (Table 3).
4 Discussion
NPs are recently employed in agriculture, because of their distinctive physicochemical features and the capacity to augment the metabolism in plants (Giraldo et al. 2014). The efficiency of NPs is subjected to their concentration, and it differs from one plant to another (Singh et al. 2015) and is determined by their surface area, size and chemical composition (Khodakovskaya et al. 2009). Plant cells, in contrast to animal cells, are enveloped by the cell wall. This extra layer has pores with diameters between 5 and 30 nm (Auffan et al. 2009; Carpita et al. 1979; Rondeau-Mouro et al. 2008). The TiO2 NPs utilized in this study were less than 22 nm in size, and hence, they were most likely able to enter the plants through these cell wall pores and produce the possible responses which were reflected in terms of escalated growth, physiology, and essential oil production (Mustafa et al. 2021; Satti et al. 2021).
Plant growth regulators, bioactive polymers, etc., are the exogenously sourced elicitors which modulate the number of physiological processes in plants and help to improve their overall performance and resulted in increasing the yield (Taiz and Zeiger 2014). Recently, the nanoparticles proved to play pivotal role in agriculture due to their elicitor effect and competence to improve growth and yield in plants (Chen et al. 2014). Moreover, besides the type as well as physical and chemical properties of nanoparticles, the effect produced in plants also depends upon the plant species (Ma et al. 2010). In the present investigation, plant growth attributes such as plant height, fresh and dry weight, were boosted by the application TiO2 NPs (Table 1), with best results by TiO2-100. Similar additive effects of TiO2 NPs, as foliar application, in growth parameters have also been recorded in Mentha piperita L. (Ahmad et al. 2018), Dracocephalum moldavica L. (Gohari et al. 2020), Calendula officinalis L. (Moaveni et al. 2011), and Zea mays L. (Morteza et al. 2013). The possible basis for improvement in growth parameters in the current study is that these NPs promoted mitotic cell division, enhanced the cell size, and may affect the content of phytohormones such as cytokinin and gibberellin (Mandeh et al. 2012). Furthermore, the enhancement in growth parameters in our study could be accredited to the recorded upsurge in chlorophyll content, chlorophyll fluorescence parameters and enzymatic activities (Fig. 3).
Elevated chlorophyll content facilitates leaves to absorb more light which promotes photosynthesis, and thus, assessment of chlorophyll content is one of the crucial aspects used to determine the photosynthesis rate of the plants. In this study, the chlorophyll and carotenoid content of leaf increased with the foliar application of TiO2 NPs (Fig. 3a, b) with maximum response shown by TiO2-100. In line with our findings, Ahmad et al. 2018; Ebrahimi et al. 2016; Gohari et al. 2020 reported encouraging effect of TiO2 NPs on photosynthetic pigments of bean, peppermint and dracocephalum, respectively. The augmentation in total chlorophyll content in the present investigation might be ascribed to TiO2 NPs facilitated increase in the content of leaf N (Fig. 3h). Because N is a component of chlorophyll's tetrapyrrole ring, an increase in leaf N could have boosted the production of chlorophyll and other photosynthetic pigments.
CA is a primary carbon metabolic enzyme found in all the tissues involved in photosynthesis and plays a central role in photosynthesis. CA increases the accessibility of CO2 to Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) in C3 plants and to phosphoenolpyruvate carboxylase (PEPC) in C4 and CAM plants by catalysing the reversible hydration of CO2 to HCO3− (carbonic acid). In higher plants the activity of CA is directly linked to photosynthesis, as it regulates the synthesis of carbon compounds and hence is thought to promote growth attributes in plants (Ahmed et al. 2020). The applied TiO2NPs in the current study also encouraged the activity of carbonic anhydrase which is consistent with the findings of Shabbir et al. 2019 in vetiver. Elevated CA activity produced by the applied TiO2 NPs might enhance the CO2 fixation in the dark reaction which is reflected in significant improvement in the fresh and dry weights of the plants treated with the TiO2 NPs (Fig. 3d).
