Abstract
Hydrogen sulfide (H2S) which is involved in plant growth, development, and the acquisition of stress tolerance including heat tolerance, is considered as the third signal molecule after nitric oxide and reactive oxygen species, while betaine is an important osmolyte with multiple physiological functions, but interaction between H2S and betaine in the acquisition of heat tolerance in plants is not clear. In this study, pretreatment with H2S donor sodium hydrosulfide (NaHS), as comparison to the control seedlings without NaHS treatment, significantly improved the activity of betaine aldehyde dehydrogenase (BADH), a key enzyme in the biosynthesis of betaine, which in turn induced the accumulation of endogenous betaine, eventually enhanced the survival percentage of maize seedlings under heat stress. In contrast, these effects induced by NaHS were eliminated by application of H2S scavenger hypotaurine and inhibitor of BADH disulfiram, respectively, indicating that H2S-improved heat tolerance of maize seedlings may be closely associated with the accumulation of endogenous betaine by activating the activity of BADH. In addition, exogenous betaine treatment enhanced the content of endogenous betaine, followed by improved the survival percentage of maize seedlings compared with the control without betaine treatment. All of the above-mentioned results showed that NaHS pretreatment could induce the accumulation of endogenous betaine by increasing BADH activity, and this accumulation may be involved in the acquisition of heat tolerance of maize seedlings, bridging a gap between H2S and betaine in the acquisition of heat tolerance in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen sulfide (H2S), a small flammable colorless gas with a characteristic odor of rotten eggs, has long been known as a phytotoxin, which may be connected with the mass extinction of species in the Permian Period (Li et al. 2011b; García-Mata and Lamattina 2013; Jin et al. 2013; Li, 2013; Lisjak et al. 2013; Calderwood and Kopriva 2014; Hancock and Whiteman 2014). For example, exposure to 3,000 parts per billion (ppb) in air H2S caused lesions on leaves, defoliation, and reduced growth of the plants (Nakamura et al. 2009). However, in animal systems, H2S has recently been identified as a third endogenous gaseous transmitter after nitric oxide (NO) and carbon monoxide (CO), and it participates in multiple physiological roles at low concentration (Li et al. 2011b). In plant systems, the positive effects of H2S are being emerged in seed germination (Zhang et al. 2010; Li et al. 2012a), organogenesis (Zhang et al. 2009a; Fang et al. 2014), stomata movement (Lisjak et al. 2010; Jin et al. 2013), osmotic stress (Zhang et al. 2009b; Christou et al. 2013), salt stress (Christou et al. 2013; Shan et al. 2014), oxidative stress (Zhang et al. 2011), and heavy metal stress (Zhang et al. 2008, 2010; Chen et al. 2013). Our previous results also showed that treatment with sodium hydrosulfide (NaHS), a H2S donor, can improve the resistance of tobacco cells (Li et al. 2012b), maize (Li et al. 2013a, b, 2014c, d), and wheat (Wu et al. 2013) seedlings to high temperature stress, as well as the acquisition of heat tolerance involved in a second messenger calcium and the accumulation of osmolytes such as proline and trehalose, as well as the enhancement of antioxidant system activity, but in maize seedlings, whether the acquisition of heat tolerance induced by NaHS is implicated in the accumulation of betaine is not clear.
