Abstract
Glaucium flavum (Papaveraceae) is a halophyte which is known for its high importance in phytomedicine and ecology. In this work, phytochemical analysis, as well as antioxidant, antiproliferative and anti-inflammatory effects were investigated for EOH, EA and PE fractions of G. flavum shoots. Results showed that total polyphenol amounts were highest in EOH fraction (158.3 mg GAE/g DR) followed by EA and PE. This latter fraction was rich in flavonoids (128.43 mg CE/g DR), however EA produced more condensed tannins (19.83 mg CE/g DR) than other fractions. In addition, seven molecules (phenolics) have been identified: kaempferol, caffeic acid, catechin hydrate, syringic acid, chlorogenic acid, isoquercitrin, and trans-hydroxycinnamic acid. Concerning antioxidant effects, ethanol fraction was distinguished by a high total antioxidant activity (432.58 mg GAE/g DR), a lower iron reducing power (EC50 = 800 µg/ml), a capacity to inhibit the β-carotene bleaching (IC50 = 48.78 µg/ml), and an important antiradical activity (IC50 = 140 µg/ml). In addition, PE, EA and EOH fractions have strong antiproliferative effect against MCF-7 cells but with superiority of EA fraction (IC50 = 135 µg/ml). EA showed also a high anti-inflammatory effect with an amount of NO which is equal to around 29 and 20 µM/ml NO at 50 and 100 µg/ml, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is caused by infectious agents, environmental exposures (sun, nuclear radiation), severs and repeated injuries, gastric fluids, bile acid and certain immune problems. Moreover, cancer is very linked to oxidative stress and inflammation. In fact, during the inflammatory reaction, various molecules are produced by immune cells as chemical mediators such as cytokines (Hanada and Yoshimura 2002). Inflammation progresses when pro-inflammatory molecules such as interleukin-1, tumour necrosis factor, gamma-interferon, IL-12, IL-18 and the granulocyte-macrophage colony-stimulating factor increase (Hanada and Yoshimura 2002). Moreover, during inflammation, hydroxyl radical (OH.), the superoxide anion radical (O2.−), alkoxyl and peroxyl radicals (RO. and RO2, respectively), hydrogen peroxide (H2O2), hypochlorite radical (−OCl), singlet oxygen (O2), nitric oxide radical (NO) and other various lipid peroxides are produced. These radicals attack normal cells causing several structural and functional damages related to deoxyribonucleic acid by the production of hypermethylation, ribonucleic acid, lipids, proteins, carbohydrates and enzymes (Craft et al. 2012). With all these molecules and others such as mitogen-activated protein (MAP) kinases, oxidative stress, inflammation and cancer can be linked (Reuter et al. 2010). Cancer is an uncontrolled, multiple and rapid proliferation of cells with the ability to escape the immune system. Traditional treatments such as chemotherapy are used but with devastating harms on human health, in addition to the psychological trauma caused on the body aspect. Alternative treatments based on plants that have at the same time antioxidant, anti-inflammatory and/or anticancer potentials such as Suaeda fruticosa (Oueslati et al. 2012) or Tamarix gallica (Boulaaba et al. 2013b) can yield to promising results.
Glaucium flavum, from the Papaveraceae family, is a facultative halophyte widespread especially in salty areas (salt marsh and littoral) such as in Europe, North Africa, Western Asia and United States. The name “Glaucium” provides from the Greek glaucos and refers to the bluish-green colour of the leaves, and “flavum” refers to the yellow flowers (Scott 1963). Previous works mentioned the antioxidant (Tawaha et al. 2007), anti-inflammatory (Arafa et al. 2016) and anti-cancer (Bournine et al. 2013) effects of G. flavum. Phytochemically, little work has been done on the phenolic composition of G. flavum. For example, phenolics such as ferulic and p-hydroxycinnamic acids were shown in Bulgarian G. flavum (Safa et al. 2013).
Due to lack of data regarding the literature of G. flavum, this study focused on (i) the phytochemical analysis of shoot Glaucium flavum fractions, and (ii) the assessment of the antioxidant, antiproliferative and anti-inflammatory effects of different solvent extracts.