Nitrogen (N) is one of the most important vital nutrient elements for crop growth. Moreover, it is a key component of chlorophyll and protein, both of which are crucial for crop growth and yield (Fageria et al. 2011), and also a component of nucleic acids, rubisco, as well as certain phytohormones. The activity of the photosynthetic machinery of the leaf is substantially determined by the availability of nitrogen in plants (Abid et al. 2016; Brennan 1992). The NR enzyme is one of the most important enzymes in the nitrogen metabolism; its activity is linked to nitrogen use and assimilation, which has an impact on plant growth (Ji et al. 2001; Yang, Gao 2002). The applied TiO2 NPs improved the NR activity and leaf nitrogen content (Fig. 3c, e). The increased NR activity mediated by TiO2 NPs in the current study could be attributed to increased leaf nitrogen content, which may have increased the amount of leaf nitrate to be acted upon by the enzyme, as substrate concentration stimulates functional enzyme activity (Hewitt, Afridi 1959). An increase in leaf nitrogen content and NR activity in mentha by the application of TiO2 NPs has also been previously reported by Ahmad et al. (2018). Not just NR, TiO2 NPs are known to influence entire nitrogen metabolism as it regulates the other key enzymes activities of nitrogen metabolism such as glutamate-pyruvate transaminase, glutamate dehydrogenase, glutamine synthase and nitrate reductase itself promoting the absorption of nitrate to the plants (Yang et al. 2006).
Photosynthesis is a two phase process, wherein during the first phase, the photonic energy is absorbed by PSII reaction centres and ultimately used for ATP and NADPH production using various electron acceptors of the electron transport chain. The energy produced in the form of ATP and NADPH is then utilised for the carbohydrate synthesis during the second phase. But plants, on the other hand, convert light to energy with just 2–4% efficiency (Kirschbaum 2011). NPs, based on their inherent light interaction capabilities, tend to interfere with and modulate photosynthetic efficiency, photochemical fluorescence, and quantum yield in plants (Mohamed and Kumar 2016). Chlorophyll fluorescence is a useful technique for studying the process of photosynthetic and PSII activity (Gohari et al. 2020; Nedbal et al. 2000; Shangguan et al. 2000). The quantity of light absorbed by PSII which is used in photochemistry is assessed by ΦPSII, known as ‘PSII operating efficiency’. The larger the ΦPSII value, greater is the quantum efficiency of PSII (Genty et al. 1989). Aside from that, it is a good way to check the linear electron transfer rate (ETR) and get a sense of overall photosynthesis. In the present study, TiO2 NPs increased the ΦPSII and ETR values, with TiO2-100 generating the maximum response (Fig. 3f, g). PSII activity is up-regulated by the applied TiO2 NPs and the ejected electrons from PSII move through various electron carriers, thus reducing PSI. TiO2 NPs-mediated increase in ETR as noted in the present study will result in faster ATP and NADPH synthesis, both of which are essential for CO2 fixation. The relationship between Fv/Fm (maximum quantum efficiency of PSII) and leaf photosynthetic efficiency is well-known (Baker and Rosenqvist 2004). The applied TiO2 NPS also escalated the Fv/Fm values (Fig. 3e). Thus, improvement in various chlorophyll fluorescence parameters in the present study reflects that applied TiO2 NPs positively influence first phase of photosynthesis by increasing light absorbance and its further usage in photochemistry. In line with our finding regarding the stimulatory role of TiO2 NPs in photosynthesis, viz. better light absorbance and increased ETR, TiO2 NPs were also previously shown to stimulate photosynthesis as they have a photo-catalysed characteristic that assists in trapping light and the conversion of light energy to chemical energy and thereby enhancing CO2 fixation (Hong et al. 2005a, 2005b; Yang et al. 2007). TiO2NPs also boosted the electron transport chain, PSII photoreduction activity, and O2-evolving and photophosphorylation activity in spinach (Mingyu et al. 2007). The second phase of photosynthesis which is associated with the reduction of CO2 or generation of carbohydrates is dependent on the products of first phase of photosynthesis, viz. ATP and NADPH. The applied TiO2 NPs might also improve the leaf carbohydrate content, which is an expected outcome as TiO2 NPs are positively influencing ETR, thereby speeding up the generation of ATP and NADPH. Furthermore, elevated CA activity as found in the current investigation (Fig. 3d) has improved the availability of CO which will be then reduced in the presence of ATP and NADPH ultimately might have increased leaf carbohydrate content. TiO2 NPs have been previously shown to increase the carbohydrates content in coriander (Khater 2015).