Land plants, being the sessile characteristic, are constantly exposed to various abiotic and biotic stress factors, such as extreme in temperature, drought, high salinity, and mechanical stress. Among these stress factors, high temperature is considered as a main environmental stress factor with a far-reaching impact on metabolism, growth, development, reproduction, and yield of crop plants including maize (Zea mays L.) (Königshofer et al. 2008; Li and Gong 2011; Saidi et al. 2011; Mittler et al. 2012; Wu et al. 2012). Many studies have illustrated high temperatures lead to increase in fluidity of membrane lipids and loss of membrane integrity; protein denaturation and aggregation; inhibition of protein synthesis and acceleration of protein degradation; inactivation of enzymes in chloroplast and mitochondria; oxidative stress; and osmotic stress (Wahid et al. 2007; Hanumappa and Nguyen 2010, Saidi et al. 2011; Mittler et al. 2012; Wu et al. 2012). Based on these negative effects, plants are forced to invest valuable resources in modifying their metabolism to prevent damage caused by heat stress, corresponding adaptive mechanism including repair and reestablishment of biomembrane; stress protein synthesis; enhancement in antioxidant capacity; and the synthesis of osmolytes with multiple functions such as betaine, proline, and soluble sugar (Wahid et al. 2007; Königshofer et al. 2008; Chen and Murata 2011; Li et al. 2011a; Mittler et al. 2012; Oukarroum et al. 2012; Saidi et al. 2011; Wu et al. 2012). In higher plants, betaine accumulation is an efficient adaptive mechanism of plants to various environmental stresses including heat stress, betaine aldehyde dehydrogenase (BADH) is a key enzyme in the biosynthesis of betaine, it plays a crucial role in betaine accumulation (Fitzgerald et al. 2009). In addition, exogenous application of betaine can increase the tolerance of some plants to heat stress (Wahid et al. 2007; Fitzgerald et al. 2009; Wu et al. 2012).
Maize not only is the third most important food grain crop after wheat and rice, but also a new model plant, heat stress is the principal cause of maize failure worldwide, global warming accentuates this problem (Leipner and Stamp 2009; Strable and Scanlon 2009). On the basis of these studies, we hypothesized that interaction between H2S and betaine may exist in the acquisition of heat tolerance of maize. In this study, effect of sodium hydrosulfide (NaHS), a H2S donor, treatment on the accumulation of betaine and its possible pathway, as well as involvement of heat tolerance in maize seedlings was investigated. The purpose was to illustrate that NaHS induced betaine accumulation and its pathway, and this accumulation was involved in the acquisition of heat tolerance.
Materials and methods
Plant material and treatment
Commercial variety of maize (Zea mays L., Huidan No. 4) was used in the present experiments. The seeds were sterilized in 0.1 % HgCl2 for 10 min, and pre-soaked in distilled water for 12 h for imbibition. The soaked seeds were sown on six layers of wetted filter papers in trays (24 cm × 16 cm, approximately 300 seeds per tray) with covers and germinated at 26 °C in the dark for 2.5 d. After germinated for 2.5 d, the seedlings with unanimous growth were irrigated with 100 ml of the following solution for 6 (for counting survival percentage) or 12 h (for determining betaine aldehyde dehydrogenase activity and betaine content), respectively: (1) 0 (control) and 0.5 mM sodium hydrosulfide (NaHS, a H2S donor, which suitable concentration was selected from our previous work, Li et al. 2013b); (2) 1 mM hypotaurine (HT, H2S scavenger) + 0.5 mM NaHS; (3) 1 mM disulfiram (DSF, inhibitor of betaine aldehyde dehydrogenase; Velasco-García et al, 2003) + 0.5 mM NaHS (pretreatment with chemicals had no significant effect on the growth of seedlings). Afterward, treated seedlings were exposed to high temperature at 47 °C for 15 h for heat stress. At the end of heat stress, seedlings were transferred to a climate chamber with 26 °C, 100 µmol m−2 s−1, and 12 h photoperiod for a week for recovery growth and irrigated with 1/2 Hoagland solution daily. Survival percentage was counted after recovery, and the seedlings which could regrow and become green during recovery were considered to have survived (Li et al. 2013a, b).
In addition, during the process of above treatment, the activity of betaine aldehyde dehydrogenase (BADH) and betaine content in maize seedlings were determined using the following methods, respectively.