Materials and methods
Plant material and fractionation
The Papaveraceae Glaucium flavum was collected from Borj-Cedria (coastal region, 25 Km southern capital Tunis, and superior semi-arid climate). Aerial parts were sampled at the reproductive stage during the appearance of yellow flowers. Fractions were obtained by magnetic stirring of 5 g of dry matter powder with 50 ml of solvent using successively the following method: firstly with petroleum ether (PE), secondly with ethyl acetate (EA) and finally with 90% ethanol (EOH). Each step of fractionation remained 48 h at room temperature (26 °C). After that, fractions were filtered through a Whatman filter paper (No. 4) and evaporated under vacuum to dryness. Dry residues (DRs) were kept at 4 °C. Dimethyl sulfoxide (DMSO) at 0.5% was used for the antiproliferative effect.
Total phenolic contents
Total polyphenols were assayed by the Folin–Ciocalteu reagent according to a previous work (Boulaaba et al. 2013a). Absorbance was checked at 760 nm. Contents were expressed as mg gallic acid equivalent per gram of DR (mg GAE/g DR) through the calibration curve with gallic acid, ranging from 0 to 500 µg/ml. All samples were analysed in triplicate.
Total flavonoid contents
Total flavonoids were measured according to Boulaaba et al. (2013a). Absorbance of the mixture was determined at 510 nm against the blank where the sample was omitted. Contents were expressed as mg catechin equivalent per gram of DR (mg CE/g DR), through the calibration curve of (+)-catechin, ranging from 0 to 500 µg/ml. All samples were analysed in triplicate.
Condensed tannin contents
Condensed tannin contents were measured using the vanillin assay (Boulaaba et al. 2013a). Contents were expressed as mg CE per gram of dry weight (mg CE/g DR). Optical density of samples was measured at 500 nm. All samples were analysed in triplicate.
Identification of natural products by liquid chromatography with diode array detector (LC-DAD)
The identification of natural products was done using high performance liquid chromatography system (consisting of a vacuum degasser, an autosampler and a binary pump with a maximum pressure of 400 bar; Agilent 1260, Agilent technologies, Germany) equipped with a reversed phase C18 analytical column of 4.6 × 100 mm and 3.5 μm particle size (Zorbax Eclipse XDB C18). The diode array detector was set to a scanning range of 200–400 nm. Column temperature was maintained at 25 °C. The injected sample volume was 2 µl and the flow rate of mobile phase was 0.4 ml/min. The mobile phase consisted of a mixture of solvent A (methanol) and solvent B (milli-Q water with 0.1% formic acid). The optimized gradient elution was illustrated as follows: 0–5 min, 10–20% A; 5–10 min, 20–30% A; 10–15 min, 30–50% A; 15–20 min, 50–70% A; 20–25 min, 70–90% A; 25–30 min, 90–50% A; 30–35 min, return to initial conditions. In this study, identification analysis was done by comparison of their retention time with those obtained from the extracts at 254 nm. The amount of each compound was expressed as microgram per gram of DR (µg/g DR). The obtained curves for two compounds showed a good linearity (with an average of r2 = 0.999). Equations for regression lines are: caffeic acid: y = 23,64x + 3,23, catechin hydrate: y = 3,73x + 0,76, chlorogenic acid: y = 9,02x − 1,55, isoquercitrin: y = 1,77x + 5,89, kaempferol: y = 9,87x − 4,32, syringic acid: y = 41,47x − 2,12 and trans-hydroxycinnamic acid: y = 49,68x + 24,60.
Antioxidant activities
DPPH radical-scavenging activity
The potential of extracts to reduce the free DPPH radical (1,1-diphenyl-2-picrylhydrazyl) was expressed as IC50 (µg/ml), the antiradical dose required to cause a 50% inhibition. For that, samples at different concentrations were added to DPPH methanolic solution (0.2 mM). The absorbance was measured at 517 nm (Boulaaba et al. 2013a).
Total antioxidant capacity
Total antioxidant capacity of methanolic extracts was evaluated through the assay of the green phosphate/Mo5+ complex according a protocol described previously (Boulaaba et al. 2013a). The absorbance was measured at 695 nm against a blank. The total antioxidant activity was expressed as mg GAE/g DR. All samples were analysed in triplicate.