In the current investigation, foliar-applied TiO2 NPs significantly accelerated the content and yield of the EO (Table 2) with 100 mg L−1 of TiO2 NPs showing the highest augmentation. The values gradually decreased when the concentration of TiO2 NPs was increased above 150 mg L−1, although the values still higher than the control treatment. The plant growth and crop yield are determined by the process of photosynthesis as it provides the substrates and energy for the growth and development of plants. The build-up of essential oils is intimately linked to CO2 fixation in dark reactions and the concentration of primary metabolites (Srivastava and Luthra 1991a, 1991b). Because carbohydrate precursors are always required for EO production, the process of photosynthesis will eventually alter the content of EO. As there is a constant necessity of carbohydrate precursors for the EO biosynthesis, the process of photosynthesis will ultimately influence the content of EO (Shabbir et al. 2019). Since EO is formed in the leaves, the test plant's leaf photosynthetic capability and growth are considered to be major factors of EO production. The probable reason for the improved synthesis of EO in the current study can be endorsed to TiO2 NPs-mediated enhancement in photosynthesis as these NPs positively modulate chlorophyll fluorescence parameters (ɸPSII, ETR and Fv/Fm), content of photosynthetic pigments, activity of carbonic anhydrase, which have resulted in better utilization of the light by the plants, and thereby, proficient CO2 assimilation might have resulted in increasing the carbohydrate content which are the precursors for the EO biosynthesis. As per Swamy and Rao (2009) escalated carbohydrate level and their possible mobilization towards secondary metabolism may have improved the EO content in plants. In line with the current investigation, improved quality and yield of the crops by exogenous application of TiO2 NPs have been previously observed by Ahmad et al. 2018; Gohari et al. 2020; Khater 2015; Morteza et al. 2013; Shabbir et al. 2019).
As per the GC reports (Fig. 4a, b), the TiO2 NPs used in the current study showed considerable enhancement in the content of menthol, a major aroma compound of Mentha arvensis L essential oil (EO) in comparison with control treatment. The EO synthesize in the leaves of Mentha arvensis L possesses monoterpenes (menthol here) derived from plastidial furnished precursors, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) using the methylerythritol phosphate (MEP) pathway (Mahmoud and Croteau 2001). Transcript abundance has been demonstrated to change in response to certain signals, e.g. sugars stimulate the synthesis of MEP pathway transcripts in plants (Hsieh and Goodman 2005). In the current investigation study, the sensitivity of genes for MEP pathway to sugars would not be surprising, considering that the leaf-applied TiO2 NPs stimulated photosynthetic machinery by augmenting light absorbance (ɸPSII, ETR and Fv/Fm), carbonic anhydrase activity and leaf chlorophyll content that eventually might increase the leaf carbohydrate which form the precursors for EO as discussed earlier might possibly have a favourable effect on the Mentha arvensis L. active constituent menthol biosynthesis. Overexpression of terpene biosynthesis enzymes (1-deoxy-d-xylulose 5-phosphate reducto-isomerase and 1-deoxy-d-xylulose 5-phosphate synthase) in transgenic peppermint has been demonstrated to boost terpene content by 100 per cent (Croteau et al. 2005). Moreover, in addition to the carbohydrate, the enhancement in the active constituent content possibly because of over expression of enzyme involved in monoterpenes biosynthesis (e.g. enhancement in menthol is possibly due to the overexpression of the reductase enzyme that convert menthone to menthol) (Ahmad et al. 2018). The foliar application of different concentrations of TiO2 NPs did not have a significant influence on physical properties of EO, viz. specific gravity and refractive index.
The present investigation indicates the growth-promoting influence of the TiO2 NPs. The physicochemical features of NPs enable them to elicit a wide range of plant responses. Exogenous treatment of TiO2 NPs enhanced the PSII activity, enzymatic activities (CA and NR), leaf carbohydrate content and leaf N status that ultimately perk up the yield qualities of Mentha arvensis L. The percentage of important active ingredients like menthol is greatly increased, which improves the quality of the plant's EO. This research could lead to agronomists determining the best TiO2 NP concentrations for various medicinal and aromatic plants (MAPs) in order to optimize EO quality and yield. Furthermore, because the NPs are only effective at low concentrations, this is a low-cost option.