Determination of activity of betaine aldehyde dehydrogenase
Betaine aldehyde dehydrogenase (BADH, E.C. 1.2.1.8) is considered to be a key enzyme in the biosynthesis of betaine in plants (Fitzgerald et al. 2009; Li et al. 2014a, b). The 2.5-day-old seedlings were irrigated with 0.5 mM NaHS, and then activity of BADH was determined as previously described the methods of Fan et al. (2012) with some modifications. Fresh weight (1.0 g) were ground with a mortar and pestle in liquid nitrogen and the soluble proteins were extracted by adding 1 ml of 50 mM potassium phosphate buffer (pH 6.5) containing 0.1 mM EDTA and 20 mM β-mercaptoethanol. After centrifugation, the protein content of the supernatant was adjusted to 100 µg ml−1 to obtain equal amounts of protein in each assay sample. BADH activity was assayed spectrophotometrically by monitoring increase in the absorbance at 340 nm (NADH formation) in a mixture (0.5 ml) consisting of 1.0 mM betaine aldehyde, 2 mM dithiothreitol (DTT), and 2 mM NAD+ in a 100 mM potassium phosphate buffer, pH 8.5. The reaction was initiated by the addition of supernatant, and BADH activity was calculated using the extinction coefficient of 6.2 mM−1 cm−1 at 340 nm and expressed as μmol min−1 g−1 fresh weight (FW).
Measurement of betaine content
In order to further understand the effect of NaHS treatment on endogenous betaine content, its content in maize seedlings treated with 0.5 mM NaHS was measured by Reinecke salt methods according to our previous method (Li et al. 2013c). Treated seedlings were dried at 80 °C to constant weight after being killed at 105 °C for 15 min, dry samples were ground in a mortar with a pestle in distilled water, the homogenates were transferred to conical flask in a rotary shaker (120 rpm) with 30 °C for 12 h to extract betaine and centrifuged at 10,000×g for 15 min, and then supernatants were mixed with Reinecke salt and generated red precipitates, which is solvable in 70 % acetone solution and the absorbance was determined at 525 nm. A calibration curve was made using known concentration of betaine according to above methods and betaine content in maize seedlings was expressed as μmol g−1 FW.
Exogenous betaine treatment, endogenous betaine content, and heat tolerance
To study the effect of exogenous betaine pretreatment on endogenous betaine and heat tolerance of maize seedlings, the 2.5-day-old seedlings were transferred to aqueous solution of 0 (control), 5, 10, 15, 20, and 25 mM betaine for 6 h, and then the content of endogenous betaine and survival percentage of maize seedlings after heat stress were measured as above-mentioned method, respectively.
Statistical analysis
The experiment was set up according to a completely randomized design with five replications. The data were processed statistically using software package SPSS version 21.0 (SPSS, Chicago, USA) based on the analysis of variance (one-way ANOVA). Figures were drawn by SigmaPlot 12.5 (Systat Software Inc., London, UK), error bars represent standard error and each data in figure represents the mean ± SE of at least three independent experiments, asterisk and double asterisks on the bars indicate significant differences (P < 0.05) and very significant differences (P < 0.01).
Results
Effect of NaHS treatment on the activity of betaine aldehyde dehydrogenase and the level of endogenous betaine in maize seedlings
The 2.5-day-old maize seedlings were treated with NaHS or H2S scavenger hypotaurine (HT), and then betaine aldehyde dehydrogenase (BADH) activity and endogenous betaine content in maize seedlings were determined. As shown in Fig. 1, NaHS pretreatments improved the activity of BADH in maize seedlings, and this improvement increased with the elongation of treatment time, 3 h of treatment showed significant difference (P < 0.05, Fig. 1), as well as treatment for 6 h or above reached very significant difference level (P < 0.01, Fig. 1). Similarly, pretreatment with NaHS also enhanced the content of endogenous betaine, and this enhancement was strengthened with the extension of treatment time, similar to the change in BADH activity (Fig. 1), which reached significant difference and very significant difference levels at 3 h or above (P < 0.01, Fig. 2), respectively. On the contrary, NaHS-induced increase in the activity of BADH and the accumulation of betaine were eliminated by the addition of hypotaurine (HT), a H2S scavenger (Figs. 1, 2). During the course of HT treatment, both enzyme activity and betaine content were lower more than those of the control slightly (Figs. 1, 2). These results displayed that NaHS treatment could trigger increase in the activity of BADH, which in turn induced the accumulation of endogenous betaine in maize seedlings, which might be the basis for NaHS-induced heat tolerance of maize seedlings.
