Abstract
Hydrogen sulfide (H2S) and methylglyoxal (MG) were supposed to be novel signaling molecules in plants. However, whether interplay between H2S and MG can initiate thermotolerance in maize seedlings and in relation to metabolism of reactive oxygen species (ROS) and osmolytes is little known. In this study, watering with MG and NaHS (H2S donor) alone or in combination elevated survival and tissue vigor of maize seedlings under heat stress and coped with an increase in the biomembrane injury (as indicated in membrane lipid peroxidation and electrolyte leakage). The above-mentioned effects were separately weakened by MG scavengers (N-acetyl cysteine: NAC; aminoguanidine: AG) and H2S inhibitor (DL-propargylglycine, PAG) and scavenger (hypotaurine, HT). These suggested that the interplay between H2S and MG initiated the thermotolerance in maize seedlings. The further data indicated that, under non-heat stress and heat stress conditions, MG and NaHS alone or in combination modulated ROS metabolism by regulating the activities of antioxidant enzymes (catalase, ascorbate peroxidase, guaiacol peroxidase, glutathione reductase, monodehydroascorbate reductase, and dehydroascorbate reductase) and the contents of non-enzymatic antioxidants (ascorbic acid, glutathione, flavonoids, and carotenoids) in maize seedlings. In addition, MG and NaHS alone or in combination also separately modulated the metabolism of osmolytes (proline, trehalose, glycine betaine, and total soluble sugar), H2S (L-cysteine desulfhydrase and O-acetylserine (thione) lyase), and MG (glyoxalase I, glyoxalase II, and MG reductase). These physiological effects also were separately impaired by NAC, AG, PAG, and HT. The current data illustrated that the interplay between H2S and MG initiated the thermotolerance in maize seedlings by modulating ROS, osmolyte, H2S, and MG metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen sulfide (H2S) is an intermediate product of sulfur in plants, which is a precursor of cysteine synthesizing sulfur-containing tripeptide glutathione (GSH) and proteins (Hancock and Whiteman 2014, 2016; Li et al. 2016). H2S, for a long time, is deemed as a toxic agent due to its high affinity to heme-containing proteins (that is, sulfhydration, also known as persulfidation, a posttranslational modification), such as cytochrome oxidase, hemoglobin, and myohemoglobin (Corpas 2019; Hancock 2019). Therefore, H2S must maintain homeostasis in plant cell under normal physiological conditions. In general, the enzymes related to H2S generation and scavenging are contributed to the homeostasis of H2S in plant cells. These enzymes include L-/D-cysteine desulfhydrase (LCD/DCD), sulfite reductase (SiR), carbonic anhydrase (CA), and cyanoalanine synthase (CAS), which undertake the biosynthesis of H2S (Li et al. 2016; Corpas 2019). In addition, cysteine synthase (CS), also known as O-acetyl serine (thione) lyase (OAS-TL), takes charge of the conversion of H2S into cysteine. Also, excessive H2S can be released to the air from plant body in the form of gas (Hancock and Whiteman 2014, 2016; Li et al. 2016). Recently, H2S has attracted more attentions in plants, which have been found to function as signaling role implicating in seed germination, seedling establishment, morphogenesis, and development, as well as response and adaptation to environmental stress (Li et al. 2016; Corpas 2019; Hancock 2019). Our previous studies also showed that H2S could increase the tolerance of maize seedlings to heat stress (Li et al. 2013, 2014, 2018a), but the upstream and/or downstream signaling events of H2S were relatively unknown.
Reactive carbonyl species (RCS), mainly methylglyoxal (MG), glyoxal, acrolein, 4-hydroxyl-2-nonenal, and malondiadehyde (MDA), are a group of byproducts of sugar and amino acid metabolism as well as lipid peroxidation. RCS can lead to the glycosylation of proteins (cysteine, histidine, arginine, lysine, and threonine), nucleic acids (guanine and cytosine), and lipids (unsaturated fatty acid), producing advanced glycation end products (AGEs) and advanced lipid peroxidation end products (ALEs), which is also known as carbonyl stress or MG stress (induced by MG) (Kaur et al. 2016; Li 2016, 2019a). MG stress can cause oxidative stress by directly triggering the excessive accumulation of reactive oxygen species and/or inhibiting the activities of antioxidant enzymes, such as peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and ascorbate peroxidase (APX) (Li 2016, 2019b; Mostofa et al. 2018). Therefore, for a long time, MG, analogous to H2S, is perceived as cytotoxic agent. In plant cells, the homeostasis of MG was critically regulated by triosephosphate isomerase (TPI), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), MG reductase (MGR), glyoxalase I (Gly I), glyoxalase II (Gly II), and even glyoxalase III (Gly III) (Kaur et al. 2016; Li 2016, 2019a). Recently, numerous works have been confirmed that MG play a dual role in the life cycle of plants, that is, as cytotoxic agent at high dose and signaling molecule at a low physiological concentration. The signaling role of MG also has become a research focus in plants in recent years (Li 2016, 2019b; Mostofa et al. 2018). In maize seedlings, MG, as a signaling molecule, could trigger the acquirement of heat tolerance (Li et al. 2018a; Wang et al. 2019). However, the interplay between MG and H2S in the development of thermotolerance in maize seedlings was poorly known.
Maize not only is a food, energy, feed, and industry crop, but also a novel model plant (Leipner and Stamp 2009; Strable and Scanlon 2009). With the aggravation of global warming, high temperature has become a critical factor limiting plant including maize production (Wahid et al. 2007; Leipner and Stamp 2009; Strable and Scanlon 2009; Ohama et al. 2017; Lawas et al. 2018; Ali et al. 2020). Therefore, understanding the mechanisms of heat injury and thermotolerance is of great significance for agricultural production. Many works have illustrated that both H2S and MG, as signaling molecules, take part in the acquirement of stress tolerance (Li et al. 2018a; Mostofa et al. 2018; Corpas 2019; Hancock 2019). Based on the current knowledge, we hypothesize that H2S might interact with MG in the development of stress tolerance. Therefore, in this study, we used maize seedlings as materials to investigate the effect of H2S-MG interaction on thermotolerance of maize seedlings and underlying mechanism.
Materials and methods
Material culture conditions and pretreatments
In this study, the healthy and same size seeds (purchased from Chuxiong Seed Company, China) of maize (Zea mays L., cv. Chenguang No. 2) were selected as materials. The selected seeds were performed surface sterilization by immersing in 0.1% HgCl2 solution for 10 min, and then rinsed six times with sterile water to remove residual HgCl2 on the seed surface. The cleansed seeds were soaked in distilled water for 12 h, and then placed in trays (with six-layer wetted filter papers and 300 seeds per tray) with covers. The soaked seeds were germinated in a climate chamber at 26 °C for 2.5 days (60 h) in the dark (the seedlings are approximately 2 cm high). Afterwards, the 60-h-old seedlings with the same size were fallen into eight groups, and then separately watered with 100 mL of the following solutions for 6 h: (1) distilled water (control), (2) 500 μM NaHS (NaHS), (3) 50 μM MG (MG), (4) 500 μM NaHS + 50 μM MG (NaHS + MG), (5) 100 μM N-acetyl-cysteine (MG scavenger) + 500 μM NaHS (NAC + NaHS), (6) 100 μM aminoguanidine (MG scavenger) + 500 μM NaHS (AG + NaHS), (7) 100 μM DL-propargylglycine (H2S inhibitor) + 50 μM MG (PAG + MG), (8) 100 μM hypotaurine (H2S scavenger) + 50 μM MG (HT + MG), and (9) 100 μM NAC, AG, PAG, or HT. In the combined treatment with chemicals, to avoid the possible reaction between chemicals, the maize seedlings were pretreated for 3 h with the first chemical prior to treatment with the second chemical. In addition, the optimal concentrations of chemicals were selected from preliminary experiments and previous studies (Li et al. 2013, 2018a; Wang et al. 2019).
