Abstract
Azospirillum brasilense Tarrand, which has the potential to stimulate plant growth, belongs to plant-growth-promoting bacteria. Many species of azospirilla colonize the rhizosphere, the portion of soil attached to the root surface. Some species can also enter the host root system and enhance their beneficial effects with an endophytic lifestyle. Depending on the specific agroecological situation, the positive effect of Azospirillum on plants may be due to different mechanisms. Azospirilla can assist in mitigation of many kinds of abiotic stress. Although they can affect antioxidant enzyme activity in abiotically stressed plants, the underlying mechanisms are not fully understood. The surface lectins of A. brasilense strains Sp7 and Sp245 differ in carbohydrate specificity and in the mode of plant root colonization. They promote plant growth and enzyme activity, and they also can alter the plant cell content of stress metabolites, which attests that they can induce adaptation processes in wheat seedling roots. Here we comparatively investigated the ability of the Sp7 and Sp245 lectins (concentration, 5–40 µg ml−1) to regulate the activities of antioxidant enzymes in roots of 4-day-old seedlings of wheat subjected to hypothermic (5 °C) and hyperthermic (42 °C) stress. Both lectins increased peroxidase and superoxide dismutase activities and decreased catalase activity, but the effects lasted for different times and the concentrations involved were also different. We conclude that the Azospirillum lectins are involved in adaptational changes in wheat seedling roots and that this involvement promotes the normal course of metabolism and ensures regulation of the plant–Azospirillum interaction in a wider range of soil and climatic factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Adverse climatic conditions, creating abiotic stresses, are among the principal factors limiting agricultural productivity (Padgham 2009; Grayson 2013). Extreme temperatures conditions can severely affect plants. Therefore, study of the mechanisms governing tolerance and adaptation in higher plants is of great scientific and practical significance. Because plants lack behavioral mechanisms of defense against unfavorable factors, the major adaptive changes occur primarily at a biochemical level (Tarchevskii 2001). A group of nonspecific responses to adverse exposures have been found recently, including (1) changes in cell membrane permeability and in intracellular pH and (2) accumulation of protective substances such as stress proteins, lipids, and soluble carbohydrates (Hasanuzzaman et al. 2013). One of the earliest effects is oxidative stress caused by the accumulation of reactive oxygen species (ROS). To protect themselves from this stress, plants have developed enzymatic antioxidative systems consisting of superoxide dismutase, catalase, and peroxidases (Almeselmani et al. 2006; Nagesh Babu and Devraj 2008).
The role of microorganisms, with their potential metabolic and genetic capabilities, in alleviating abiotic stress in plants has been studied intensely in the past few decades (Nadeem et al. 2007; Turner et al. 2013; Gopalakrishnan et al. 2015; Souza et al. 2015). Many researchers have argued that plant-growth-promoting rhizobacteria (PGPR) can reduce the consequences of abiotic stress in plants (El-Komy et al. 2003; Pereyra et al. 2006; Arzanesh et al. 2009). These PGPR include Pseudomonas (Ali et al. 2009; Sorty et al. 2016), Azotobacter (Sahoo et al. 2014), Azospirillum (Creus et al. 2004; Omar et al. 2009), Rhizobium (Remans et al. 2008; Sorty et al. 2016), Bacillus (Vardharajula et al. 2011; Sorty et al. 2016), Enterobacter (Nadeem et al. 2007; Sorty et al. 2016), Bradyrhizobium (Panlada et al. 2013), Methylobacterium (Madhaiyan et al. 2007; Meena et al. 2012), and Burkholderia (Oliveira et al. 2009).
The application of PGPR to abiotically stressed plants significantly increases the content of defense-related enzymes such as superoxide dismutase, peroxidase, catalase, polyphenol oxidase, phenylalanine ammonia-lyase, and lipoxygenase (Liang et al. 2011; Chakraborty et al. 2015). However, few data are available about the mechanisms of the bacteria-mediated antioxidative protection of plants.