Effect of NaHS and hypotaurine (HT) treatments on the activity of betaine aldehyde dehydrogenase (BADH) of maize seedlings. The 2.5-day-old seedlings of maize were treated with 0 (control), 0.5 mM NaHS alone or in combination with 1 mM HT for 12 h, and then the activity of BADH were determined. Error bars represent standard error and each data in figure represents the mean ± SE of three experiments, and asterisk and double asterisks indicate significant difference (P < 0.05) and very significant difference (P < 0.01) from the control without NaHS treatment, respectively
Effect of NaHS and hypotaurine (HT) treatments on the accumulation of endogenous betaine of maize seedlings. The 2.5-day-old seedlings of maize were treated with 0 (control), 0.5 mM NaHS alone or in combination with 1 mM HT for 12 h, and then the content of endogenous betaine was measured. Error bars represent standard error and each data in figure represents the mean ± SE of three experiments, and asterisk and double asterisks indicate significant difference (P < 0.05) and very significant difference (P < 0.01) from the control without NaHS treatment, respectively
Effect of disulfiram (DSF) treatment on NaHS-induced increase in BADH activity and betaine accumulation in maize seedlings
As mentioned above, NaHS treatment could improve the activity of BADH (Fig. 1), which in turn induced the accumulation of endogenous betaine in maize seedlings (Fig. 2). The combination of disulfiram (DSF), an inhibitor of BADH, with NaHS inhibited increase in BADH activity induced by NaHS in maize seedlings, and the enzyme activity was slightly lower than that of the control from beginning to end (Fig. 3a). Analogously, DSF treatment further eliminated NaHS-induced the accumulation of betaine, consisting with the change in the activity of BADH (Fig. 3a), betaine accumulation was low than that of the control seedlings (Fig. 3b). These results suggested that inhibitor of BADH disulfiram treatment could eliminate the accumulation of betaine induced by NaHS, and this elimination might be achieved by inhibiting BADH activity in maize seedlings.
Effect of disulfiram (DSF) pretreatments on the activity of betaine aldehyde dehydrogenase (BADH, a) and endogenous betaine content (b) of maize seedlings. The 2.5-day-old seedlings of maize were treated with 0 (control), 0.5 mM NaHS in combination with 1 mM DSF for 12 h, and then the activity of BADH and endogenous betaine content were determined. Error bars represent standard error and each data in figure represents the mean ± SE of three experiments
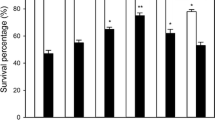
Effect of pretreatment with NaHS, H2S scavenger, and BADH inhibitor on survival percentage of maize seedlings
Sodium hydrosulfide (NaHS) was commonly used as donors for H2S, it releases H2S, giving a relatively short burst of H2S, when dissolved in water (Wang 2012; García-Mata and Lamattina 2013, Lisjak et al. 2013). Pretreatment with NaHS improved the survival percentage of maize seedlings under heat stress, but this improvement was weakened by application of hypotaurine (HT, H2S scavenger) and disulfiram (DSF, inhibitor of BADH), respectively, especially addition of HT showed more efficient (Fig. 4), indicating that NaHS treatment could improve heat tolerance of maize seedlings, and the acquisition of heat tolerance induced by NaHS might be involved, at least partly, in the accumulation of endogenous betaine through inducing increase in the activity of BADH by NaHS.
Effect of NaHS, hypotaurine (HT) and disulfiram (DSF) pretreatments on the survival percentage of maize seedlings. The 2.5-day-old seedlings of maize were treated with 0 (control), 0.5 mM NaHS alone or in combination with 1 mM HT or DSF for 6 h, and the survival percentage of maize seedlings after heat stress was counted. Error bars represent standard error and each data in figure represents the mean ± SE of three experiments, and asterisk and double asterisks indicate significant difference (P < 0.05) and very significant difference (P < 0.01) from the control without NaHS treatment, respectively
Effect of exogenous betaine treatment on endogenous betaine and survival percentage of maize seedlings
To further understand effect of exogenous betaine on endogenous betaine and heat tolerance of maize seedlings, the 2.5-day-old maize seedlings were irrigated with 100 ml of betaine with different concentration. As shown in Fig. 5, exogenous betaine treatment increased the content of endogenous betaine in maize seedlings, and the increase was enhanced with increasing concentration of betaine, 10 mM betaine or above reached significant difference and very significant difference levels compared with the control without betaine treatment among the five concentrations (P < 0.05, P < 0.01, Fig. 5). Similarly, after seedlings were treated with betaine, the increase in survival percentage of the seedlings after heat stress at 47 °C was in agreement with the accumulation of endogenous betaine, that is, the improvement of survival percentage was enhanced with the increase of the concentration of betaine, treatment with 10 mM betaine or above reached significant difference and very significant difference levels compared with the control without betaine treatment among the five concentrations (P < 0.05, P < 0.01, Fig. 5). These data implied that betaine treatment could induce the accumulation of endogenous betaine, followed by improved heat tolerance of maize seedlings.