Data Availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Abdel Latef AAH, Srivastava AK, El-sadek MSA, Kordrostami M, Tran LSP (2018) Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad Dev 29:1065–1073
Abdelaal KA, EL-MaghrabyElansaryHafezIbrahimEl-BannaEl-EsawiElkelish LMHYMEIMMA (2019) Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy 10:26
Abid M, Tian Z, Ata-Ul-Karim ST, Cui Y, Liu Y, Zahoor R, Jiang D, Dai T (2016) Nitrogen nutrition improves the potential of wheat (Triticum aestivum L.) to alleviate the effects of drought stress during vegetative growth periods. Front Plant Sci 7:981. https://doi.org/10.3389/fpls.2016.00981
Adams PR (2007) Identification of essential oil components by gas chromatography/ mass spectrometry. Allured Publishing Corporation, Carol Stream, IL, USA
Afaq SH, Siddiqui MMH, Tajuddin SA (1994) Standardization of herbal drugs. Aligarh Muslim University, Aligarh
Ahmad B, Shabbir A, Jaleel H, Khan MM, Sadiq Y (2018) Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Curr Plant Biol 13:6–15. https://doi.org/10.1016/j.cpb.2018.04.002
Ahmad B, Khan M, Jaleel H, Shabbir A, Sadiq Y, Uddin M (2020) Silicon nanoparticles mediated increase in glandular trichomes and regulation of photosynthetic and quality attributes in Mentha piperita L. J Plant Growth Regul 39:346–357
Ahmad B, Zaid A, Zulfiqar F, Bovand F, Dar TA (2022) Nanotechnology: a novel and sustainable approach towards heavy metal stress alleviation in plants. Nanotechnol Environ Eng 24:1–4
Ahmed KB, Khan MM, Siddiqui H, Jahan A (2020) Chitosan and its oligosaccharides, a promising option for sustainable crop production-a review. Carbohydr Polym 227:115331. https://doi.org/10.1016/j.carbpol.2019.115331
Ahmed KB, Khan M, Shabbir A, Ahmad B, Uddin M, Azam A (2022) Comparative effect of foliar application of silicon, titanium and zinc nanoparticles on the performance of vetiver—a medicinal and aromatic plant. SILICON 14:1–4
Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR (2009) Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol 10:634–641. https://doi.org/10.1038/nnano.2009.242
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621. https://doi.org/10.1093/jxb/erh196
Batsmanova LM, Gonchar LM, Taran NY, Okanenko AA (2013) Using a colloidal solution of metal nanoparticles as micronutrient fertiliser for cereals (Doctoral dissertation, Sumy StateUniversity) https://essuir.sumdu.edu.ua/bitstreamdownload/123456789/35441/1/princon_2013_2_4_38.pdf
Bharti AS, Sharma S, Shukla N, Uttam KN (2018) Steady state and time resolved laser-induced fluorescence of garlic plants treated with titanium dioxide nanoparticles. Spectrosc Lett 51:45–54
Brennan RF (1992) The role of manganese and nitrogen nutrition in the susceptibility of wheat plants to take-all in western Australia. Fertil Res 31:35–41. https://doi.org/10.1007/BF01064225
Carpita N, Sabularse D, Montezinos D, Delmer DP (1979) Determination of the pore size of cell walls of living plant cells. Science 205:1144–1147. https://doi.org/10.1126/science.205.4411.1144
Chen H, Seiber JN, Hotze M (2014) ACS select on nanotechnology in food and agriculture: a perspective on implications and applications. J Agric Food Chem 62:1209–1212. https://doi.org/10.1021/jf5002588
Croteau RB, Davis EM, Ringer KL, Wildung MR (2005) (-)-Menthol biosynthesis and molecular genetics. Naturwissenschaften 92:562–577. https://doi.org/10.1007/s00114-005-0055-0
Dubchak S, Ogar A, Mietelski JW, Turnau K (2010) Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radiocaesium in Helianthus annuus. Span J Agric Res 1:103–108. https://doi.org/10.1007/s00114-005-0055-0
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Dwivedi RS, Randhawa NS (1974) Evaluation of rapid test for the hidden hunger of zinc in plants. Plant Soil 40:445–451. https://doi.org/10.1007/BF00011531
Ebrahimi A, Galavi M, Ramroudi M, Moaveni P (2016) Study of agronomic traits of pinto bean (Phaseolus vulgaris L.) under nano TiO2 spraying at various growth stages. Int J Pharm Res Allied Sci 5:458–471