Heat stress and evaluation of survival percentage, lipid peroxidation, electrolyte leakage, and tissue vigor in maize seedlings
After chemical treatment for 6 h, the residual chemicals on the seedling roots were washed clearly with distilled water and the seedlings were carried out heat stress in a climate chamber at 46 °C for 16 h (this heat strength was optimized according to the preliminary experiments). After heat stress, the heated seedlings were recovered for 7 days in a climate chamber at 26 °C, 200 μmol m−2 s−1, and 14-h photoperiod. During the recovery growth, the maize seedlings were watered with 1/2 Hoagland solution. After recovery, the survival percentage of seedlings was calculated as survival percentage (%) = survived seedlings/total seedlings × 100%.
After chemical treatments for 6 h (non-heat stress) and heat stress at 46 °C for 16 h (heat stress), the whole seedling samples (the same as below) were used to evaluate lipid peroxidation (that is, the content of malondialdehyde: MDA), electrolyte leakage (indicating biomembrane integrity), and tissue vigor (namely reducing power of triphenyl tetrazolium chloride: TTC) as per the procedure reported by Wang et al. (2019). The MDA content, electrolyte leakage, and tissue vigor were separately indicated by μmol g−1 fresh weight (FW), %, and A485.
Antioxidant enzyme and superoxide radical production assay
After chemical treatments and heat stress, antioxidant enzymes catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (POD), and superoxide dismutase (SOD) in maize seedlings were extracted and assayed as the previously described methods (Li et al. 2019; Wang et al. 2019). The increase (for POD) and decrease (for CAT, APX, and SOD) in optical density (OD) at 470, 240, 290, and 560 nm were separately recorded, as well as their activities were calculated according to the extinction coefficient of 26.6 (for four-guaiacol), 40 (for hydrogen peroxide), and 2.8 (for ascorbic acids: AsA) mM−1 cm−1, while one unit of SOD was defined as the amount of enzyme inhibiting 50% photochemical reduction of nitroblue tetrazolium.
Similarly, the enzymes related to glutathione (GSH)-AsA cycle, namely glutathione reductase (GR), dehydroascorbate reductase (DHAR), and monodehydroascorbate reductase (MDHAR) in maize seedlings, were extracted and determined as per the methods of Wang et al. (2019). The decrease (for GR) and increase (for DHAR and MDHAR) in absorbance at 340 and 265 nm were separately noted, and their activities were counted on the basis of extinction coefficient of 6.2 (for NADPH) and 14 (for AsA) mM−1 cm−1.
In addition, the productive rate of superoxide radical (O2·−) in maize seedlings treated with chemical and heat stress was quantified by measuring the formation of formazan using Na,3′-[1-[(phenylamino)-carbonyl]-3, 4-tetrazolium] (4-methoxy-6-nitro) benzene sulfonic acid hydrate (XTT) method (Li et al. 2019). O2·− production was calculated according to the molar extinction coefficient of 2.16 × 104 M−1 cm−1 (for formazan) at 470 nm and expressed in nmol g−1 FW min−1.
Measurement of water-soluble and lipid-soluble antioxidants
After chemical treatments and heat stress, water-soluble antioxidants GSH, oxidized GSH (GSSG), AsA, and oxidized AsA (DHA) in maize seedlings were extracted and measured in line with the methods of Wang et al. (2019). Their contents were expressed as μmol g−1 FW.
Additionally, lipid-soluble antioxidants flavonoids (FLA) and carotenoids (CAR) in maize seedlings were extracted and quantified in the light of the procedure previously described by Wang et al. (2019). The contents of FLA and CAR were expressed in μg g−1 FW.
Determination of methylglyoxal and its metabolic enzymes
After chemical treatments and heat stress, the activities of glyoxalase I (Gly II), glyoxalase II (Gly II), and MG reductase (MGR) as well as MG content in maize seedlings were determined according to the methods (Chen et al. 2003; Wang et al. 2019). The activities of Gly I, Gly II, and MGR were computed using the extinction coefficient of 3.37 (for S-D-lactoylglutathione at 240 nm), 13.6 (for 5,5′-dithio-bis (2-nitrobenzoic acid) at 412 nm), and 6.2 (for NADPH at 340 nm) mM−1 cm−1, and their activities and MG content were expressed in μmol g−1 FW min−1 and μmol g−1 FW, respectively.
Determination of H2S and its metabolic enzymes
After chemical treatments and heat stress, H2S content and the activities of LCD and OAS-TL in maize seedlings were determined as per the methods previously reported by Wang et al. (2019) and Li (2015). The content of H2S and the activities of LCD and OAS-TL in maize seedlings were expressed in μmol g−1 FW and μmol g−1 FW min−1, respectively.
Evaluation of osmolytes
After chemical treatments and heat stress, osmolytes proline (Pro), glycine betaine (GB), trehalose (Tre), and total soluble sugar (TSS) contents were evaluated according to the methods of Li et al. (2019) and Wang et al. (2019) and expressed as μmol g−1 FW and μg g−1 FW, respectively.
Statistical analysis
All experiments were designed according to the requirement of random experiment and repeated at least three times. The data were analyzed with one-way analysis of variance and the figures were made using software sigmaplot 14. The significance compared with the control was evaluated using the least significant difference (LSD) test. In figures, the data were means ± standard error (SE); the asterisk (*) and double asterisks (**) denoted significant differences (P < 0.05) and very significant differences (P < 0.01). In addition, all data were subjected to Pearson correlation analysis to test relationship each other; significance is the same as above.
Results
Interplay between H2S and MG initiates thermotolerance in maize seedlings
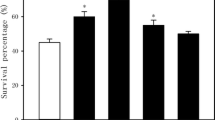
To explore the effect of interplay between H2S and MG on the thermotolerance of maize seedlings, the 2.5-day-old maize seedlings were watered with distilled water (control), NaHS, MG, NaHS + MG, NAC + NaHS, AG + NaHS, PAG + MG, and HT + MG, and then subjected to heat stress at 46 °C for 16 h. As expected, compared with the control, NaHS and MG alone obviously elevated the survival of maize seedlings under heat stress (P < 0.01), and NaHS-induced survival was enhanced by MG (Fig. 1a; P < 0.01). In addition, NaHS-induced survival was significantly weakened by MG scavengers NAC and AG, respectively (Fig. 1a; P < 0.01). Similarly, MG-induced survival was also signally impaired by H2S biosynthetic inhibitor (PAG) and scavenger (HT), respectively (Fig. 1a; P < 0.01). Also, compared with the control, the survival rate of maize seedlings was significantly lowered by NAC, AG, PAG, or HT (Fig. 1a; P < 0.05).
Effect of irrigation of maize seedlings with methylglyoxal (MG), NaHS, N-acety cysteine (NAC) + NaHS, aminoguanidine (AG) + NaHS, DL-propargylglycine (PAG) + MG, hypotaurine (HT) + MG, NAC, AG, PAG, and HT on the survival percentage (a), malondialdehyde (MDA) content (b), electrolyte leakage (c), and tissue vigor (d) under non-heat stress and heat stress. The 2.5-day-old maize seedlings were watered with 100 mL of distilled water (control), 500 μM NaHS (NaHS), (3) 50 μM MG (MG), (4) 500 μM NaHS + 50 μM MG (NaHS + MG), (5) 100 μM NAC + 500 μM NaHS (NAC + NaHS), (6) 100 μM AG + 500 μM NaHS (AG + NaHS), (7) 100 μM PAG + 50 μM MG (PAG + MG), (8) 100 μM HT + 50 μM MG (HT + MG), and (9) 100 μM NAC, AG, PAG, or HT for 6 h, and then subjected to heat stress at 46 °C for 16 h. After chemical treatment and heat stress, the survival percentage, MDA content, electrolyte leakage, and tissue vigor were evaluated. In figures, the data were means ± standard error (SE), and the asterisk and double asterisks separately denoted significant differences (P < 0.05) and very significant differences (P < 0.01) compared with the control
For MDA content and electrolyte leakage in maize seedlings, under non-heat stress conditions (at 26 °C), compared with the control, chemical treatments had no significant difference (Fig. 1b, c). Under heat stress, compared with the non-heat stress seedlings, the heated seedlings had a higher MDA content and electrolyte leakage (P < 0.01), but the seedlings treated with NaHS, MG, and their combination showed a lower MDA content and electrolyte leakage compared with the control (Fig. 1b, c; P < 0.01). Analog to the change in survival, heat stress-induced increase in MDA content, and electrolyte leakage was separately aggravated by NaHS in combination with MG scavengers (NAC and AG), MG in combination with H2S inhibitor (PAG), and scavenger (HT), as well as NAC, AG, PAG, and HT only (Fig. 1b, c; P < 0.01).