Effect of exogenous betaine pretreatments on the content of endogenous betaine and the survival percentage of maize seedlings. The 2.5-day-old seedlings of maize were treated with 0 (control), 5, 10, 15, 20, and 25 mM betaine for 6 h, and the content of endogenous betaine and the survival percentage of maize seedlings after heat stress were determined. Error bars represent standard error and each data in figure represents the mean ± SE of three experiments, and asterisk and double asterisks indicate significant difference (P < 0.05) and very significant difference (P < 0.01) from the control without betaine treatment, respectively
Discussion
Numerous studies illustrated that H2S is a multifunctional signal molecule, which is involved in plant growth, development, and the acquisition of biotic and abiotic stress tolerance (Papenbrock et al. 2007; Li 2013; Wang 2012; Lisjak et al. 2013; Calderwood and Kopriva 2014; Hancock and Whiteman 2014). As mentioned above, H2S participates in the tolerance to multiple stress, such as osmotic stress (Zhang et al. 2009b; García-Mata and Lamattina, 2010; Christou et al. 2013), salt stress (Christou et al. 2013; Shan et al. 2014), chilling stress (Fu et al. 2013), and heat stress (Christou et al. 2014). Our previous and present results also found that NaHS pretreatment could improve heat tolerance of tobacco suspension cultured cells (Li et al. 2012b), maize (Li et al. 2013a, b, 2014c, d; Fig. 4), and wheat (Wu et al. 2013) seedlings, and the acquisition of heat tolerance induced by NaHS is involved in a second messenger Ca2+ and the accumulation of osmolytes such as proline and trehalose, as well as the enhancement of antioxidant system activity, but whether heat tolerance induced by NaHS is involved in the accumulation of betaine, an osmolyte with multiple physiological functions, is waiting for being further investigated.
Heat injury and the acquisition of heat tolerance in plants is a complex event, which is involved in all of aspects of biomembrane, protein, water and ion balance, as well as redox homeostasis (Wahid et al. 2007; Königshofer et al. 2008; Saidi et al. 2011; Mittler et al. 2012; Wu et al. 2012). During the process of evolution, plants have been developed adaptive mechanism, including repair and reestablishment of biomembrane, synthesis of stress protein (mainly heat shock proteins, HSPs), enhancement of antioxidant system, and accumulation of osmolytes such as betaine, proline, and soluble sugar, to prevent damage caused by heat stress (Wahid et al. 2007; Königshofer et al. 2008; Saidi et al. 2011; Mittler et al. 2012; Wu et al. 2012). Accumulation of osmolyte betaine is an important adaptive mechanism of plant to heat stress due to its multiple protective functions, such as osmotic adjustment, protein and biomembrane stabilizing, ROS-scavenging, and redox buffering (Szabados and Savoure 2010; Theocharis et al. 2012). In higher plants, betaine aldehyde dehydrogenase (BADH) is a key enzyme in the biosynthesis of betaine, it plays a crucial role in betaine accumulation (Fitzgerald et al. 2009). In sweet potato seedlings, the overexpression of Spinacia oleracea (SoBADH) increased BADH activity, which in turn accumulated betaine under normal and multiple environmental stresses, followed by increased protection against cell damage through the maintenance of cell membrane integrity, stronger photosynthetic activity, reduced reactive oxygen species (ROS) production, and induction or activation of ROS scavenging by the increased activity of antioxidant enzymes, eventually improved tolerance to various abiotic stresses (Fan et al. 2012). Our present results also showed that NaHS treatment could increase the activity of betaine aldehyde dehydrogenase (BADH), which in turn induced the accumulation of endogenous betaine (Figs. 1, 2), followed by improved the resistance of maize seedlings to high temperature stress (Fig. 4), while these effects were eliminated by addition of H2S scavenger HT and BADH inhibitor DSF, respectively (Fig. 3). In addition, transgenic tomato lines expressing BADH exhibited higher capabilities for betaine accumulation under normal conditions and showed higher photosynthetic capacities by improving D1 protein content under heat treatment at 42 °C (Li et al. 2014b). Similarly, betaine overaccumulation in transgenic wheat expressing BADH led to the enhancement of antioxidant activity and the improvement of water status, followed by increased photosynthesis under heat stress (Wang et al. 2010).