Fageria NK, Moreira A, Coelho AM (2011) Yield and yield components of upland rice as influenced by nitrogen sources. J of Plant Nutr 34:361–370. https://doi.org/10.1080/01904167.2011.536878
Faizan M, Bhat JA, Chen C, Alyemeni MN, Wijaya L, Ahmad P, Yu F (2021a) Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol Biochem 161:122–130
Faizan M, Faraz A, Mir AR, Hayat S (2021b) Role of zinc oxide nanoparticles in countering negative effects generated by cadmium in Lycopersicon esculentum. J Plant Growth Regul 40(1):101–115
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92. https://doi.org/10.1016/S0304-4165(89)80016-9
Ghasemnezhad A, Ghorbanpour M, Sohrabi O, Ashnavar M (2019) A general overview on application of nanoparticles in agriculture and plant science. Compr Anal Chem 87:85–110
Giraldo JP, Landry MP, Faltermeier SM, McNicholas TP, Iverson NM, Boghossian AA, jmjReuelHilmerSenBrewStrano NFAJFJAMS (2014) Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat Mater 13:400. https://doi.org/10.1038/nmat3890
Gohari G, Mohammadi A, Akbari A, Panahirad S, Dadpour MR, Fotopoulos V, Kimura S (2020) Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci Rep 10:1–4. https://doi.org/10.1038/s41598-020-57794-1
Guenther E (1972) The essential oils: history-origin in plants production-analysis. Krieger Publishing Company, Huntington, New York Robert E
Gupta AK, Mishra R, Singh AK, Srivastava A, Lal RK (2017) Genetic variability and correlations of essential oil yield with agro-economic traits in Mentha species and identification of promising cultivars. Ind Crop Prod 95:726–734. https://doi.org/10.1016/j.indcrop.2016.11.041
Hewitt EJ, Afridi MMRK (1959) Adaptive synthesis of nitrate reductase in higher plants. Nature 183:57–59. https://doi.org/10.1038/183057a0
Hong F, Zhou J, Liu C, Yang FC, Wu CL, Zheng L, Yang P (2005a) Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem Res 105:269–279. https://doi.org/10.1385/BTER:105:1-3:269
Hong FS, Yang P, Gao FQ, Liu C, Zheng L, Zhou J (2005b) Effect of nano-anatase TiO2 on spectral characterization of photosystem II particles from spinach. Chem Res Chin Univ 21:196–200
Jaberzadeh A, Moaveni P, Moghadam HRT, Zahedi H (2013) Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not Bot Horti Agrobot 41:201–207
Jaworski EJ (1971) Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun 43:1247–1279. https://doi.org/10.1016/S0006-291X(71)80010-4
Jenkins GL, Knevel AM, Digangi FE (1967) Quantitative pharmaceutical chemistry, 6th edn. McGraw Hill Book Company, London
Ji LN, Huang JJ, Mo TH (2001) Bioinorganic chemistry: introduction. Zhongshan University Press, Guangzhou, pp 119–149
Jing H, Xinyi W, Fan W, Chen W, White JC, Yang Y, Wang B, Xing B, Tao S, Wang X (2020) Potential application of titanium dioxide nanoparticles to improve the nutritional quality of coriander (Coriandrum sativum L.). J Hazard Mater 389:121837. https://doi.org/10.1016/j.jhazmat.2019.121837
Khater MS (2015) Effect of titanium nanoparticles (TiO2) on growth, yield and chemical constituents of coriander plants. Arab J Nucl Sci App 48:187–194
Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F, Biris AS (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 3:3221–3227. https://doi.org/10.1021/nn900887m
Kirschbaum MUF (2011) Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies. Plant Physiol 155:117. https://doi.org/10.1104/pp.110.166819
Lal RK (2013) Adaptability patterns and stable cultivar selection in menthol mint (Mentha arvensis L.). Ind Crop Prod 50:176–181. https://doi.org/10.1016/j.indcrop.2013.07.008
Lawrence BM (2007) Mint: the genus mentha. CRC Press, Boca Raton, FL
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 1(1):F4.3.1-F4.3.8. https://doi.org/10.1002/0471142913.faf0403s01
Lindner RC (1944) Rapid analytical methods for some of the common inorganic constitutes of plant tissue. Plant Physiol 19:76–89. https://doi.org/10.1104/pp.19.1.76