For tissue vigor in maize seedlings, under both non-heat stress and heat stress conditions (at 46 °C for 16 h), compared with the control, the treatment with NaHS, MG, and their combination significantly improved the tissue vigor of maize seedlings (Fig. 1d; P < 0.01). In addition, NaHS combined with MG scavengers (NAC and AG), MG combined with H2S inhibitor PAG and scavenger HT, as well as NAC, AG, PAG, and HT alone markedly decreased the tissue vigor of maize seedlings under both non-heat stress and heat stress conditions compared with the control (Fig. 1d; P < 0.01).
Interplay between H2S and MG modulates ROS-metabolic enzymes in maize seedlings under non-heat stress and heat stress conditions
The interplay between H2S and MG could induce the thermotolerance in maize seedlings (Fig. 1); to further understand its underlying mechanisms, the enzymatic and non-enzymatic components related to ROS metabolism in maize seedlings were measured after chemical treatments and heat stress. The data showed that, under non-heat stress conditions, compared with the control, antioxidant enzymes showed the different trends: MG, NaHS, and their combination significantly up-regulated the activity of CAT (Fig. 2a; P < 0.01), while markedly down-regulated APX except for NaHS alone (no significance) (Fig. 2b; P < 0.01), but no significant effect on POD and SOD activities (Fig. 2c, d; P < 0.01). Additionally, NAC + NaHS and AG + NaHS significantly declined CAT and APX activities (Fig. 2a, b; P < 0.01), but the significant difference on POD and SOD was not observed (Fig. 2c, d). Similarly, PAG + MG and HT + MG obviously decreased APX and CAT activities except for HT + MG for CAT (no significance) (Fig. 2a, b; P < 0.01), while no effect on POD and SOD (Fig. 2a, b). Also, NAC, AG, PAG, and HT alone had no significant effect on the activities of CAT, APX, POD, and SOD in maize seedlings (Fig. 2).
Effect of irrigation of maize seedlings with methylglyoxal (MG), NaHS, N-acety cysteine (NAC) + NaHS, aminoguanidine (AG) + NaHS, DL-propargylglycine (PAG) + MG, and hypotaurine (HT) + MG, NAC, AG, PAG, and HT on the activity of catalase (CAT, a), ascorbate peroxidase (APX, b), guaiacol peroxidase (POD, c), and superoxide dismutase (SOD, d) under non-heat stress and heat stress. The 2.5-day-old maize seedlings were watered with 100 mL of different chemicals for 6 h (referring to Fig. 1 and “Materials and methods”), and then subjected to heat stress at 46 °C for 16 h. After chemical treatment and heat stress, the activities of CAT, APX, POD, and SOD were assayed. In figures, the data were means ± standard error (SE), and the asterisk and double asterisks separately denoted significant differences (P < 0.05) and very significant differences (P < 0.01) compared with the control
Under heat stress conditions, compared with the control, MG, NaHS, and their combination obviously stimulated CAT, APX, and POD activities except for NaHS for CAT (no significance) (Fig. 2a, b, c; P < 0.01), whereas these treatments had no significant difference on SOD (Fig. 2d). In addition, NAC + NaHS signally reduced CAT and POD activities (Fig. 2a, c; P < 0.01), but no significant effect on APX and SOD (Fig. 2a, d); also, the significant difference on CAT, APX, POD, and SOD was not noted in maize seedlings treated with AG + NaHS under heat stress compared with the control (Fig. 2a, b, c, d). Besides, compared with the control, PAG + MG significantly descended CAT and POD activities (Fig. 2a, c; P < 0.01), but the significant effect on APX and SOD was not recorded (Fig. 2b, d) under heat stress. Similarly, under heat stress, HT + MG had no significant difference on CAT, APX, POD, and SOD activities compared with the control (Fig. 2a, b, c, d). In addition, NAC, AG, PAG, and HT alone obviously declined CAT and POD activities (Fig. 2a, c; P < 0.05) in maize seedlings, but no significant effect on APX and SOD (Fig. 2b, d) compared with the control.
For water-soluble and lipid-soluble antioxidants, under non-heat stress conditions, compared with the control, treatment with MG, NaHS, and their combination signally increased the contents of AsA (P < 0.01), GSH (P < 0.05), FLA (P < 0.01), and CAR (P < 0.05) in maize seedlings except for NaHS + MG for GSH and NaSH for FLA (Fig. 3a, c, e, f), while it obviously decreased the levels of DHA (P < 0.01) and GSSG (P < 0.05) except for NaHS for GSSG (Fig. 3b, d). In addition, NAC + NaHS, AG + NaHS, PAG + MG, and HT + MG markedly reduced the contents of DHA (P < 0.01), FLA (P < 0.01), and CAR (P < 0.05), whereas significantly ascended the GSSG level except for NAC + NaHS (Fig. 3d; P < 0.01), but no significant effect on GSH content (Fig. 3c). Also, compared with the control, NAC + NaHS and PAG + MG obviously improved the content of AsA in maize seedlings (Fig. 3a; P < 0.01), but the significant effect on AsA was not observed in maize seedlings treated with AG + NaHS and HT + MG (Fig. 3a). In addition to these, significant difference of the contents of AsA, DHA, GSH, GSSG, FLA, and CAR was not noted in maize seedlings treated with NAC, AG, PAG, and HT only compared with the control (Fig. 3).
Effect of irrigation of maize seedlings with methylglyoxal (MG), NaHS, N-acety cysteine (NAC) + NaHS, aminoguanidine (AG) + NaHS, DL-propargylglycine (PAG) + MG, and hypotaurine (HT) + MG, NAC, AG, PAG, and HT on the contents of ascorbic acid (AsA, a), dehydroascorbate (DHA, b), glutathione (GSH, c), oxidized GSH (GSSG, d), flavonoids (FLA, e), and carotenoids (CAR, f) under non-heat stress and heat stress. The 2.5-day-old maize seedlings were watered with 100 mL of different chemicals for 6 h (referring to Fig. 1 and “Materials and methods”), and then subjected to heat stress at 46 °C for 16 h. After chemical treatment and heat stress, the contents of AsA, DHA, GSH, GSSG, FLA, and CAR were tested. In figures, the data were means ± standard error (SE), and the asterisk and double asterisks separately denoted significant differences (P < 0.05) and very significant differences (P < 0.01) compared with the control
Under heat stress conditions, compared with the control, MG and NaHS alone or in combination memorably increased the contents of AsA (P < 0.01), GSH (P < 0.05), FLA (P < 0.05), and CAR (P < 0.05) in maize seedlings (Fig. 3a, c, e, f), while MG and NaHS + MG observably reduced the contents of DHA (P < 0.01) and GSSG (P < 0.05) (Fig. 3b, d), but NaHS had no significant effect on DHA and GSSG (Fig. 3b, d). In addition, NAC + NaHS, AG + NaHS, PAG + MG, and HT + MG obviously declined the contents of GSH and CAR except for AG + NaHS for CAR (Fig. 3c, f; P < 0.01) in maize seedlings under heat stress. Also, NAC + NaHS significantly increased the AsA content (Fig. 3a; P < 0.05), decreased DAH (Fig. 3b; P < 0.05), while no significant effect on GSSG and FLA (Fig. 3d, e) under heat stress. Furthermore, under heat stress, AG + NaHS obviously reduced the GSSG content (Fig. 3d; P < 0.05), but the significant difference was not noted for AsA, DHA, and FLA (Fig. 3d). Similarly, PAG + MG markedly elevated the AsA level (Fig. 3a; P < 0.01), descended FLA content (Fig. 3e; P < 0.01), but had no significant effect on DHA and GSSG (Fig. 3b, d) under heat stress. Also, HT + MG significantly declined the levels of DHA and FLA (Fig. 3b, e; P < 0.01), but the significant difference on AsA and GSSG was not recorded in maize seedlings under heat stress compared with the control (Fig. 3a, d). Additionally, compared with the control, NAC, AG, PAG, and HT alone increased the contents of DHA, GSH, and GSSG in maize seedlings (Fig. 3b, c, d; P < 0.05) and decreased the levels of AsA (Fig. 3a; P < 0.05), FLA (Fig. 3e; P < 0.05) and CAR (Fig. 3f; P < 0.01).