Azospirillum spp. are the most studied PGPR and are a common model for research on plant–bacterial interactions. These bacteria take advantage of many plant-growth-promoting mechanisms (Bashan et al. 2014) and have been used as inoculants in crop production, initially with cereals but later with other plants. They stimulate plant growth through fixation of N2, synthesis of phytohormones, solubilization of phosphates, improvement of plant water and mineral status, production of compounds that increase membrane activity and proliferation of root tissues, and decrease in stressor influence (Bashan et al. 2004; Baldani and Baldani 2005; Alen’kina et al. 2006). Although research in this area is active, an open question remains on which of the above factors, explaining the benefit of N2-fixing bacteria to plant growth and performance, has priority over the others.
Azospirillum can colonize roots externally and/or internally, or it can colonize the stem as an endophyte, as seen in rice (Oryza sativa L.), with some strains doing both (Ramos et al. 2002; Xie and Yokota 2005). By use of fluorescently labeled probes and monoclonal antibodies, Assmus et al. (1995) and Schloter et al. (1997) detected Azospirillum in both the plant interior and the rhizosphere. Specifically, A. brasilense Tarrand Sp245 (Tarrand et al. 1978) was found in the root xylem, whereas Sp7 (Tarrand et al. 1978) was detected only on the root surface (Schloter et al. 1997). Endophytic bacteria are of particular research interest, because they can lead a mutualistic life in the plant tissue interior. This ability permits them to depend less on extrinsic environmental factors, as compared with other microorganisms, and to manifest a complex of economically useful properties. When inside the plant tissue, endophytes contribute to sustained plant defense against environmental stress.
Among the high molecular weight and specific substances implicated in interorganismal communication, an important part is played by lectins, glycoproteins that bind strictly specified carbohydrate groups on the surface of a target cell. There is ample evidence that plant lectins are implicated in bacterial colonization of plants and in the restructuring of the metabolism of the bacterial symbiont. Plant lectins act as adaptogens for plants; for example, wheat germ agglutinin changes antioxidant enzyme activity in seeds and broadens plant adaptability (Kruhova et al. 1999). Less is known about the role of bacterial lectins, which nonetheless are involved in the important “molecular dialog” during the development of a symbiosis (Nikitina et al. 1996; Castellanos et al. 1998).
Previously, we have reported the isolation of surface lectins from two A. brasilense strains, Sp7 (epiphyte) and Sp245 (endophyte), differing in the mode of plant colonization. The lectins have been found to be glycoproteins with different molecular masses and carbohydrate specificities (Nikitina et al. 2005; Shelud’ko et al. 2009). The 36-kDa Sp7 lectin was specific for l-fucose (1.87 mM) and d-galactose (20 mM). The Sp245 lectin had an affinity for the bacterium’s own polysaccharide, an acidic d-rhamnan, and had a molecular mass of 67 kDa.
Both Sp7 and Sp245 lectins are polyfunctional. Apart from functioning as adhesins, they can influence plant cell metabolism by promoting seed germination (Nikitina et al. 2004) and by expressing mitogenic and enzyme-modifying activities toward the plant cell (Chernyshova et al. 2005; Alen’kina et al. 2006; Alen’kina and Nikitina 2015, 2017). In addition, they can alter the plant cell content of stress metabolites (Alen’kina et al. 2014). Finally, lectin activity in Azospirillum can be promoted by adverse effects and even by stress, possibly also owing to the adaptogenic function of lectins (Nikitina et al. 2005).
Here we comparatively evaluated the ability of the lectins from A. brasilense Sp7 and Sp245 to regulate the activities of peroxidase, catalase, and superoxide dismutase in roots of wheat seedlings subjected to short-term hypothermic and hyperthermic stress.
2 Materials and methods
Strains and growth conditions
– Azospirillum brasilense Sp7 (epiphytic strain) was obtained from the culture collection of Winogradsky Institute of Microbiology, Russian Academy of Sciences, Moscow. A. brasilense Sp245 (IBPPM 219; endophytic strain) was from the IBPPM RAS Collection of Rhizosphere Microorganisms (http://collection.ibppm.ru). The cultures were grown in the minimal salts medium described by Sadasivan and Neyra (1985) at 37 °C for 18 h.