Iron reducing power
This antioxidant activity was focused on the reduction of the trivalent iron produced by the FeCl3: Iron(III) chloride anhydrous (Boulaaba et al. 2013a). The intensity of the appearing blue-green colour was measured at 700 nm. The EC50 value (µg/ml) for the reducing power is the extract concentration at which the absorbance was 0.5, and ascorbic acid was used as a positive control. All samples were analysed in triplicate.
β-carotene bleaching test
The antioxidant activity of the extracts, compared with references such as Butylated hydroxytoluene and Butylated hydroxyanisole, was evaluated in terms of β-carotene blanching effect (Falleh et al. 2011). The absorbance was measured at 470 nm on a multidetection microplate reader. Readings of all samples were performed immediately (t = 0 min) and after 120 min of incubation. Three replicates were prepared for each of the samples and the results are expressed as IC50 values (µg/ml).
Anti-inflammatory activity
The anti-inflammatory effect of the G. flavum fractions was evaluated on the murine macrophage RAW 264.7 cell line (American Type Culture Collection, ATCC) through the accumulation of nitrite (NO). Cells were grown in 24-well plates at a concentration of 2 × 105 cells/ml during 24 h after which G. flavum shoot fractions at 12.5, 25, 50 and 100 µg/ml were added for 1 h of treatment. EOH and PE residues were dissolved in DMSO, and EA in EOH. Lipopolysaccharide (LPS) (1 µg/ml) was added to the treatment group of plates, while medium or LPS alone was added to the control group. After a 24-h LPS-stimulation, the cell-free supernatants were collected and assayed for nitric oxide (NO) levels using Griess’s reagent (1% sulfanilamide, 5% phosphoric acid and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride). The resazurin test was performed to check the cytotoxicity of samples on cells. The absorbance was measured at 540 nm, and the nitrite concentration in the samples was determined through a standard curve of sodium nitrite at 10, 20 and 50 µM.
Cell maintenance and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
The hormone-dependent human MCF-7 breast cancer cells were obtained from ATCC, and cultured at 37 °C in humidified 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium (Sigma), supplemented with 10% heat-inactivated foetal bovine serum (Gibco) and antibiotics (100 µg/ml streptomycin and 100 U/ml penicillin). The antiproliferative effect was investigated using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma) test. In fact, cells were seeded in 96-well plates at a concentration of 2 × 104 cells/ml during 24 h after which G. flavum shoot fractions at 31.25, 62.5, 125, 250 and 500 µg/ml were added. DMSO and all solvent solutions diluted in medium at the same percentage (0.5%) were used as negative controls. After 72 h of treatment, medium was removed and MTT (5 mg/ml) was added. After 24 h incubation, the blue formazan produced was dissolved using 10% sodium dodecyl sulfate (Wako). The absorbance was measured at 570 nm on a microplate reader. Results shown represent the mean of three independent experiments.
Statistical analysis
For all plant parameters, three replicates were used. A two-way analysis of variance was achieved for whole data, using the XLSTAT 2014.5.03 statistical program. Means were compared using the Duncan’s multiple range test at the P < 0.05 level, when significant differences were found. Pearson correlation coefficients were obtained by linear curve.
Results and discussion
Phenolic contents and phytochemical analysis
PE, EA and 90% EOH fractions were analysed for their phenolic contents. Results showed a significant difference among them (Table 1). EOH extract produced the highest of total polyphenol amounts (158.3 mg GAE/g DR) followed by EA and PE (132.72 and 96.24 mg GAE/g DR, respectively). However, flavonoid contents showed a contrarily tendency with a high presence of molecules in PE (128.43 mg CE/g DR) followed by EA and EOH (85.05 and 36.7 mg CE/g DR, respectively). Condensed tannins were more produced in EA (19.83 mg CE/g DR) than in PE and EOH (12 and 5.27 mg CE/g DR, respectively).