Interplay between H2S and MG modulates GSH-AsA cycle and superoxide radical production in maize seedlings under non-heat stress and heat stress conditions
Beside ROS-metabolic enzymes, after chemical treatments and heat stress, the activity of enzymes related to GSH-AsA cycle and the rate of superoxide radical production in maize seedlings were evaluated. As shown in Fig. 4, under non-heat stress conditions, treatment with MG and NaHS alone or in combination significantly activated GR (Fig. 4a; P < 0.01), reduced DHAR activity (Fig. 4c; P < 0.05), but had no significant effect on MDHAR compared with the control (Fig. 4b). In addition, NAC + NaHS and AG + NaHS observably declined the activities of GR (Fig. 4a; P < 0.05, except for NAC + NaHS), MDHAR (Fig. 4b; P < 0.05), and DHAR (Fig. 4c; P < 0.01) compared with the control. Similarly, PAG + MG and HT + MG also signally descended MDHAR activity in maize seedlings (Fig. 4b; P < 0.01), but had no significant difference on GR and DHAR compared with the control (Fig. 4a, c). In addition, compared with the control, MG + NaHS markedly declined the productive rate of superoxide radical (P < 0.01), while NaHS, MG, NAC + NaHS, AG + NaHS, PAG + MG, and HT + MG had no significant effect on its production (Fig. 4d). Also, NAC, AG, PAG, and HT alone had no significant effect on GR, MDHAR, and DHAR activities and O2·− production in maize seedlings (Fig. 4) except prominent decrease in O2·− production by NAC (Fig. 4d; P < 0.01).
Effect of irrigation of maize seedlings with methylglyoxal (MG), NaHS, N-acety cysteine (NAC) + NaHS, aminoguanidine (AG) + NaHS, DL-propargylglycine (PAG) + MG, and hypotaurine (HT) + MG, NAC, AG, PAG, and HT on the activity of glutathione reductase (GR, A), monodehydroascorbate reductase (MDHAR, B), and dehydroascorbate reductase (DHAR, C) and superoxide radical production (O2·−, d) under non-heat stress and heat stress. The 2.5-day-old maize seedlings were watered with 100 mL of different chemicals for 6 h (referring to Fig. 1 and “Materials and methods”), and then subjected to heat stress at 46 °C for 16 h. After chemical treatment and heat stress, the activity of GR, MDHAR, and DHAR, as well as the production of superoxide radical were measured. In figures, the data were means ± standard error (SE), and the asterisk and double asterisks separately denoted significant differences (P < 0.05) and very significant differences (P < 0.01) compared with the control
Under heat stress conditions, the treatment with MG and NaHS alone or in combination markedly upregulated the activities of GR, MDHAR, and DHAR in maize seedlings compared with the control (Fig. 4a, b, c; P < 0.01). In addition, NAC + NaHS and AG + NaHS had no significant effect on GR (Fig. 4a) and MDHAR (Fig. 4b) except signal decrease in DHAR (Fig. 4c; P < 0.01) in maize seedlings under heat stress compared with the control. Similarly, treatment with PAG + MG and HT + MG significantly reduced the activities of GR (Fig. 4a; P < 0.05) and MDHAR (Fig. 4b; P < 0.05, except for PAG + MG), but no significant difference on DHAR activity (Fig. 4c) in maize seedlings under heat stress compared with the control. Also, heat stress triggered the production of superoxide radical, which was significantly alleviated by MG, NaHS, NaHS + MG, NAC + NaHS, AG + NaHS, PAG + MG, and HT + MG compared with the control (Fig. 4d; P < 0.05). Additionally, compared with the control, NAC, AG, PAG, and HT alone significantly reduced the activities of GR (P < 0.05 for NAC, PAG, and HT; P < 0.01 for AG), MDHAR (P < 0.05), and DHAR (P < 0.01) in maize seedlings (Fig. 4a, b, c), but no significant effect on O2·− production except significant reduction in by NAC (Fig. 4d; P < 0.05).
Interplay between H2S and MG modulates H2S metabolism in maize seedlings under non-heat stress and heat stress conditions
To further understand the effect of interplay between H2S and MG on H2S metabolism in maize seedlings, the key enzymes related to H2S metabolism were measured after the maize seedlings were treated with different chemicals and subsequent heat stress. As indicated in Fig. 5, under non-heat stress conditions, compared with the control, treatment with NaHS signally increased the endogenous H2S content in maize seedlings (P < 0.05), but MG alone or in combination with NaHS had no significant effect on LCD and OAS-TL activities, as well as endogenous H2S content (Fig. 5a, b, c). In addition, treatment with NAC + NaHS, AG + NaHS, PAG + MG, and HT + MG was not significantly different on LCD and OAS-TL activities, as well as the endogenous content of H2S in maize seedlings in comparison to the control (Fig. 5a, b, c). Also, compared with the control, NAC and AG alone had no significant effect on LCD and OAS-TL activities and H2S content in maize seedlings (Fig. 5a, b, c), while PAG inhibited LCD activity and H2S content (Fig. 5a, c; P < 0.01), but no significant effect on OAS-TL (Fig. 5b). Similarly, HT obviously declined H2S level (Fig. 5d; P < 0.01), but significant difference of LCD and OAS-TL was not observed in maize seedlings (Fig. 5a, b).
Effect of irrigation of maize seedlings with methylglyoxal (MG), NaHS, N-acety cysteine (NAC) + NaHS, aminoguanidine (AG) + NaHS, DL-propargylglycine (PAG) + MG, and hypotaurine (HT) + MG, NAC, AG, PAG, and HT on the activity of L-cysteine desulfhydrase (LCD, a) and O-acetyl serine (thione) lyase (OAS-TL, b), as well as H2S content (c) under non-heat stress and heat stress. The 2.5-day-old maize seedlings were watered with 100 mL of different chemicals for 6 h (referring to Fig. 1 and “Materials and methods”), and then subjected to heat stress at 46 °C for 16 h. After chemical treatment and heat stress, the activity of LCD and OAS-TL, as well as H2S content were measured. In figures, the data were means ± standard error (SE), and the asterisk and double asterisks separately denoted significant differences (P < 0.05) and very significant differences (P < 0.01) compared with the control
Under heat stress conditions, in comparison to the control, the treatment with MG and NaHS alone or in combination observably activated LCD (P < 0.05) and OAS-TL (P < 0.05, except for NaHS), as well as ascended the endogenous content of H2S (P < 0.05) in maize seedlings (Fig. 5a, b, c). In addition, NAC + NaHS, AG + NaHS, PAG + MG, and HT + MG markedly reduced the activities of LCD (P < 0.01) and OAS-TL (P < 0.05) in maize seedlings under heat stress in comparison to the control. Similarly, NAC + NaHS and PAG + MG obviously declined the content of endogenous H2S (P < 0.05) in maize seedlings under heat stress compared with the control, but AG + NaHS and HT + MG had no significant difference on the endogenous content of H2S (Fig. 5a, b, c). Also, compared with the control, NAC and AG significantly decreased OAS-TL activity (Fig. 5b; P < 0.01), but no significant effect on LCD and H2S (Fig. 5a, c). Similarly, PAG and HT inhibited the activities of LCD (Fig. 5a; P < 0.01) and OAS-TL (Fig. 5b; P < 0.01) as well as the content of H2S (Fig. 5c; P < 0.05) except no effect of HT on LCD.