Lectin isolation
– Lectins were isolated from the surface of Sp7 and Sp245 and were purified by gel filtration on a 30 × 2.2-cm column of Sephadex G-75 (particle diameter, 40–120 µm). The emergence of protein fractions was followed at 278 nm with a Uvicord SII apparatus (LKB, Sweden). The eluents were 0.1 M CH3COOH (pH 4.8) and 0.05 M phosphate-buffered saline (PBS; pH 7.0) containing 0.15 M NaCl. The flow rate was 1.5 ml min−1 (Alen’kina et al. 2006). To confirm the lectin nature of the purified material, we conducted a hemagglutination assay as described by Lakhtin (1989). Fifty-microliter portions of successive twofold dilutions of lectin solutions were added to the wells of a microtitration plate, with PBS as a control. Washed trypsin-treated rabbit erythrocytes were added at a concentration of 2% in PBS and were incubated at room temperature for 2 h. The hemagglutination titer was the minimum lectin concentration that gave hemagglutination.
Animals were cared for and handled in compliance with the Guide for the Care and Use of Laboratory Animals, the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes, and the legislation of the Russian Federation. The use of the animals was also approved by the institution where the experiments were done.
Protein assay
– Protein was estimated by the Bradford method (1976).
Seedling growth and stress treatments
– Seeds of wheat (Triticum aestivum L. cv. Saratovskaya 29) [Agricultural Research Institute for South-East Region (ARISER), Saratov, Russia] were surface-sterilized in 70% (v/v) ethanol for 1 min and were washed five times with sterile water. For seedling roots, seeds were grown aseptically in petri dishes on sterile distilled water and were incubated in the dark at 25 °C. Seedlings used for experiments were 4 days old. In all experimental treatments, the initial and experimental seedlings were adjusted for physiological age. A root length of 25–30 mm was the criterion that the initial plant material was homogeneous.
For stress experiments, roots were simultaneously exposed for 2 h to either Sp7 or Sp245 lectin (concentration, 5–40 µg ml−1) and temperatures of 42 and 5 °C. The roots were then homogenized in 0.15 M PBS (pH 7.8), the homogenate was centrifuged at 7000× g for 10 min, and the supernatant liquid was used to determine enzyme activities. Seedlings grown at 25 °C were the control group.
Peroxidase assay
– Peroxidase (EC 1.11.1.7.) was assayed by Khairullin et al.’s (2001) micromethod, based on the oxidation of o-phenylenediamine (OPD). A 50-µl portion of supernatant liquid prediluted 20-fold with PBS (pH 5.6) and 25 µl of OPD solution (concentration, 0.5 mg ml−1) were added to each well of a flat-bottomed immunoassay plate (Nunc, USA). Two min after 25 µl of 0.43 mM H2O2 was added, color development was stopped with 50 µl of 4 N H2SO4. The absorption of the samples was measured at 492 nm with an AIF-Ts-01S ELISA reader (ZAO ILIP, St. Petersburg, Russia). Peroxidase activity was expressed as absorption units per g of root wet weight and, for comparative purposes, as relative units.
Catalase assay
– Catalase (EC 1.11.1.6) activity was assayed as described by Aebi (1984). The decrease in H2O2 was measured at 240 nm, and the activity was calculated as units (μM H2O2 consumed per min) per g of root weight (extinction coefficient, 39.4 mM−1 cm−1). For comparative purposes, it was also expressed as relative units.
Superoxide dismutase assay
– The activity of superoxide dismutase (SOD; EC 1.15.1.11) was assayed by the inhibition of the reduction rate for tetrazolium nitroblue in a nonenzymatic system containing phenazine methosulfate and NADH (Alscher et al. 2002). The absorbance of formazan (oxidation product of tetrazolium nitroblue) was measured at 560 nm and was used to calculate the enzyme activity. The results are presented as relative units.
Statistics
– The analysis was run with the AGROS program package for statistical and biometrical–genetic analysis in plant breeding and selection (version 2.09; Department of Statistical Analysis, Russian Academy of Agricultural Sciences). Least significant differences (LSD0.05) were determined at a significance level of P = 0.05. Values followed by different letters (a, b, c, d) differ significantly at P ≤ 0.05, according to Duncan’s multiple range test. The figures show arithmetic mean ± standard error (SE) of three independent experiments, done in five biological replications.
3 Results
Lectin effects on antioxidant enzyme activity in wheat seedling roots exposed to hypothermic and hyperthermic stress were investigated with three enzymes. These included (1) SOD, catalyzes the dismutation of superoxide radicals to hydrogen peroxide and molecular oxygen and (2) peroxidase and catalase, which degrade hydrogen peroxide.