Ma X, Geiser-Lee J, Deng Y, Kolmakov A (2010) Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ 408:3053–3061. https://doi.org/10.1016/j.scitotenv.2010.03.031
Mahmoodzadeh H, Nabavi M, Kashefi H (2013) Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus). J Ornam Hortic Plants 3:25–32
Mahmoud SS, Croteau RB (2001) Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc Natl Acad Sci 98:8915–8920. https://doi.org/10.1073/pnas.141237298
Mandeh M, Omidi M, Rahaie M (2012) In vitro influences of TiO2 nanoparticles on barley (Hordeum vulgare L.) tissue culture. Biol Trace Elem Res 150:376–380. https://doi.org/10.1007/s12011-012-9480-z
Mingyu S, Hong F, Liu C, Wu X, Liu X, Chen L, Gao F, Yang F, Li Z (2007) Effects of nano-anatase TiO2 on absorption, distribution of light and photoreduction activities of chloroplast membrane of spinach. Biol Trace Elem Res 118:120. https://doi.org/10.1007/s12011-007-0006-z
Mishra V, Mishra RK, Dikshit A, Pandey AC (2014) Interactions of nanoparticles with plants. Emerging technologies and management of crop stress tolerance. Elsevier, pp 159–180. https://doi.org/10.1016/B978-0-12-800876-8.00008-4
Moaveni P, Farahani AH, Maroufi K (2011) Investigation of TiO2 nanoparticles affected on some of enzymes in calendula (Calendula Officinalis L.) under field condition. Adv Environ Biol l5: 2238–2241
Mohammad Mukarram M, Khan MA, Corpas FJ (2021) Silicon nanoparticles elicit an increase in lemongrass (Cymbopogon flexuosus (Steud.) Wats) agronomic parameters with a higher essential oil yield. J Hazard Mater 412:125254. https://doi.org/10.1016/j.jhazmat.2021.125254
Morteza E, Moaveni P, Farahani HA, Kiyani M (2013) Study of photosynthetic pigments changes of maize (Zea mays L.) under nano TiO2 spraying at various growth stages. Springerplus. https://doi.org/10.1186/2193-1801-2-247
Mukarram M, Petrik P, Mushtaq Z, Khan MM, Gulfishan M, Lux A (2022) Silicon nanoparticles in higher plants: uptake, action, stress tolerance, and crosstalk with phytohormones, antioxidants, and other signalling molecules. Environ Pollut 5:119855
Mustafa N, Raja NI, Ilyas N, Ikram M, Ehsan M (2021) Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress. Green Process Synth 10:246–257. https://doi.org/10.1515/gps-2021-0025
Nedbal L, Soukupova J, Whitmarsh J, Trtılek M (2000) Postharvest imaging of chlorophyll fluorescence from lemons can be used to predict fruit quality. Photosynthetica 38:571–579. https://doi.org/10.1023/A:1012413524395
Novozamsky I, Houba VJG, van Eck R, van Vark W (1983) A noval digestion technique for multi-element plant analysis. Commun Soil Sci Plant Anal 14:239–248. https://doi.org/10.1080/00103628309367359
Pestovsky YS, Martínez-Antonio A (2017) The use of nanoparticles and nano formulations in agriculture. J Nanosci Nanotechnol 17:8699–8730
Prakash OM, Naik M, Katiyar R, Naik S, Kumar D, Maji D, Shukla A, Nannaware AD, Kalra A, Rout PK (2018) Novel process for isolation of major bio-polymers from Mentha arvensis distilled biomass and saccharification of the isolated cellulose to glucose. Ind Crop Prod 119:1–8. https://doi.org/10.1016/j.indcrop.2018.03.063
Rastogi A, Tripathi DK, Yadav S, Chauhan DK, Živčák M, Ghorbanpour M, El-Sheery NI, Brestic M (2019) Application of silicon nanoparticles in agriculture. Biotech 9(3):1–1
Rondeau-Mouro C, Defer D, Leboeuf E, Lahaye M (2008) Assessment of cell wall porosity in Arabidopsis thaliana by NMR spectroscopy. Int J Biol Macromol 42:83–92. https://doi.org/10.1016/j.ijbiomac.2007.09.020
Satti SH, Raja NI, Javed B, Akram A, Z-u-R M, Ahmad MS, Ikram M (2021) Titanium dioxide nanoparticles elicited agromorphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE 16:e0246880. https://doi.org/10.1371/journal.pone.0246880
Shabbir A, Khan MMA, Ahmad B, Sadiq Y, Jaleel H, Uddin M (2019) Efficacy of TiO2 nanoparticles in enhancing the photosynthesis, essential oil and khusimol biosynthesis in Vetiveria zizanioides L. Nash Photosynthetica 57:599–606. https://doi.org/10.32615/ps.2019.071