Interplay between H2S and MG modulates MG metabolism in maize seedlings under non-heat stress and heat stress conditions
To deeply investigate the effect of interplay between H2S and MG on MG metabolism in maize seedlings, the key enzymes of MG metabolism were assayed after chemical treatment (non-heat stress) and heat stress. The results indicated that, under non-heat stress conditions, compared with the control, treatment with MG and NaHS alone or in combination significantly increased the Gly II (Fig. 6b; P < 0.01) and MGR (Fig. 6c; P < 0.05, except for NaHS + MG) activities, as well as MG content (Fig. 6d; P < 0.01, except for NaHS + MG) in maize seedlings, while markedly decreased Gly I activity (Fig. 6a; P < 0.01) except for NaHS + MG (no significance). In addition, treatment with NAC + NaHS, AG + NaHS, PAG + MG, and HT + MG all significantly reduced the Gly II and MGR activities (Fig. 6b, c; P < 0.01), as well as MG level (Fig. 6d; P < 0.05) compared with the control, whereas it had no significant effect on Gly I activity (Fig. 6a). Also, compared with the control, NAC, AG, PAG, and HT significantly decreased MG content in maize seedlings (P < 0.01), but no significant effect on Gly I and Gly II (Fig. 6).
Effect of irrigation of maize seedlings with methylglyoxal (MG), NaHS, N-acety cysteine (NAC) + NaHS, aminoguanidine (AG) + NaHS, DL-propargylglycine (PAG) + MG, and hypotaurine (HT) + MG, NAC, AG, PAG, and HT on the activity of glyoxalase I (Gly I, a), glyoxalase II (Gly II, b), and MG reductase (MGR, c), as well as the content of MG (d) under non-heat stress and heat stress. The 2.5-day-old maize seedlings were watered with 100 mL of different chemicals for 6 h (referring to Fig. 1 and “Materials and methods”), and then subjected to heat stress at 46 °C for 16 h. After chemical treatment and heat stress, the activity of Gly I, Gly II, and MGR, as well as MG content were evaluated. In figures, the data were means ± standard error (SE), and the asterisk and double asterisks separately denoted significant differences (P < 0.05) and very significant differences (P < 0.01) compared with the control
Under heat stress conditions, compared with the control, treatment with MG and NaHS alone or in combination observably activated Gly I (Fig. 6a; P < 0.05, except for NaHS), Gly II (Fig. 6b; P < 0.01), and MGR (Fig. 6c; P < 0.01, except for MG), as well as increased endogenous MG content (Fig. 6d; P < 0.05, except for MG treatment). In addition, treatment with NAC + NaHS and AG + NaHS markedly declined Gly I (Fig. 6a; P < 0.01), Gly II (Fig. 6b; P < 0.05, except for AG + NaHS), and MGR (Fig. 6c; P < 0.05, except for AG + NaHS) activities, as well as endogenous MG level (Fig. 6d; P < 0.05) under heat stress compared with the control. Similarly, under heat stress, treatment with PAG + MG and HT + MG also signally reduced Gly I (Fig. 6a; P < 0.01), Gly II (Fig. 6b; P < 0.05, except for HT + MG), and MGR (Fig. 6c; P < 0.05, except for HT + MG) activities, as well as endogenous MG content (Fig. 6d; P < 0.01) compared with the control. In addition, under heat stress conditions, compared with the control, NAC, AG, PAG, and HT obviously reduced Gly I (P < 0.01) and Gly II (P < 0.01) activities as well as MG content (P < 0.01 for NAC and AG, P < 0.05 for PAG and HT) in maize seedlings (Fig. 6).
Interplay between H2S and MG modulates osmolyte metabolism in maize seedlings under non-heat stress and heat stress conditions
To further answer the possible mechanisms of H2S and MG interplay-initiated the thermotolerance in maize seedlings, after chemical treatment and heat stress, the contents of osmolytes proline (Pro), trehalose (Tre), glycine betaine (GB), and total soluble sugar (TSS) were quantified. As expected, under non-heat stress conditions, in comparison to the control, treatment with NaHS and NaHS + MG all significantly accumulated Pro (P < 0.05), Tre (P < 0.05, except for NaHS + MG), and GB (P < 0.05), but no significant effect on TSS content in maize seedlings (Fig. 7a, b, c, d). In addition, MG treatment only increased the content of GB (P < 0.01) in maize seedlings under heat stress compared with the control, while the significant difference on Pro, Tre, and TSS was not noted (Fig. 7a, b, c, d). Also, under heat stress, NAC + NaHS signally reduced Pro (P < 0.05), Tre (P < 0.05), and GB (P < 0.05) contents in maize seedlings compared with the control, but no significant effect on TSS (Fig. 7a, b, c, d). Similarly, AG + NaHS also markedly declined the content of GB (P < 0.01) in maize seedlings compared with the control, while the significant effect on Pro, Tre, and TSS was not recorded (Fig. 7a, b, c, d). In addition to these, PAG + MG obviously increased the content of GB (P < 0.05) in maize seedlings compared with the control, decreased Tre level (P < 0.01), while no significant effect on Pro and TSS (Fig. 7a, b, c, d). Similarly, HT + MG signally decreased GB level (P < 0.01) in maize seedlings compared with the control, but the significant difference on Pro, Tre, and TSS was not obtained (Fig. 7a, b, c, d). Additionally, NAC and AG had no significant effect on Pro, Tre, GB, and TSS except significant decrease in GB (P < 0.01) by NAC (Fig. 7). Analogously, PAG and HT obviously reduced Pro, Tre, and GB in maize seedlings (P < 0.01), but significant difference of TSS was not found (Fig. 7).
Effect of irrigation of maize seedlings with methylglyoxal (MG), NaHS, N-acety cysteine (NAC) + NaHS, aminoguanidine (AG) + NaHS, DL-propargylglycine (PAG) + MG, and hypotaurine (HT) + MG, NAC, AG, PAG, and HT on the contents of proline (Pro, a), trehalose (Tre, b), glycine betaine (GB, c), and total soluble sugar MG (TSS, d) under non-heat stress and heat stress. The 2.5-day-old maize seedlings were watered with 100 mL of different chemicals for 6 h (referring to Fig. 1 and “Materials and methods”), and then subjected to heat stress at 46 °C for 16 h. After chemical treatment and heat stress, the contents of Pro, Tre, GB, and TSS were quantified. In figures, the data were means ± standard error (SE), and the asterisk and double asterisks separately denoted significant differences (P < 0.05) and very significant differences (P < 0.01) compared with the control
Under heat stress conditions, in comparison to the control, the treatment with MG and NaHS single or in combination all significantly elevated the contents of Pro (P < 0.01), Tre (P < 0.01), GB (P < 0.01), and TSS (P < 0.01) in maize seedlings (Fig. 7a, b, c, d). Similarly, NAC + NaHS also increased GB (P < 0.05) and TSS (P < 0.01) levels in maize seedlings under heat stress, decreased the contents of Pro (P < 0.05) and Tre (P < 0.01) (Fig. 7a, b, c, d). Also, AG + NaHS declined the level of Pro (P < 0.01) in maize seedlings under heat stress, but no significant effect on Tre, GB, and TSS (Fig. 7a, b, c, d). Furthermore, PAG + MG ascended the contents of Pro (P < 0.05) and TSS (P < 0.05) in maize seedlings under heat stress, descended the Tre level (P < 0.01), but the significant effect on GB was not gotten (Fig. 7a, b, c, d). Also, HT + MG also increased Pro (P < 0.01) and TSS (P < 0.05) contents in maize seedlings under heat stress, decreased the levels of Tre (P < 0.05) and GB (P < 0.01) compared with the control. In addition to these, under heat stress conditions, compared with the control, NAC and AG decreased the contents of Tre, BA, and TSS (P < 0.05), but no significant effect on Pro (Fig. 7). Similarly, PAG and HT also obviously reduced Pro (P < 0.01), Tre (P < 0.01), and GB (P < 0.05) contents in maize seedlings, but significant difference of TSS was not noted (Fig. 7).