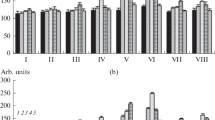
Both Sp7 and Sp245 lectins increased peroxidase activity in roots exposed to hypothermic and hyperthermic stress. The picture was the same under both types of stress. With all four concentrations of the Sp7 lectin, the activity rose after 30 min of incubation, peaking at 20 µg ml−1, and then declined gradually to the control value. With the Sp245 lectin, peroxidase activity increased after 60 min of incubation and the increase was proportional to the lectin concentration (Fig. 1).
Effect of the Azospirillum brasilense Sp7 and Sp245 lectins on the activities of peroxidase in wheat seedling roots exposed to 5 and 42 °C. Results are expressed as mean ± SE (n = 5). Mean separation among treatments was done by Duncan test at P ≤ 0.05. Mean values followed by different letters are significantly different
Both lectins also enhanced the activity of SOD in the stressed roots (Fig. 2). Under hypothermic conditions, the activity of SOD increased with all concentrations of either lectin after the roots were exposed for 1 h. The most effective lectin concentrations were 20 µg ml−1 (Sp7 lectin) and 10 µg ml−1 (Sp245 lectin). The same picture emerged under hyperthermic conditions: the enzyme activity rose after the roots were incubated with the lectins for 1 h. The most effective lectin concentrations were 10 µg ml−1 (Sp7 lectin) and 5 µg ml−1 (Sp245 lectin).
Effect of the Azospirillum brasilense Sp7 and Sp245 lectins on the activities of SOD in wheat seedling roots exposed to 5 and 42 °C. Results are expressed as mean ± SE (n = 3). Mean separation among treatments was done by Duncan test at P ≤ 0.05. Mean values followed by different letters are significantly different
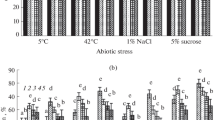
Under hypothermic conditions, both Sp7 and Sp245 lectins decreased root catalase activity. The inhibition peaked as early as 15 min after exposure. Thirty min into exposure, the inhibition slowly decreased, and by 1 h of incubation, it was back to the control value. The effect was maximal with 5 µg ml−1 of either lectin (Fig. 3). The same was observed when the stress was changed to hyperthermic: catalase activity declined with both lectins, and the effect was maximal with 5 µg ml−1 (Fig. 3).
Effect of the Azospirillum brasilense Sp7 and Sp245 lectins on the activities of catalase in wheat seedling roots exposed to 5 and 42 °C. Results are expressed as mean ± SE (n = 3). Mean separation among treatments was done by Duncan test at P ≤ 0.05. Mean values followed by different letters are significantly different
The Sp7 and Sp245 lectins regulated the enzyme activities differently. Under both types of stress, the Sp245 lectin promoted peroxidase and SOD activities more than did the Sp7 lectin.
As noted above, both lectins decreased catalase activity in the temperature-stressed roots. Under both types of stress, the inhibition achieved with the Sp245 lectin was greater than that attained with the Sp7 lectin.
4 Discussion
Temperatures stress is a serious problem in agriculture and a critical factor for plant survival. Both high and low temperatures affect plant metabolism: high temperature disrupts the quaternary structure of protein complexes, whereas low temperature greatly decreases plant performance (Timperio et al. 2008; Zhestkova et al. 2009).
For most wheat cultivars, the highest germination temperature is, on average, 38° and the best germination temperature lies between 20 and 32 °C. Temperatures beyond these limits are considered unfavorable and have adverse effects on plants, including decreased yields and grain quality.
PGPR have been used mostly to promote plant growth, because they can stimulate plants through different means, including production of plant growth regulators and fixation of N2 (Bashan et al. 2014). Studies have reported additional beneficial effects of PGPR on plants through their ability to improve tolerance for abiotic (including temperature) stress (El-Komy et al. 2003; Pereyra et al. 2006; Arzanesh et al. 2009).
Several abiotic stresses are related to the accumulation of ROS in plant cells. Reactions of these compounds with proteins, membrane lipids, and DNA may cause severe oxidative damage. Avoiding oxidative stress is necessary for plant survival under temperature stress. ROS are removed by several enzymes such as catalase, peroxidases, and SOD, which together form the plant antioxidant system. However, few data are available about the mechanisms of the bacteria-mediated antioxidative protection of plants (Reddy et al. 2004).