In addition, exogenous application of betaine increased the tolerance of barley to thermal stress at 45 °C by exerting a protective effect on the oxygen evolution complex (OEC) and by increasing connectivity between PSII-antennae, thus inducing a greater stability of the PSII (Oukarroum et al. 2012). In our present work, application of exogenous betaine increased the level of endogenous betaine, which in turn improved survival percentage of maize seedlings under heat stress (Fig. 5). Addition of betaine to the germination medium or imbibition of seeds in a solution of betaine improved the expression of HSP genes and accumulated more HSP70 than controls. The results of (Li et al. 2011a) showed that betaine, either applied exogenously or accumulated in vivo in codA-transgenic seeds, enhanced the expression of HSP genes, which in turn improved the tolerance to high temperature of tomato seeds during germination. Interestingly, H2S treatment also induced the acquisition of heat tolerance (Li et al. 2013a, b, 2014c; Christou et al. 2013; Fig. 4) and the expression of HSP genes (Christou et al. 2014). These data indicated that the acquisition of heat tolerance induced by NaHS might be involved in the accumulation of betaine by either activating BADH activity in vivo or applying exogenously, further bridging a gap between H2S and betaine in the acquisition of heat tolerance in plants.
In conclusion, pretreatment with NaHS significantly improved the activity of BADH and endogenous betaine accumulation, which in turn enhanced the survival percentage of maize seedlings under heat stress compared with the control without NaHS treatment, these effects were eliminated by application of H2S scavenger HT and BADH inhibitor DSF, respectively. In addition, in comparison with the control without betaine treatment, supplement of exogenous betaine also could enhance the content of endogenous betaine, followed by improved the survival percentage of maize seedlings. All of the above-mentioned results indicated that NaHS treatment could improve the accumulation of betaine by activating the activity of BADH, and this accumulation may be involved in the acquisition of heat tolerance in maize seedlings. However, with the progress of omics, responses of transcriptome, proteome, and metabolome to application of NaHS and betaine in the acquisition of heat tolerance in plants need to be illustrated in the future.
References
Calderwood A, Kopriva S (2014) Hydrogen sulfide in plants: from dissipation of excess sulfur to signalling molecule. Nitric Oxide 41:72–78
Chen THH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34:1–20
Chen J, Wang WH, Wu FH, You CY, Liu WT, Dong XK, He JX, Zheng HL (2013) Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362:301–318
Christou A, Filippou P, Manganaris GA, Fotopouls V (2014) Sodium hydrosulfide induces systemic thermotolerance to strawberry plants through transcriptional regulation of heat shock proteins and aquaporin. BMC Plant Biol 14:42
Christou A, Manganaris GA, Papadopoulos I, Fotopouls V (2013) Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot 64:1953–1966
Fan W, Zhang M, Zhang H, Zhang P (2012) Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS One 7:e37344
Fang T, Cao ZY, Li JL, Shen WB, Huang LQ (2014) Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol Biochem 76:44–51
Fitzgerald TL, Waters DLE, Henry RJ (2009) Betaine aldehyde dehydrogenase in plants. Plant Biol 11:119–130
Fu PN, Wang WJ, Hou LX, Liu X (2013) Hydrogen sulfide is involved in the chilling stress response in Vitis vinifera L. Acta Soc Bot Pol 82:295–302