Relationship between physiological parameters
The results from Fig. 1 showed that the survival rate (SR) of maize seedlings treated with H2S combined with MG reached the most significant difference (P ˂ 0.01) compared with other treatments. To further understand the relationship between thermotolerance (SR) and antioxidant, osmotic adjustment, and MG-/H2S-metabolic systems, these indices were evaluated by Pearson correlation analysis. The relationship, as shown in Tables 1, 2, and 3, SR was positively related with APX (P ˂ 0.01), POD (P ˂ 0.01), SOD (P ˂ 0.01), AsA (P ˂ 0.05), Pro (P ˂ 0.05), TSS (P ˂ 0.05), Gly II (P ˂ 0.05), and LCD (P ˂ 0.05). Similarly, APX was positively related with SOD (P ˂ 0.05), AsA (P ˂ 0.05), and CAR (P ˂ 0.05), negatively with DHA (P ˂ 0.05). In addition, POD, SOD, CAT, GR, DHAR, and GSH were separately positive correlation with SOD (P ˂ 0.05), FLA (P ˂ 0.05), GSH (P ˂ 0.05), and CAR (P ˂ 0.05), while FLA was negative correlation with DHA (P ˂ 0.05) (Table 1).
Similarly, Pro was positively related with TSS, Gly I, Gly II, LCD, and OAS-TL (P ˂ 0.05); GB was positively related with MG, MGR, and OAS-TL (P ˂ 0.05); Tre was positively correlated with MGR (P ˂ 0.05) and Gly II (P ˂ 0.01); TSS was positively related with MG (P ˂ 0.01), Gly I (P ˂ 0.01), LCD (P ˂ 0.05), and OAS-TL (P ˂ 0.01); MG was positively related with Gly I and OAS-TL (P ˂ 0.01); OAS-TL also was positively related with MGR (P ˂ 0.01), Gly I (P ˂ 0.01), and LCD (P ˂ 0.05); and H2S was positively correlated with LCD (P ˂ 0.05) (Tables 2 and 3).
Discussion
The acquirement of plant stress tolerance including thermotolerance is implicated in many messenger molecules (such as Ca2+, H2O2, NO, H2S, and MG) and their interplays. However, the interplays among messenger molecules are poorly known. In tobacco cells, germinating maize seeds, as well as maize, strawberry, wheat, and poplar seedlings, H2S pretreatment elevated the thermotolerance of seedlings (Li et al. 2012, 2013; Christou et al. 2014; Yang et al. 2016; Cheng et al. 2018). Similarly, MG-induced stress tolerance including thermotolerance was found in maize, wheat, and Brassica rapa seedlings (Bless et al. 2017; Li et al. 2018a, 2018b; Wang et al. 2019). These data preliminarily implied the interplay between H2S and MG in the formation of thermotolerance in plants. The current study forcefully supported this hypothesis; that is, both NaHS (H2S donor) and MG alone or in combination increased the survival of maize seedlings and tissue vigor (as indicated in increase in TTC reductivity) under heat stress and reduced the destruction of biological membrane integrity, fluidity, and compartmentalization (as shown in reduction in ion leakage) and over-accumulation of lipid peroxidation products (as illustrated in decrease in malondialdehyde) (Fig. 1a, b, c, d). In addition, the H2S- or MG-triggered positive effects were separately weakened by NaHS in combination with MG scavengers (NAC and AG), MG in combination with H2S inhibitor (PAG) and scavenger (HT), as well as NAC, AG, PAG, or HT alone in maize seedlings under non-heat stress and heat stress conditions (Fig. 1a, b, c, d). Interestingly, under non-heat stress conditions, compared with the control, treatment with NAC, AG alone, or in combination with NaHS as well as PAG, HT alone or in combination with MG could significantly reduce the tissue vigor, which was expressed in dehydrogenase activity (Ishikawa et al. 1995). This reduction might be MG scavengers (NAC and AG), H2S inhibitor (PAG), and scavenger (HT) that could inactivate dehydrogenase. This hypothesis needs to be identified in the future. The current results and previous data (Li et al. 2018a) further verified the interplay between H2S and MG in the generation of thermotolerance in plants.
A key strategy to acquire thermotolerance is ROS homeostasis in plant cells. ROS homeostasis is closely regulated by antioxidant system, which includes antioxidant enzymes and non-enzymatic antioxidants (Gill and Tuteja 2010; Sewelam et al. 2016). Antioxidant system is a group of ROS-scavenging enzymes (such as SOD, CAT, APX, and POD), ROS-scavengers (GSH, AsA, FLA, and CAR), and antioxidant-producing enzymes (GR, HAR, and MDHAR). ROS-scavenging enzymes and ROS-scavengers could directly scavenge ROS, while antioxidant-producing enzymes could convert oxidized antioxidants (such as DHA and GSSG) into reduced antioxidants (AsA and GSH) (Agati et al. 2012; Foyer 2018; Waszczak et al. 2018; Olson 2019; Arunkumar et al. 2020. In this work, treatment with MG and NaHS alone or in combination elevated the activities of ROS-scavenging enzymes (CAT, APX, and POD) (Fig. 2a, b, c) and antioxidant-producing enzymes (GR, MDHAR, and DHAR) (Fig. 4a, b, c), as well as the contents of scavengers (AsA, GSH, FLA, and CAR) (Fig. 3a, b, c) in maize seedlings, thus alleviating the production of superoxide radical (Fig. 4d) and oxidative damage (Fig. 1b) under non-heat stress and heat stress conditions. In addition, Pearson correlation analysis showed that the positive relationship between antioxidant system was observed in maize seedlings treated with H2S and MG under heat stress (Table 1). Under non-heat stress and heat stress conditions, H2S combined with MG scavengers (NAC and AG) or MG in combination with H2S inhibitor (PAG) and scavenger (HT) reduced the activities of CAT, APX, POD, GR, MDHAR, and DHAR (Figs. 2a, b, c and 4b, c), as well as the contents of DHA, GSSG, FLA, and CAR (Fig. 3b, d, e, f), but had no significant effect on SOD (Fig. 2d). Similarly, NAC, AG, PAG, and HT alone weakened the activities of CAT, POD, GR, MDHAR, and DHAR, as well as the contents of AsA, FLA, and CAR in maize seedlings under heat stress conditions (Figs. 2, 3, and 4). In addition, H2S can maintain ROS homeostasis by inactivating NADPH oxidase (which is a dominating source of ROS production) and CAT, as well as activating APX through a posttranscriptional modification (S-sulfhydration, also known as persulfidation) (Hancock and Whiteman 2014, 2016; Aroca et al. 2015, 2018; Li et al. 2016; Corpas 2019; Hancock 2019). Also, the excessive MG is able to induce the accumulation of ROS by inhibiting the activities of antioxidant enzymes (CAT, APX, POD, SOD, and GR) and/or induces ROS production (Kaur et al. 2016; Li 2016, 2019a; Mostofa et al. 2018). Based on these evidences, the steady state of ROS in plant cells can be achieved by maintaining the homeostasis of H2S and MG. Thus, the interplay between H2S and MG initiated the thermotolerance in maize seedlings by modulating ROS-metabolic pathways.