Temperature stress affects the growth of plants throughout their ontogeny, although the threshold level varies considerably at different developmental stages. For instance, during seed germination, high and low temperatures may slow down or totally inhibit germination, depending on the plant species and the stress intensity. The events determining the entire course of resistance development occur in this very period of adaptation to unfavorable factors.
The large body of experimental data in the literature indicates that lectins are polyfunctional proteins. In addition to being able to reversibly and specifically bind to target cells, they can express biological activity. This means that low concentrations of lectins can induce cellular responses (Messina et al. 1987; Antonyuk et al. 1993). This ability was confirmed in our previous work on the effect of Azospirillum lectins on seed germination (Nikitina et al. 2004), mitogenic and enzyme-modifying activities (Chernyshova et al. 2005; Alen’kina et al. 2006; Alen’kina and Nikitina 2015, 2017), and alteration of the plant cell content of stress metabolites (Alen’kina et al. 2014). The lectin effects found in this work were recorded in the same concentration range as used in those previous studies.
Our research has shown that both Sp7 and Sp245 lectins substantially modified the enzyme activities as early as several minutes into stress. Both lectins increased peroxidase and SOD activities but decreased catalase activity in the stressed roots. In all cases, the two lectins regulated the enzyme activities differently, a finding in good agreement with our earlier results (Alen’kina et al. 2006, 2010, 2014; Alen’kina and Nikitina 2015, 2017). These differences may have been caused by the differences in structure and in carbohydrate specificity (Nikitina et al. 2005; Shelud’ko et al. 2009), resulting in differences in the interaction with the plant cell surface, which are of deciding importance for the “switch on” of the subsequent stages.
The differences in the concentration at which the lectins were effective may have been due to the action of adverse temperatures on lectin binding to the root receptors. Our data attest to the complex character of growth regulation, which is reflected in the complex concentration effects. Concentration dependences may be conducive to high physiological heterogeneity even when concentrations vary slightly for natural reasons. In view of this, concentration dependence studies are important for understanding the processes occurring during plant adaptation to environmental conditions and for the correct application of lectins as plant growth regulators.
Our data confirm the results of Arzanesh et al. (2009) and Baniaghil et al. (2013) that azospirilla can increase the activities of plant peroxidase and SOD at different abiotic stresses. The decrease in catalase activity could have been due to the effect of salicylic acid, whose synthesis is induced by Azospirillum lectins (Scandalios 2005).
Together with our earlier data, the findings of this study indicate that the Azospirillum lectins are implicated in plant adaptation and can induce plant defense mechanisms. These lectin properties, in combination with the growth-promoting activity of Azospirillum bacteria, conduce to plant resistance to adverse factors and to increased plant productivity.
References
Aebi H (1984) Catalase in vitro. In: Packer L (ed) Methods in enzymology, vol 105. Academic Press, San Diego, pp 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Alen’kina SA, Nikitina VE (2015) Effect of Azospirillum lectins on the activity of proteolytic enzymes and their inhibitors in wheat seedling roots. Microbiology 84:630–635. https://doi.org/10.1134/s0026261715050021
Alen’kina SA, Nikitina VE (2017) Change in the ratio of the activities of different types of proteases and their inhibitors in plant roots exposed to Azospirillum lectins. J Plant Regul 381:337–349. https://doi.org/10.1007/s00344-016-9658-2