García-Mata C, Lamattina L (2010) Hydrogen sulfide, a novel gasotransmitter involved in guard cell signalling. New Phytol 188:977–984
García-Mata C, Lamattina L (2013) Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci 201:66–73
Hancock JT, Whiteman M (2014) Hydrogen sulfide and cell signaling: team player or referee? Plant Physiol Biochem 78:37–42
Hanumappa M, Nguyen HT (2010) Genetic approaches toward improving heat tolerance in plants. In: Jenks MA, Wood AJ (eds) Genes for plant abiotic stress. Wiley-Blackwell, Oxford, pp 221–260
Jin ZP, Xue SW, Luo YN, Tian BH, Fang HH, Li H, Pei YX (2013) Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol Biochem 62:41–46
Königshofer H, Tromballa HW, Loppert HG (2008) Early events in signalling high-temperature stress in tobacco BY2 cells involve alterations in membrane fluidity and enhanced hydrogen peroxide production. Plant Cell Environ 31:1771–1780
Leipner J, Stamp P (2009) Chilling stress in maize seedlings. In: Bennetzen JL, Hake SC (eds) Handbook of maize: its biology. Springer, New York, pp 291–344
Li ZG, Gong M (2011) Mechanical stimulation-induced cross-adaptation in plants: an overview. J Plant Biol 54:358–364
Li SF, Li F, Wang JW, Zhang W, Meng QW, Chen THH, Murata N, Yang XH (2011a) Glycine betaine enhances the tolerance of tomato plants to high temperature during germination of seeds and growth of seedlings. Plant Cell Environ 34:1931–1943
Li L, Rose P, Moore PK (2011b) Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51:169–187
Li ZG, Gong M, Liu P (2012a) Hydrogen sulfide is a mediator in H2O2-induced seed germination in Jatropha Curcas. Acta Physiol Plant 34:2207–2213
Li ZG, Gong M, Xie H, Yang L, Li J (2012b) Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci 185:185–189
Li ZG (2013) Hydrogen sulfide: a multifunctional gaseous molecule in plants. Russ J Plant Physiol 60:733–740
Li ZG, Ding XJ, Du PF (2013a) Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J Plant Physiol 170:741–747
Li ZG, Yang SZ, Long WB, Yang GX, Shen ZZ (2013b) Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ 36:1564–1572
Li ZG, Yuan LX, Wang QL, Ding ZL, Dong CY (2013c) Combined action of antioxidant defense system and osmolytes in chilling shock-induced chilling tolerance in Jatropha curcas seedlings. Acta Physiol Plant 35:2127–2136
Li MF, Guo SJ, Xu Y, Meng QW, Li G, Yang XH (2014a) Glycine betaine-mediated potentiation of HSP gene expression involves calcium signaling pathways in tobacco exposed to NaCl stress. Physiol Plant 150:63–75
Li MF, Li ZM, Li SF, Guo SJ, Meng QM, Li G, Yang XH (2014b) Genetic engineering of glycine betaine biosynthesis reduces heat-enhanced photoinhibition by enhancing antioxidative defense and alleviating lipid peroxidation in tomato. Plant Mol Biol Rep 32:42–51
Li ZG, Luo LJ, Zhu LP (2014c) Involvement of trehalose in hydrogen sulfide donor sodium hydrosulfide-induced the acquisition of heat tolerance in maize (Zea mays L.) seedlings. Bot Stu 55:20
Li ZG, Yi XY, Li YT (2014d) Effect of pretreatment with hydrogen sulfide donor sodium hydrosulfide on heat tolerance in relation to antioxidant system in maize (Zea mays) seedlings. Biologia 69:1001–1009
Lisjak M, Srivastava N, Teklic T, Civale L, Lewandowski K, Wilson I, Wood ME, Whiteman M, Hancock JT (2010) A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol Biochem 48:931–935
Lisjak M, Teklic T, Wilson ID, Whiteman M, Hancock JT (2013) Hydrogen sulfide: environmental factor or signalling molecule? Plant, Cell Environ 36:1607–1616
Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends Biochem Sci 37:118–125
Nakamura M, Kuramata M, Kasugai I, Abe M, Youssefian S (2009) Increased thiol biosynthesis of transgenic poplar expressing a wheat O-acetylserine(thiol) lyase enhances resistance to hydrogen sulfide and sulfur dioxide toxicity. Plant Cell Rep 28:313–323