Generally, H2S also shows dual role of toxicity and signaling in plants (Hancock and Whiteman 2014, 2016; Li et al. 2016). Therefore, the homeostasis of H2S in plant cells plays a key role in plant growth, development, and stress tolerance including thermotolerance (Corpas 2019; Hancock 2019). In plants, the H2S homeostasis is closely regulated by LCD and OAS-TL (Li et al. 2016; Corpas 2019; Hancock 2019). In this work, under non-heat stress conditions, treatment with MG had no significant effect on the activity of LCD and OAS-TL as well as the endogenous H2S level in maize seedlings (Fig. 5a, b, c). Similarly, NaHS treatment also was not significantly different on LCD and OAS-TL activity, but increased the content of endogenous H2S in maize seedlings under non-heat stress (Fig. 5a, b, c). Therefore, the increase in the endogenous H2S was achieved by the entry of external H2S into the cells across plasma membrane, because H2S is a lipophilic signaling molecule (Hancock and Whiteman 2014, 2016; Li et al. 2016; Corpas 2019; Hancock 2019). Under heat stress conditions, compared with the control, treatment with MG activated LCD and OAS-TL (Fig. 5a, b), which in turn induced the accumulation of endogenous H2S in maize seedlings (Fig. 5c). The effects of MG were separately eliminated by PAG and HT (Fig. 5a, b, c), indicating that H2S exerted its signaling role in the downstream of MG. Similarly, NaHS treatment also activated LCD and OAS-TL (Fig. 5a, b) in maize seedlings under heat stress, followed by triggered H2S signaling (Fig. 5c), thus initiating the thermotolerance (Fig. 1a, b, c, d). In addition, under heat stress conditions, NaHS treatment increased the accumulation of endogenous MG, while this accumulation was separately removed by MG scavengers NAC and AG (Fig. 6d), suggesting that H2S might be a signaling molecule in the upstream of MG. Also, under both non-heat stress and heat stress conditions, treatment with NAC + NaHS, AG + NaHS, PAG + MG, and HT + MG inactivated the activity of LCD and OAS-TL, followed by inhibiting the overaccumulation of endogenous H2S in maize seedlings compared with the control (Fig. 5a, b, c). Similar results could be observed in maize seedlings treated with NAC, AG, PAG, and HT alone under both non-heat stress and heat stress conditions (Fig. 5a, b, c). In addition, Pearson correlation analysis also showed that H2S metabolism was positively related with that of MG in maize seedlings treated with H2S in combination with MG under heat stress, vice versa (Table 3). These data further supported the hypothesis that the interplay between H2S and MG initiated the thermotolerance in maize seedlings by regulating H2S metabolism.
Similar to oxidative stress induced by ROS, excessive MG also can trigger MG stress (inhibiting antioxidant enzyme activity and promoting the production of ROS) (Kaur et al. 2016; Li 2016, 2019b). Therefore, the homeostasis of MG in plant cells is another important strategy to adapt to high-temperature stress. During MG homeostasis, MG-scavenging system plays a key role (Li 2016, 2019a; Mostofa et al. 2018). MG-scavenging system is mainly composed of three members (Gly I, Gly II, and MGR), which maintains the homeostasis of MG in plant cells by the synergistic action with GSH (Li 2016, 2019b; Mostofa et al. 2018). In this study, under non-heat stress conditions, treatment with MG and NaHS inhibited the activity of Gly I (Fig. 6a), which in turn triggered the accumulation of MG (Fig. 6d), as signaling, followed by initiated the downstream signaling events (ROS, H2S, MG, and osmolyte metabolism) related to the themotolerance in maize seedlings. Under heat stress conditions, treatment with MG and NaHS activated Gly I, Gly II, and MGR (Fig. 6a, b, c), followed by alleviating the increase in the endogenous MG level to avoid MG stress (Fig. 6d) in maize seedlings, thus maintaining MG homeostasis. Adversely, under heat stress condition, NAC + NaHS, AG + NaHS, PAG + MG, and HT + MG inactivated Gly I, Gly II, and MGR, as well as inhibited the excessive accumulation of endogenous MG in maize seedlings compared with the control (Fig. 6a, b, c). Similar inhibiting effect was noted in maize seedlings treated with NAC, AG, PAG, and HT alone, especially treatment with MG scavengers NAC and AG (Fig. 6a, b, c). These further supported the fact that the interplay between H2S and MG initiated the thermotolerance in maize seedlings by modulating MG metabolism.
Due to their multifaceted functions, osmolytes such as Pro, Tre, GB, and TSS play a vital role in the development of thermotolerance in plants. These osmolytes not only act as osmotic adjustment substances to cope with osmotic stress induced by abiotic stress including heat stress, but also as ROS-scavengers, biomembrane and protein stabilizers, ion chelators, molecular chaperones, and signaling molecules (Ashraf and Foolad 2007; Rosas-Rodríguez and Valenzuela-Soto 2010; Szabados and Savouré 2010; Fedotova 2019; Khatibi et al. 2019). In this study, under non-heat stress conditions, NaHS treatment ascended the contents of endogenous Pro, Tre, and GB in maize seedlings in comparison to the control, while H2S inhibitor PAG and scavenger HT combined with MG remitted the increase in endogenous Pro, Tre, and GB to some extent (Fig. 7a, b, c). Similarly, MG treatment also increased the content of GB in maize seedlings under heat stress (Fig. 7c), but H2S in combination with MG scavengers NAC and AG reduced the accumulation of GB (Fig. 7c) and further coped with the increase in Pro, Tre, and TSS compared with the control (Fig. 7a, b, d). Under heat stress conditions, in comparison to the control, MG and NaHS alone or in combination increased the contents of Pro, Tre, GB, and TSS (Fig. 7a, b, c, c), but this increase was separately impaired by MG scavengers NAC and AG alone or in combination with NaHS, as well as H2S inhibitor PAG and scavenger HT alone or in combination with MG in maize seedlings, especially treatment with scavengers or inhibitor alone was more significant (Fig. 7a, b, c, d). Interestingly, Pearson correlation analysis indicated that the positive relationship between SR, antioxidant, osmotic adjustment, and H2S-/MG-metabolic systems was noted in maize seedlings treated with H2S combined with MG under heat stress (Tables 1, 2, and 3). These results further forcefully supported the speculation that the interplay between H2S and MG initiated the thermotolerance in maize seedlings by adjusting the osmolyte metabolism.
To summarize, the treatment with H2S and MG alone or in combination could initiate the thermotolerance in maize seedlings by increasing the survival and tissue vigor, as well as relieving the increase in superoxide radical production and biomembrane injury (as shown in MDA content and electrolyte leakage). Furthermore, H2S and MG alone or in combination further modulated ROS metabolism by regulating CAT, APX, POD, SOD, GR, MDHAR, DHAR, AsA, GSH, FLA, and CAR; osmolyte metabolism by accumulating Pro, Tre, GB, and TSS; H2S metabolism by activating LCD and OAS-TL; and MG metabolism by stimulating Gly I, Gly II, and MGR in maize seedlings under non-heat stress and heat stress conditions (Fig. 8). These positive physiological effects could be weakened by NAC, AG alone, or in combination with H2S, as well as PAG, HT alone, or in combination with MG. Therefore, the interplay between H2S and MG initiated the thermotolerance in maize seedlings by modulating ROS, osmolyte, H2S, and MG metabolism.