Alen’kina SA, Payusova OA, Nikitina VE (2006) Effect of Azospirillum lectins on the activities of wheat-root hydrolytic enzymes. Plant Soil 283:147–151. https://doi.org/10.1007/s11104-005-4890-8
Alen’kina SA, Bogatyrev VA, Matora LYu, Sokolova MK, Chernysheva MP, Trutneva KA, Nikitina VE (2014) Signal effects of the lectin from the associative nitrogen-fixing bacterium Azospirillum brasilense Sp7 in bacterial–plant root interactions. Plant Soil 381:337–349. https://doi.org/10.1134/s0026261715050021
Alen’kina SA, Matora LY, Nikitina VE (2010) Assessment of the effect of azospirilla lectins on c-AMP level in plant cells. Microbiology 79:853–855. https://doi.org/10.1134/S0026261710060202
Ali SZ, Sandhya V, Grover M, Kishore N, Rao LV, Venkateswarlu B (2009) Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol Fertil Soil 46:45–55. https://doi.org/10.1007/s00374-009-0404-9
Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP (2006) Protective role of antioxidant enzymes under high temperature stress. Plant Sci 171:382–388. https://doi.org/10.1007/s10535-015-0539-5
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress plants. J Exp Bot 53:1331–1341. https://doi.org/10.1093/jexbot/53.372.1331
Antonyuk LP, Fomina OR, Galkin MA, Ignatov VV (1993) The effect of wheat germ agglutinin on dinitrogen fixation, glutamine synthetase activity and ammonia excretion in Azospirillum brasilense Sp245. FEMS Microbiol Lett 110:285–290. https://doi.org/10.1111/j.1574-6968.1993.tb06336.x
Arzanesh MH, Alikhani HA, Khavazi K, Rahimian HA, Miransari M (2009) In vitro growth of wheat (Triticum aestivum L.) seedlings, inoculated with Azospirillum sp., under drought stress. Int J Bot 5:244–249. https://doi.org/10.3923/ijb.2009.244.249
Assmus B, Hutzler P, Kirchhof G, Amann R, Lawrence JR, Hartmann A (1995) In situ localization of Azospirillum brasilense in the rhizosphere of wheat with fluorescently labeled, rRNA-targeted oligonucleotide probes and scanning confocal laser microscopy. Appl Environ Microb 61:1013–1019
Baldani JI, Baldani VLD (2005) History on the biological nitrogen fixation research in graminaceous plants: special emphasis on the Brazilian experience. An Acad Bras Cienc 77:549–579. https://doi.org/10.1590/S0001-37652005000300014
Baniaghil N, Arzanesh MH, Ghorbanli M, Shahbazi M (2013) The effect of plant growth promoting rhizobacteria on growth parameters, antioxidant enzymes and microelements of canola under salt stress. J Appl Environ Biol Sci 3:17–27
Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003). Can J Microbiol 50:521–577. https://doi.org/10.1139/w04-035
Bashan Y, de-Bashan LE, Prabhu SR, Hernandez J-P (2014) Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013). Plant Soil 378:1–33. https://doi.org/10.1007/s11104-013-1956-x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Castellanos T, Ascencio F, Bashan Y (1998) Cell-surface lectins of Azospirillum spp. Curr Microbiol 36:241–244. https://doi.org/10.1007/s002849900302
Chakraborty K, Joseph D, Praveen NK (2015) Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the Gulf of Mannar of Peninsular. J Food Sci Technol 52:1924–1935. https://doi.org/10.1007/s13197-013-1189-2
Chernyshova MP, Alen’kina SA, Nikitina VE, Ignatov VV (2005) Extracellular proteolytic enzymes of Azospirillum brasilense strain Sp7 and regulation of their activity by homologous lectin. Appl Biochem Microbiol 41:390–393. https://doi.org/10.1007/s10438-005-0066-9
Creus CM, Sueldo RJ, Barassi CA (2004) Water relations and yield in Azospirillum-inoculated wheat exposed to drought in the field. Can J Bot 82:273–281. https://doi.org/10.1139/b03-119
El-Komy HM, Hamdia MA, Abd El-Baki GK (2003) Nitrate reductase in wheat plants grown under water stress and inoculated with Azospirillum spp. Biol Plant 46:281–287. https://doi.org/10.1023/A:1022819114860
Gopalakrishnan S, Sathya A, Vijayabharathi R, Varshney RK, Gowda CL, Krishnamurthy L (2015) Plant growth promoting rhizobia: challenges and opportunities. 3Biotech 5:355–377