Phenolic contents were previously shown in many halophyte extracts (Boulaaba et al. 2013a, b; Falleh et al. 2011; Oueslati et al. 2012). In this work and independently of fractions, G. flavum was richer on total polyphenol, flavonoids and condensed tannin contents than A. indicum (Boulaaba et al. 2013a). In addition, a solvent effect has been well demonstrated in a comparative study of Helianthus annuus extracts using solvents with different polarities (Ye et al. 2015). The analysis of the PE, EA and 90% EOH G. flavum extracts revealed that this Papaveraceae plant was rich in phenolic acid and flavonoid compounds. The Table 2 showed seven principle phenolics detected in the three plant fractions by referring to the standards. The identified molecules in each fraction were: kaempferol (1.557 µg/g DR) in the PE fraction; and catechin hydrate (0.757 µg/g DR), chlorogenic acid (0.223 µg/g DR) and trans-hydroxycinnamic acid (0.795 µg/g DR) in the EA one. EOH fraction was distinguished by the presence of caffeic acid (2.810 µg/g DR), chlorogenic acid (2.526 µg/g DR), syringic acid (1.111 µg/g DR), and high levels of catechin hydrate (30.664 µg/g DR), isoquercitrin (or isoquercetin) (27.395 µg/g DR), and trans-hydroxycinnamic acid (20.904 µg/g DR). Then, EOH fraction is considered as the main enriched extract in phenolic compounds especially in catechin hydrate. Independently of fractions, catechin hydrate and isoquercitrin were the main components. The presence of phenolics in our G. flavum confirmed a previous research on a Bulgarian Glaucium, which showed the presence of phenolics such as trans-hydroxycinnamic acids (Safa et al. 2013). Moreover, Papaveraceae family plants were previously reported to produce kaempferol compound as Meconopsis spp (Tanaka et al. 2001), and Papaver nudicaule (Schliemann et al. 2006). Furthermore, the presence of hydroxycinnamic and syringic acids in G. flavum in mentioned in another Papaveraceae plant such as Corydalis cheiranthifolia (Hagel et al. 2015). Hydroxycinnamic acid represents a main group considering that many important phenolics derived from it such as cinnamic acid, p-coumaric acid, ferulic acid, caffeic acid, chlorgenic acid and rosmarinic acid. Our study showed the presence of isoquercitrin, also named isoquercetin or isotrifoliin which is a flavonoid glycoside of quercetin (quercetin-3-glucoside). These two metabolites were previously detected in another Papaveraceae plant, Meconopsis horridula (Liu et al. 2014). However, catechin and chlorogenic acid seem not to be know, in the Glaucium species. Finally, caffeic acid, presented in small amount in EOH fraction (2.810 µg/g DR) provided from the phenylpropanoid and playing an important role in the synthesis of the lignin, represents one of the main metabolite of plants. It came from the cinnamic acid by the intermediate of the p-coumaric acid in its esterified form. The combination of the ester forms of caffeic and quinic acids forms the chlorogenic acid. Once the phytochemical analysis has been done and in order to better understand the effects of G. flavum enriched in phenolic compounds, some biological tests were further investigated.
Antioxidant activities
Independently of tests, EOH fraction presented the most important antioxidant effects (Table 3). This study showed that this extract had an important antiradical activity (IC50 = 140 µg/ml), a high capacity to inhibit the bleaching of β-carotene (IC50 = 48.78 µg/ml), a lower iron reducing power (EC50 = 800 µg/ml) and an important total antioxidant activity (432.58 mg GAE/g DR). In fact, it is evident that halophytes enriched in phenolics present an important antioxidant activity (Boulaaba et al. 2013a, b; Falleh et al. 2011; Oueslati et al. 2012). The relation between high antioxidant activities of G. flavum and the amount of phenolics is evident. Natural products exhibited in this plant were in simple forms (caffeic acid, chlorogenic acid, kaempferol and syringic acid) and derivate forms (catechin hydrate, isoquercitrin and trans-hydroxycinnamic acid) but all have antioxidant effects. In a previous works, hydroxycinnamic acid and its derivative caffeic acid presented an important in vitro antioxidant activity (Gallardo et al. 2006; Maurya and Devasagayam 2010). Moreover, caffeic acid and also chlorogenic acid are well known for their in vitro and in vivo antioxidant effects. In this context, and as a function of the comparative study of Marinova et al. (2009), the chlorogenic acid seems to be more responsible of the antioxidant effects than caffeic acid due to its specific interactions with the reactive oxygen species. Moreover, flavonoids act as primary antioxidants to stabilise the radicals such as the physiological anion ONOO− and the synthetic radical DPPH.. For example, it is the case of catechin, epicatechin and quercetin that have been found in EOH fraction of G. flavum and already analysed from grape for this effect (Iacopini et al. 2008). Moreover, isoquercitrin from Thuja orientalis plant reduced the inhibition level of glutathione (GSH) caused by elevation of reactive oxygen species in the transformed retinal ganglion cells RGC-5 (Jung et al. 2010).