The underlying mechanisms of the interplay between hydrogen sulfide (H2S) and methylglyoxal initiated the thermotolerance in maize seedlings. Treatment with exogenous H2S donor (NaHS) and methylglyoxal (MG) alone or in combination modulated the reactive oxygen species (ROS) metabolism (catalase: CAT; ascorbate peroxidase: APX; guaiacol peroxidase: POD; superoxide dismutase: SOD; glutathione reductase: GR; monodehydroascorbate reductase: MDHAR; dehydroascorbate reductase: DHAR; ascorbic acid: AsA; glutathione: GSH; flavonoids: FLA; and carotenoids: CAR), H2S metabolism (L-cysteine desulfhydrase: LCD; O-acetyl serine thione lyase: OAS-TL), MG metabolism (glyoxalase I: Gly I; glyoxalase II: Gly II; and MG reductase: MGR), and osmolyte metabolism (proline: Pro; trehalose: Tre; glycine betaine: GB; and total soluble sugar: TSS) in maize seedlings under non-heat stress and heat stress, thus initiating the thermotolerance in maize seedlings. These positive effects could be weakened by MG scavengers (N-acety cysteine: NAC; aminoguanidine: AG) and H2S inhibitor DL-propargylglycine (PAG) and hypotaurine (HT). The blunted arrows (┬) denote inhibitory effects
References
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76
Ali S, Rizwan M, Arif MS, Ahmad R, Hasanuzzaman M, Ali B, Hussain A (2020) Approaches in enhancing thermotolerance in plants: an updated review. J Plant Growth Regul , In press 39:456–480
Aroca A, Serna A, Gotor C, Romero LC (2015) S-sulfhydration: a cysteine posttranslational modification in plant systems. Plant Physiol 168:334–342
Aroca A, Gotor C, Romero LC (2018) Hydrogen sulfide signaling in plants: emerging roles of protein persulfidation. Front Plant Sci 9:1369
Arunkumar A, Gorusupudi A, Bernstein PS (2020) The macular carotenoids: a biochemical overview. Biochim Biophys Acta-Mol Cell Biol Lipids. https://doi.org/10.1016/j.bbalip.2020.158617
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Bless Y, Ndlovu L, Gokul A, Keyster M (2017) Exogenous methylglyoxal alleviates zirconium toxicity in Brassica rapa L. seedling shoots. South Afr J Bot 109:327
Chen CN, Porubleva L, Shearer G, Svrakic M, Holden LG, Dover GL, Johnston M, Chitnis PR, Kohl FH (2003) Associating protein activities with their genes: rapid identification of a gene encoding a methylglyoxal reductase in the yeast Saccharomyces cerevisiae. Yeast 20:545–554
Cheng T, Shi J, Dong Y, Ma Y, Peng Y, Hu X, Chen J (2018) Hydrogen sulfide enhances poplar tolerance to high-temperature stress by increasing S-nitrosoglutathione reductase (GSNOR) activity and reducing reactive oxygen/nitrogen damage. Plant Growth Regul 84:11–23
Christou A, Filippou P, Manganaris GA, Fotopoulos V (2014) Sodium hydrosulfide induces systemic thermotolerance to strawberry plants through transcriptional regulation of heat shock proteins and aquaporin. BMC Plant Biol 14:42
Corpas FJ (2019) Hydrogen sulfide: a new warrior against abiotic stress. Trends Plant Sci 24:983–988
Fedotova MV (2019) Compatible osmolytes - bioprotectants: is there a common link between their hydration and their protective action under abiotic stresses? J Mol Liq 292:111339
Foyer CH (2018) Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Exp Bot 154:134–142
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Hancock JH (2019) Hydrogen sulfide and environmental stresses. Environ Exp Bot 161:50–56
Hancock JT, Whiteman M (2014) Hydrogen sulfide and cell signaling: team player or referee? Plant Physiol Biochem 78:37–42
Hancock JT, Whiteman M (2016) Hydrogen sulfide signaling: interactions with nitric oxide and reactive oxygen species. Ann N Y Acad Sci 1365:5–14
Ishikawa M, Robertson AJ, Gusta LV (1995) Comparison of viability tests for assessing cross-adaptation to freezing, heat and salt stresses induced by abscisic acid in bromegrass (Bromus inermis Leyss) suspension cultured cells. Plant Sci 107:83–93
Kaur C, Sharma S, Singla-Pareek SL, Sopory SK (2016) Methylglyoxal detoxification in plants: role of glyoxalase pathway. Indian J Plant Physiol 21:377–390
Khatibi SMH, Vahed FZ, Sharifi S, Ardalan M, Shoja MM, Vahed SZ (2019) Osmolytes resist against harsh osmolarity: something old something new. Biochimi 159:156–164
Lawas LMF, Zuther E, Jagadish SVK, KHincha D (2018) Molecular mechanisms of combined heat and drought stress resilience in cereals. Curr Opin Plant Biol 45:212–217
Leipner J, Stamp P (2009) Chilling stress in maize seedlings. In: Bennetzen JL, Hake SC (eds) Handbook of maize: its biology. Springer, Berlin, pp 291–344
Li ZG (2015) Analysis of some enzymes activities of hydrogen sulfide metabolism in plants. Methods Enzymol 555:253–269
Li ZG (2016) Methylglyoxal and glyoxalase system in plants: old players, new concepts. Bot Rev 82:183–203
Li ZG (2019a) Methylglyoxal: a novel signaling molecule in plant responses to abiotic stresses. In: Khan MR, Reddy PS, Ferrante A, Khan NA (eds) Plant signaling molecules: role and regulation under stressful environments. Elsevier, Cambridge, pp 219–233
Li ZG (2019b) Measurement of signaling molecules calcium ion, reactive sulfur species, reactive carbonyl species, reactive nitrogen species, and reactive oxygen species in plants. In: Khan MR, Reddy PS, Ferrante A, Khan NA (eds) Plant signaling molecules: role and regulation under stressful environments. Elsevier, Cambridge, pp 83–103
Li ZG, Gong M, Xie H, Yang L, Li J (2012) Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci 185(186):185–189
Li ZG, Yang SZ, Long WB, Yang GX, Shen ZZ (2013) Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ 36:1564–1572
Li ZG, Yi XY, Li YT (2014) Effect of pretreatment with hydrogen sulfide donor sodium hydrosulfide on heat tolerance in relation to antioxidant system in maize (Zea mays) seedlings. Biologia 69:1001–1009
Li ZG, Min X, Zhou ZH (2016) Hydrogen sulfide: a signal molecule in plant cross-adaptation. Front Plant Sci 7:1621
Li ZG, Long WB, Yang SZ, Wang YC, Tang JH (2018a) Signaling molecule methylglyoxal-induced thermotolerance is partly mediated by hydrogen sulfide in maize (Zea mays L.) seedlings. Acta Physiol Plant 40:76
Li ZG, Nie Q, Yang CL, Wang Y, Zhou ZH (2018b) Signaling molecule methylglyoxal ameliorates cadmium injury in wheat (Triticum aestivum L) by a coordinated induction of glutathione pool and glyoxalase system. Ecotoxicol Environ Saf 149:101–107
Li ZG, Xu Y, Bai LK, Zhang SY, Wang Y (2019) Melatonin enhances thermotolerance of maize seedlings (Zea mays L.) by modulating antioxidant defense, methylglyoxal detoxification, and osmoregulation systems. Protoplasma 256:471–490
Mostofa MG, Ghosh A, Li ZG, Siddiqui MN, Fujitad M, Trane LSP (2018) Methylglyoxal–a signaling molecule in plant abiotic stress responses. Free Rad Biol Med 122:96–109
Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K (2017) Transcriptional regulatory network of plant heat stress response. Trends Plant Sci 22:53–65
Olson KR (2019) Hydrogen sulfide, reactive sulfur species and coping with reactive oxygen species. Free Rad Biol Med 140:74–83
Rosas-Rodríguez JA, Valenzuela-Soto EM (2010) Enzymes involved in osmolyte synthesis: how does oxidative stress affect osmoregulation in renal cells? Life Sci 87:515–520
Sewelam N, Kazan K, Schenk PM (2016) Global plant stress signaling: reactive oxygen species at the cross-road. Front Plant Sci 7:187
Strable J, Scanlon M (2009) Maize (Zea mays): a model organism for basic and applied research in plant biology. Cold Spring Harb. Protoc. 10 pdb.emo132
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang Y, Ye XY, Qiu XM, Li ZG (2019) Methylglyoxal triggers the heat tolerance in maize seedlings by driving AsA-GSH cycle and reactive oxygen species-/methylglyoxal-scavenging system. Plant Physiol Biochem 138:91–99
Waszczak C, Carmody M, Kangasjarvi J (2018) Reactive oxygen species in plant signaling. Ann Rev Plant Biol 69:209–236
Yang M, Qin B, Ma X, Wang P, Li M, Chen L, Chen L, Sun A, Wang Z, Yin Y (2016) Foliar application of sodium hydrosulfide (NaHS), a hydrogen sulfide (H2S) donor, can protect seedlings against heat stress in wheat (Triticum aestivum L.). J Integr Agric 15:2745–2758
Funding
This work is funded by National Natural Science Foundation of China (31760069, 31360057).
Author information
Authors and Affiliations
Contributions
ZGL conceived and designed experiments and wrote manuscript, XYY performed experiments and wrote manuscript, XMQ and YYS assisted the whole experiment process. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Néstor Carrillo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ye, XY., Qiu, XM., Sun, YY. et al. Interplay between hydrogen sulfide and methylglyoxal initiates thermotolerance in maize seedlings by modulating reactive oxidative species and osmolyte metabolism. Protoplasma 257, 1415–1432 (2020). https://doi.org/10.1007/s00709-020-01516-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01516-x