Grayson M (2013) Agriculture and drought. Nature 501:S1. https://doi.org/10.1038/501S1a
Hasanuzzaman M, Nahar K, Alam MdM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684. https://doi.org/10.3390/ijms14059643
Khairullin RM, Yarullina LG, Troshina NB, Akhmetova IE (2001) Chitooligosaccharide-induced activation of o-phenylenediamine oxidation by wheat seedlings in the presence of oxalic acid. Biochemistry (Moscow) 66:286–289. https://doi.org/10.1023/A:1010247712723
Kruhova OD, Mandrovs’ka NM, Kyrychenko OV (1999) Effect of exogenous lectin on the endogenous lectin and antioxidant enzymes activity and flavonoid content in wheat. Ukr Biochem J 78:106–112
Lakhtin VM (1989) Lectins and aspects of their study. Microbiol J 51:69–74
Liang FS, Ho WQ, Crabtree GR (2011) Engineering the ABA plant stress pathway for regulation of induced proximity. Sci Signal 15:RS2. https://doi.org/10.1126/scisignal.2001449
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69:220–228. https://doi.org/10.1016/j.chemosphere.2007.04.017
Meena KK, Kumar M, Kalyuzhnaya MG, Yandigeri MS, Singh DP, Saxena AK, Arora DK (2012) Epiphytic pink-pigmented methylotrophic bacteria enhance germination and seedling growth of wheat (Triticum aestivum) by producing phytohormone. Antonie Van Leeuwenhoek 101:777–786. https://doi.org/10.1007/s10482-011-9692-9
Messina JL, Hamlin J, Larner J (1987) Insulin-mimetic actions of wheat germ agglutinin and concanavalin A on specific mRNA levels. Arch Biochem Biophys 254:110–115
Nadeem SM, Zahir ZA, Naveed M, Arshad M (2007) Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can J Microbiol 53:1141–1149. https://doi.org/10.1139/W07-081
Nagesh Babu R, Devaraj VR (2008) High temperature and salt stress response in French bean (Phaseolus vulgaris). Aust J Crop Sci 2:40–48
Nikitina VE, Alen’kina SA, Ponomareva EG, Savenkova NN (1996) Role of lectins of the cell surface of azospirilla in association with wheat roots. Microbiology (Moscow) 65:144–148
Nikitina VE, Bogomolova NV, Ponomareva EG, Sokolov OI (2004) Effect of azospirilla lectins on germination capacity of seeds. Biol Bull 31:354–357. https://doi.org/10.1023/B:BIBU.0000036939.98396.24
Nikitina VE, Ponomareva EG, Alen’kina SA (2005) Azospirillum cell surface lectins and their role in associative plant–bacterial interactions. In: Ignatov VV (ed) Molecular bases of the relationships between associative microorganisms and plants. Nauka, Moscow, pp 70–97 (in Russian)
Oliveira CA, Alves VMC, Marriel IE, Gomes EA, Scotti MR, Carneiro NP, Guimara˜es CT, Schaffert RE, Sa´ NMH (2009) Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado biome. Soil Biol Biochem 41:1782–1787. https://doi.org/10.1016/j.soilbio.2008.01.012
Omar MNA, Osman MEH, Kasim WA, Abd El-Daim IA (2009) Improvement of salt tolerance mechanisms of barley cultivated under salt stress using Azospirillum brasiliense. Tasks Veg Sci 44:133–147. https://doi.org/10.1007/978-1-4020-9065-3_15
Padgham J (2009) Agricultural development under a changing climate: opportunities and challenges for adaptation. Agriculture and Rural Development & Environmental Departments, The World Bank, Washington, DC
Panlada T, Pongdet P, Aphakorn L, Rujirek N-N, Nantakorn B, Neung T (2013) Alleviation of the effect of environmental stresses using co-inoculation of mungbean by Bradyrhizobium and rhizobacteria containing stress-induced ACC deaminase enzyme. Soil Sci Plant Nut 59:559–571. https://doi.org/10.1080/00380768.2013.804391
Pereyra MA, Zalazar CA, Barassia CA (2006) Root phospholipids in Azospirillum-inoculated wheat seedlings exposed to water stress. Plant Physiol Biochem 44:873–879. https://doi.org/10.1016/j.plaphy.2006.10.020
Ramos I, Esteban E, Lucena JJ, Gárate A (2002) Cadmium uptake and subcellular distribution in plants Latuca sp. Cd–Mn interaction. Plant Sci 162:761–767. https://doi.org/10.1016/S0168-9452(02)00017-1
Reddy AR, Chaitanyaa KV, Vivekanandanb M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202. https://doi.org/10.1016/j.jplph.2004.01.013