Evaluation of the anti-inflammatory activity of G. flavum fractions on LPS-activated RAW 264.7 macrophages
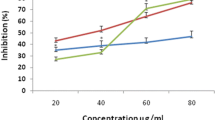
The anti-inflammatory effect of PE, EA and 90% EOH shoot fractions were evaluated through the NO production inhibition in LPS-stimulated RAW 264.7 macrophages (Fig. 1). Independently of fractions, the percentage of NO inhibition decreased with concentrations from 90 to 20%. In fact, the NO amount decreased significantly as a function of EA concentrations to attend around 29 and 20 µM/ml NO at 50 and 100 µg/ml, respectively. Same tendency but with less efficiency was observed with PE fraction. Using EOH fraction, we observe a decreasing effect of the NO production in treated cells (12.5, 25 and 50 µg/ml of extracts) compared with the untreated cells (control) without noting a significant dose effect. However, at 100 µg/ml the anti-inflammatory effect became significant when the NO level reaches a value of 28.42 µM/ml.
Effect of petroleum ether (PE), ethyl acetate (EA) and 90% ethanol (EOH) shoot fractions from G. flavum on NO production inhibition (%) in LPS-stimulated RAW 264.7 macrophages. Cells were grown in 24-well plates at a concentration of 2 × 105 cells/ml during 24 h after which G. flavum extracts at 12.5, 25, 50 and 100 µg/ml were added. Each value represents the mean ± standard deviation of three determinations
The richness of fraction on polyphenols seems to be responsible of the anti-inflammatory effect. For instance, the abundance of chlorogenic acid in the EA fraction was probably the origin of the inhibition of some inflammatory mediators such as the LPS-induced cyclooxygenase-2 or nitric oxide synthases (iNOS) through the decreasing of the nuclear factor kappa B expression (Shan et al. 2009; Hwang et al. 2014). Many flavonoids have been studied for their high anti-inflammatory activity, such as catechin, which possesses an important capacity to block the O2 radical formation and/or inhibit prostaglandins synthesis enzymes. This product and its derivatives were previously isolated from many plants such as Cinnamomum sieboldii, Ceiba pentandra, Anacardium occidentale Linn, Atuna racemosa and Syzygium corynocarpum mainly for their anti-inflammatory effects (Perez 2001). In addition, in corn bran 80% EOH extract, trans-hydroxycinnamic acid and its derivatives showed a high anti-inflammatory effect since it can inhibit the NO production and iNOS expression in a dose-dependent manner in LPS-stimulated Raw 264.7 cells (Kim et al. 2012).
Antiproliferative activity of Glaucium flavum
Antiproliferative effects of G. flavum fractions were investigated against MCF-7 cells (Fig. 2). All extracts inhibited breast cancer cell proliferation in a dose-dependent manner. For both PE and 90% EOH extracts, a moderate decreasing of cell growth was observed until 125 µg/ml (Fig. 2a, c). However, EA fraction inhibited cell growth by around 48% using 31.25 to 125 µg/ml of extracts (Fig. 2b). From the dose 125 to 500 µg/ml, all fractions significantly inhibited the growth of MCF-7 cells as compared with the control ones. In fact, 500 µg/ml inhibited cell growth by 52%, 87% and 73%, respectively, for PE (Fig. 2a), EA (Fig. 2b) and EOH (Fig. 2c) fractions. The IC50 values of antiproliferative effect were equal to 250, 135 and 225 µg/ml for PE, EA and EOH fractions, respectively.