Shangguan ZP, Shao MA, Dyckmans J (2000) Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J Plant Physiol 156:46–51. https://doi.org/10.1016/S0176-1617(00)80271-0
Sharma S, Chaudhary S, Kashyap SC, Sharma SK (2011) Room temperature ferromagnetism in Mn doped TiO2 thin films: electronic structure and raman investigations. J Appl Phys 109:083905
Sharma A, Vishwakarma K, Singh NK, Prakash V, Ramawat N, Prasad R, Sahi S, Singh VP, Tripathi DK, Sharma S (2022) Synergistic action of silicon nanoparticles and indole acetic acid in alleviation of chromium (CrVI) toxicity in Oryza sativa seedlings. J Biotechnol 343:71–82
Sheikh Mohamed M, Sakthi Kumar D (2016) Effect of nanoparticles on plants with regard to physiological attributes. In: Chittaranjan Kole D, Kumar S, Khodakovskaya MV (eds) Plant nanotechnology. Springer International Publishing, Cham, pp 119–153. https://doi.org/10.1007/978-3-319-42154-4_6
Singh A, Singh NB, Hussain I, Singh H, Singh SC (2015) Plant-nanoparticle interaction: an approach to improve agricultural practices and plant productivity. Int J Pharm Sci Invent 4:25–40
Singh P, Arif Y, Siddiqui H, Sami F, Zaidi R, Azam A, Alam P, Hayat S (2021) Nanoparticles enhances the salinity toxicity tolerance in Linum usitatissimum L. by modulating the antioxidative enzymes, photosynthetic efficiency, redox status and cellular damage. Ecotoxicol Environ Saf 213:112020
Srivastava NK, Luthra R (1991a) Distribution of photosynthetically fixed 14CO2 into essential oil in relation to primary metabolites in developing peppermint (Mentha piperita) leaves. Plant Sci 76:153–157
Srivastava NK, Luthra R (1991b) Interspecific variation in mints for photosynthetic efficiency, and 14-C primary metabolic pool in relation to essential oil accumulation. J Plant Physiol 138:650–654. https://doi.org/10.1016/S0176-1617(11)81310-6
Swamy KN, Rao SSR (2009) Effect of 24-epibrassinolide on growth, photosynthesis, and essential oil content of Pelargonium graveolens (L.) Herit. Russ J Plant Physiol 56:616–620. https://doi.org/10.1134/S1021443709050057
Taiz L, Zeiger E, Moller IM, Murphy A (2014) Plant physiology, 6th edn. Sinauer Associates Inc., Sunderland, Massachusetts
Vargas-Hernandez M, Macias-Bobadilla I, Guevara-Gonzalez RG, Rico-Garcia E, Ocampo-Velazquez RV, Avila-Juarez L, Torres-Pacheco I (2020) Nanoparticles as potential antivirals in agriculture. Agriculture 10:444
Yang P, Gao F (2002) Bioinorganic chemistry: theory. Science Press, Beijing, pp 186–189
Yang F, Hong F, You W, Liu C, Gao F, Wu C, Yang P (2006) Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol Trace Elem Res 110:179–190. https://doi.org/10.1385/bter:110:2:179
Yang F, Liu C, Gao F, Su M, Wum Z, Zheng L, Hong F, Yang P (2007) The Improvement of Spinach growth by nano-anatase TiO2 treatment is related to nitrogen photo-reduction. Biol Trace Elem Res 119:77–88. https://doi.org/10.1007/s12011-007-0046-4
Acknowledgements
The authors thank CIMAP, Lucknow, India, for supplying legitimate planting material, and USIF, Aligarh Muslim University, Aligarh, India, for providing NPs SEM and EDX, and Department Applied Physics, AMU, Aligarh for providing the TiO2 NPs.
Funding
All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Contributions
AJ conducted and analysed the data of the experiments and also prepared the first draft of manuscript. MMAK assisted in drafting the manuscript. KBMA helped in characterization and study of PSII parameters. BA, YS and MG helped in manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jahan, A., Khan, M.M.A., Ahmad, B. et al. Influence of titanium dioxide nanoparticles on growth, physiological attributes and essential oil production of Mentha arvensis L.. Braz. J. Bot 46, 337–349 (2023). https://doi.org/10.1007/s40415-023-00880-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-023-00880-1