Remans R, Beebe S, Blair M, Manrique G, Tovar E, Rao IM, Croonenborghs A, Torres-Gutierrez R, El-Howeity M, Michiels J, Vanderleyden J (2008) Physiological and genetic analysis of root responsiveness to auxin-producing plant growth-promoting bacteria in common bean (Phaseolus vulgaris L.). Plant Soil 302:149–161. https://doi.org/10.1007/s11104-007-9462-7
Sadasivan L, Neyra CA (1985) Flocculation in Azospirillum brasilense and Azospirillum lipoferum. J Bacteriol 163:716–723. https://doi.org/0021-9193/85/080716-08$02.00/0
Sahoo RK, Ansari MW, Pradhan M, Dangar TK, Mohanty S, Tuteja N (2014) A novel Azotobacter vinellandii (SRIAz 3) functions in salinity stress tolerance in rice. Plant Signal Behav 9:e29377. https://doi.org/10.4161/psb.29377
Scandalios JG (2005) Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res 38:995–1014. https://doi.org/10.1590/S0100-879X2005000700003
Schloter M, Wiehe W, Assmus B, Steindl H, Becke H, Hoftich G, Hartmann A (1997) Root colonization of different plants by plant-growth-promoting Rhizobium leguminosarum bv. trifolii R39 studied with monospecific polyclonal antisera. Appl Environ Microbiol 63:2038–2046
Shelud’ko AV, Ponomareva EG, Varshalomidze OE, Vetchinkina EP, Katsy EI, Nikitina VE (2009) Hemagglutinating activity and motility of the bacterium Azospirillum brasilense in the presence of various nitrogen sources. Microbiology 78:696–702. https://doi.org/10.1134/s0026261709060058
Sorty AM, Meena KK, Choudhary K, Bitla UM, Minhas PS, Krishnani KK (2016) Effect of plant growth promoting bacteria associated with halophytic weed (Psoralea corylifolia L.) on germination and seedling growth of wheat under saline conditions. Appl Biochem Biotechnol 180:872–882. https://doi.org/10.1007/s12010-016-2139-z
Souza RD, Ambrosini A, Passaglia LMP (2015) Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol 38:401–419. https://doi.org/10.1590/S1415-475738420150053
Tarchevskii IA (2001) Plant metabolism under stress. Fen, Kazan
Tarrand JJ, Krieg NR, Döbereiner J (1978) A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol 24:967–980. https://doi.org/10.1139/m78-160
Timperio AM, Egidi MG, Zolla L (2008) Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP). J Proteom 71:391–411. https://doi.org/10.1016/j.jprot.2008.07.005
Turner TR, James EK, Poole PS (2013) The plant microbiome. Genome Biol 14:209. https://doi.org/10.1186/gb-2013-14-6-209
Vardharajula S, Ali SZ, Grover M, Reddy G, Bandi V (2011) Drought-tolerant plant growth promoting Bacillus spp.: effect on growth osmolytes, and antioxidant status of maize under drought stress. J Plant Interact 6:1–14. https://doi.org/10.1080/17429145.2010.535178
Xie CH, Yokota A (2005) Azospirillum oryzae sp. nov., a nitrogen-fixing bacterium isolated from the roots of the rice plant Oryza sativa. Int J Syst Evol Microbiol 55:1435–1438. https://doi.org/10.1099/ijs.0.63503-0
Zhestkova IM, Ampilogova YN, Shevyreva TA, Trofimova MS (2009) Effect of chilling temperatures on osmotic water permeability and aquaporin activity in the plasma membranes from pea roots. Russ J Plant Physiol 6:635–641. https://doi.org/10.1134/S1021443709050082
Acknowledgements
We thank Dmitry N. Tychinin (this institute) for the English version of this manuscript.
Author information
Authors and Affiliations
Contributions
VN is guarantor of integrity of entire study; SA and VN were involved in study concepts; NR was involved in study design, experimental device and data acquisition ; SA was involved in literature research, data analysis, statistical analysis, manuscript preparation, and manuscript editing ; VN reviewed the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Alen’kina, S.A., Romanov, N.I. & Nikitina, V.E. Regulation by Azospirillum lectins of the activity of antioxidant enzymes in wheat seedling roots under short-term stresses. Braz. J. Bot 41, 579–587 (2018). https://doi.org/10.1007/s40415-018-0489-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-018-0489-1