Measurements of cell proliferation using MTT assay in breast MCF-7 cell line treated with G. flavum aerial part fractions. Cells at 2 × 104 cells/ml were cultured in the absence (control) or in the presence of 31.25, 62.5, 125, 250 or 500 µg/ml of G. flavum for 72 h. The fractions petroleum ether (a), ethyl acetate (b) and 90% ethanol (c) correspond to the plant collected during the flowering period. Values represent the means of three independent experiments ± standard deviation. The bars marked with different letters are significantly different at p < 0.05
Many phenolic compounds are known to have this activity. Caffeic acid, a derivative of cinnamic acid and very present in many plants as in G. flavum (with an amount equal to 2.810 µg/g DR), has this antiproliferative effect. This was observed with Ocimum gratissimum caffeic acid on the human cervical cancer cell line HeLa (Ye et al. 2010). This property is due to the structure of molecules characterized by the presence of two OH groups on phenyl giving them the same properties of polyphenols. The structure (simple or not) of the molecule can influence the intensity of the activity. Further research on the chemical structures of the compounds found in G. flavum could give more precision. In addition, the inhibitory effect of EOH fraction can be explained by the presence of the chlorogenic acid. This compound already showed an inhibited activity on the invasion of rat ascites hepatoma AH109A cells (Yagasaki et al. 2000). Moreover, the antiproliferative effect of G. flavum on MCF-7 cells may be explained by the apoptosis effect of kaempferol through the activations of extracellular MAP kinase (Aiyer et al. 2012) and tumor suppressor protein P53 in human HCT116 colon cancer cells (Li et al. 2009). EOH G. flavum fraction presented a high amount of catechin hydrate (30.664 µg/g DR). This is in accordance with a previous work that showed an important antiproliferative effect in the presence of 500 µg/ml of catechin-rich extract of Cocos nucifera on lymphocytes associated with a cell cycle arrest in the S/G2M phase (Kirszberg et al. 2003).
Conclusions
The results of this study suggest that G. flavum, as food medicinal halophyte, could be a promising source of natural products for industrial applications such as production of pharmaceuticals, nutraceuticals and functional foods. However, such a study would be incomplete without a thorough study of the mechanisms of action of the anti-proliferative and anti-inflammatory activities of the most interesting fractions.
References
Aiyer HS, Warri AM, Woode DR, Hilakivi-Clarke L, Clarke R (2012) Influence of berry polyphenols on receptor signaling and cell-death pathways: implications for breast cancer prevention. J Agric Food Chem 60(23):5693–5708
Arafa AM, Mohamed MES, Eldahmy SI (2016) The aerial parts of yellow horn poppy (Glaucium flavum Cr.) growing in Egypt: isoquinoline alkaloids and biological activities. J Pharm Sci Res 8(5):323–332
Boulaaba M, Mkadmini K, Tsolmon S, Han J, Smaoui A, Kawada K, Ksouri R, Isoda H, Abdelly C (2013a) In vitro antiproliferative effect of Arthrocnemum indicum extracts on Caco-2 cancer cells through cell cycle control and related phenol LC-TOF-MS identification. Evid Based Complement Altern Med. https://doi.org/10.1155/2013/529375
Boulaaba M, Tsolmon S, Ksouri R, Han J, Kawada K, Smaoui A, Abdelly C, Isoda H (2013b) Anticancer effect of Tamarix gallica extracts on human colon cancer cells involves Erk1/2 and p38 action on G2/M cell cycle arrest. Cytotechnology 65:927–936
Bournine L, Bensalem S, Wauters J-N, Iguer-Ouada M, Maiza-Benabdesselam F, Bedjou F, Castronovo V, Bellahcène A, Tits M, Frédérich M (2013) Identification and quantification of the main active anticancer alkaloids from the root of Glaucium flavum. Int J Mol Sci 14:23533–23544
Craft BD, Kerrihard AL, Amarowicz R, Pegg RB (2012) Phenol-based antioxidant and the in vitro methods used for their assessment. Compr Rev Food Sci Food Saf 11(2):148–173
Falleh H, Ksouri R, Medini F, Guyot S, Abdelly C, Magné C (2011) Antioxidant activity and phenolic composition of the medicinal and edible halophyte Mesembryanthemum edule L. Ind Crops Prod 34:1066–1071
Gallardo C, Jiménez L, García-Conesa M-T (2006) Hydroxycinnamic acid composition and in vitro antioxidant activity. Food Chem 99:455–463