Oukarroum A, Madidi SE, Strasser RJ (2012) Exogenous glycine betaine and proline play a protective role in heat-stressed barley leaves (Hordeum vulgare L.): a chlorophyll a fluorescence study. Plant Biosyst 146:1037–1043
Papenbrock J, Riemenschneider A, Kamp A, Schulz-Vog HN, Schmidt A (2007) Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants—from the field to the test tube and back. Plant Biol 9:582–588
Saidi Y, Finka A, Goloubinoff P (2011) Heat perception and signalling in plants: a tortuous path to thermotolerance. New Phytol 190:556–565
Shan C, Liu H, Zhao L, Wang X (2014) Effects of exogenous hydrogen sulfide on the redox states of ascorbate and glutathione in maize leaves under salt stress. Biol Plant 58:169–173
Strable J, Scanlon MJ (2009) Maize (Zea Mays): A model organism for basic and applied research in plant biology. Cold Spring Harb Protoc 10 (2009), pdb.emo132
Szabados L, Savoure A (2010) Proline: A multifunctional amino acid. Trends Plant Sci 15:89–97
Theocharis A, Clément C, Barka EA (2012) Physiological and molecular changes in plants grown at low temperatures. Planta 235:1091–1105
Velasco-García R, Chacón-Aguilar VM, Hervert-Hernández D, Muñoz-Clares RA (2003) Inactivation of betaine aldehyde dehydrogenase from Pseudomonas aeruginosa and Amaranthus hypochondriacus L. leaves by disulfiram. Chem Biol Int 143/144:149–158
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang R (2012) Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92:791–896
Wang GP, Zhang XY, Li F, Luo Y, Wang W (2010) Overaccumulation of glycine betaine enhances tolerance to drought and heat stress in wheat leaves in the protection of photosynthesis. Photosynthetica 48:117–126
Wu HC, Luo DL, Vignols F, Jinn TL (2012) Heat shock-induced biphasic Ca2+ signature and OsCaM1-1 nuclear localization mediate downstream signalling in acquisition of thermotolerance in rice (Oryza sativa L.). Plant Cell Environ 35:1543–1557
Wu DH, Li YL, Xia X, Pu ZP, Liao JM, Huang K, Li ZG (2013) Hydrogen sulfide donor sodium hydrosulfide pretreatment improved multiple resistance abilities of wheat to high temperature and drought stress. J Yunnan Normal Univ 33:29–35
Zhang H, Hu LY, Hu KD, He YD, Wang SH, Luo JP (2008) Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 50:1518–1529
Zhang H, Tang J, Liu XP, Wang Y, Yu W, Peng WP, Fang F, Ma DF, Wei ZJ, Hu LY (2009a) Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J Integr Plant Biol 51:1084–1092
Zhang H, Ye YK, Wang SH, Luo JP, Tang J, Ma DF (2009b) Hydrogen sulfide counteracts chlorophyll loss in sweet potato seedling leaves and alleviates oxidative damage against osmotic stress. Plant Growth Regul 58:243–250
Zhang H, Wang MF, Hua LY, Wang SH, Hua KD, Bao LJ, Luo JP (2010) Hydrogen sulfide promotes wheat seed germination under osmotic stress. Russ J Plant Physiol 57:532–539
Zhang H, Hua SL, Zhang ZJ, Hua LY, Jiang CX, Wei ZJ, Liu J, Wang H, Jiang ST (2011) Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol Technol 60:251–257
Acknowledgments
This research is supported by National Natural Science Foundation of China (31360057). We appreciate the reviewers and editors for their exceptionally helpful comments about the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, ZG., Zhu, LP. Hydrogen sulfide donor sodium hydrosulfide-induced accumulation of betaine is involved in the acquisition of heat tolerance in maize seedlings. Braz. J. Bot 38, 31–38 (2015). https://doi.org/10.1007/s40415-014-0106-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-014-0106-x