Hagel JM, Mandal R, Han B, Han J, Dinsmore DR, Borchers CH, Wishart DS, Facchini PJ (2015) Metabolome analysis of 20 taxonomically related benzylisoquinoline alkaloid-producing plants. BMC Plant Biol 15:220
Hanada T, Yoshimura A (2002) Regulation of cytokine signalling and inflammation. Cytokine Growth Factor Rev 13:413–421
Hwang SJ, Kim YW, Park Y, Lee HJ, Kim KW (2014) Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm Res 63:81–90
Iacopini P, Baldi M, Storchi P, Sebastiani L (2008) Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J Food Compos Anal 21:589–598
Jung SH, Kim BJ, Lee EH, Osborne NN (2010) Isoquercitrin is the most effective antioxidant in the plant Thuja orientalis and able to counteract oxidative-induced damage to a transformed cell line (RGC-5 cells). Neurochem Int 57:713–721
Kim EO, Min KJ, Kwon TK, Um BH, Moreau RA, Choi SW (2012) Anti-inflammatory activity of hydroxycinnamic acid derivatives isolated from corn bran in lipopolysaccharide-stimulated Raw 264.7 macrophages. Food Chem Toxicol 50:1309–1316
Kirszberg C, Esquenazi D, Alviano CS, Rumjanek VM (2003) The effect of a catechin-rich extract of Cocos nucifera on lymphocytes proliferation. Phytother Res 17(9):1054–1058
Li W, Du B, Wang T, Wang S, Zhang J (2009) Kaempferol induces apoptosis in human HCT116 colon cancer cells via the ataxia-telangiectasia mutated-p53 pathway with the involvement of p53 upregulated modulator of apoptosis. Chem Biol Inter 177:121–127
Liu J, Wu H, Zheng F, Liu W, Feng F, Xie N (2014) Chemical constituents of Meconopsis horridula and their simultaneous quantification by high-performance liquid chromatography coupled with tandem mass spectrometry. J Sep Sci 37:2513–2522
Marinova EM, Toneva A, Yanishlieva N (2009) Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem 114:1498–1502
Maurya DK, Devasagayam TPA (2010) Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem Toxicol 48:3369–3373
Oueslati S, Ksouri R, Falleh H, Pichette A, Abdelly C, Legault J (2012) Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem 132:943–947
Perez GRM (2001) Anti-inflammatory activity of compounds isolated from plants. Sci World 1:713–784
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49:1603–1616
Safa O, Soltanipoor MA, Rastegar S, Kazemi M, Dehkordi KN, Ghannadi A (2013) An ethnobotanical survey on hormozgan province, Iran. Avicenna J Phytomed 3:64–81
Schliemann W, Schneider B, Wray V, Schmidt J, Nimtz M, Porzel A, Böhm H (2006) Flavonols and an indole alkaloid skeleton bearing identical acylated glycosidic groups from yellow petals of Papaver nudicaule. Phytochem 67:191–201
Scott GAM (1963) Glaucium Flavum Crantz. J Ecol 51(3):743–754
Shan J, Fu J, Zhao Z, Kong X, Huang H, Luo L, Yin Z (2009) Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-kB and JNK/AP-1 activation. Int Immunopharmacol 9:1042–1048
Tanaka M, Fujimori T, Uchida I, Yamaguchi S, Takeda K (2001) A malonylated anthocyanin and flavonols in blue Meconopsis flowers. Phytochem 56(4):373–376
Tawaha K, Alali F, Gharaibeh M, Mohammad M, El-Elimat T (2007) Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem 104:1372–1378
Yagasaki K, Miura Y, Okauchi R, Furuse T (2000) Inhibitory effects of chlorogenic acid and its related compounds on the invasion of hepatoma cells in culture. Cytotechnology 33:229–235
Ye F, Liang Q, Li H, Zhao G (2015) Solvent effects on phenolic content, composition, and antioxidant activity of extracts from florets of sunflower (Helianthus annuus L.). Ind Crops Prod 76:574–581
Ye JC, Hsiao MW, Hsieh CH, Wu WC, Hung YC, Chang WC (2010) Analysis of caffeic acid extraction from Ocimum gratissimum linn. by high performance liquid chromatography and its effects on a cervical cancer cell line. Taiwan J Obstet Gynecol 49(3):266–271
Acknowledgements
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research and by the Japanese JICA/JST Science and Technology Research Partnership for Sustainable Development Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boulaaba, M., Kalai, F.Z., Dakhlaoui, S. et al. Antioxidant, antiproliferative and anti-inflammatory effects of Glaucium flavum fractions enriched in phenolic compounds. Med Chem Res 28, 1995–2001 (2019). https://doi.org/10.1007/s00044-019-02429-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02429-y