Abstract
Purpose
Prostate-specific membrane antigen (PSMA) PET/CT has an established reliable diagnostic performance for detecting metastases in prostate cancer. However, there are increasing instances of scans demonstrating equivocal bone lesions, with non-specific uptake and without a definite benign or malignant CT correlate. To date, the prevalence, malignancy rate, and relationship with radioligand type ([18F] PSMA-1007 vs. others ([68Ga]Ga-PSMA-11 and [18F] DCFPyL) for these equivocal lesions have not been extensively established.
Methods
A systematic review and meta-analysis was conducted on equivocal bone lesions. Pubmed and EMBASE were searched up to December 11, 2023. Quality of the studies was evaluated using QUADAS-2. The following proportions were pooled using random-effects model: (1) prevalence of equivocal bone lesions (i.e., number of patients with one or more equivocal bone lesions/number of patients with PSMA PET/CT) and (2) their malignancy rates (i.e., number of metastases/number of equivocal bone lesions). Subgroup analyses based on radioligand type, clinical setting, and definition of equivocal bone lesion were performed.
Results
Twenty-five studies (4484 patients) were included. Pooled prevalence of equivocal bone lesions was 20% (95%CI, 12–31%). [18F]PSMA-1007 was associated with a greater prevalence of equivocal lesions compared with other radioligands: 36% (95%CI 26–48%) vs. 8% (95%CI, 4–14%), respectively, p < 0.01. Pooled malignancy rate of equivocal bone lesions was 14% (95%CI, 7–25%). [18F]PSMA-1007 was associated with a lower malignancy rate compared to other radioligands: 8% (95%CI, 3–19%) vs. 29% (95%CI, 17–44%), respectively, p = 0.01. There were no signficant difference in prevalence or malignancy rate between subgroups stratified to clinical setting or definition of equivocal bone lesions (p = 0.32–0.60).
Conclusions
Equivocal bone lesions are often encountered on PSMA PET/CT but exihibit a low malignancy rate. Compared to other radioligands, [18F]PSMA-1007 requires special attention as it is associated with a higher frequency and lower rate of metastasis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) has revolutionized the way prostate cancer is diagnosed and managed. The high level of PSMA expression in prostate cancer cells allows PSMA PET/CT to outperform conventional imaging in detecting not only recurrent and metastatic disease in the setting of biochemical failure, but also for initial staging of newly diagnosed intermediate- and high-risk prostate cancer [1]. Over the past decade, multiple clinical trials have demonstrated high accuracy of PET/CT using various PSMA-targeted radioligands and studies have shown that PSMA PET/CT findings impact the clinical management in approximately half of prostate cancer patients evaluated with such studies [2,3,4,5,6,7]. As a result, there has been widespread adoption of PSMA PET/CT across the globe along with integration into clinical practice guidelines [8, 9].
Despite its initial success, there is increasing awareness of several pitfalls of PSMA PET/CT, one of which is the presence of equivocal bone lesions with PSMA uptake. These often lead to further workup, and it has been suggested that many are ultimately benign (or represent a “false positive” finding for metastasis) [10]. There is also concern that certain PSMA-targeted radioligands such as [18F]F-PSMA-1007 – which was initially considered advantageous for better assessing the prostate (or prostatectomy bed) due to its predominant excretion through the liver, are associated with a higher frequency of indeterminate bone lesions due to “unspecific bone uptake” [11]. Ambiguity of the significance of these equivocal lesions interpreted as being malignant may in some cases exclude such patients from receiving curative-intent local definitive therapy (e.g., prostatectomy or radiation treatment). As a means to address this issue, standardized reporting schemes such as PSMA Reporting and Data System (PSMA-RADS), Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE), and European Association of Nuclear Medicine PSMA (E-PSMA) standardized reporting guidelines have been proposed to improve the interpretation and communication of findings on PSMA PET [12,13,14,15]. In PSMA-RADS, a bone lesion is assigned a score of “3B” if it demonstrates equivocal uptake on PET along with CT appearance which is not definitive but also not atypical for malignancy [12]. These lesions are recommended to undergo additional imaging, biopsy, or follow-up to determine their clinical significance. Nevertheless, the clinical significance of equivocal bone lesions on PSMA PET/CT and how to best use this information to manage the patient’s clinical treatment is still unclear.
Therefore, we conducted a systematic review and meta-analysis to evaluate (1) the prevalence of equivocal bone lesions on PSMA PET/CT, (2) the malignancy rate of equivocal bone lesions, and (3) the relationship between type of radioligand and equivocal bone lesions.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [16]. A study protocol was registered a priori to the Prospective Register of Systematic reviews (no. CRD42023486697) [17].
Search strategy
PubMed and EMBASE databases were searched up to December 11, 2023 to identify studies investigating equivocal bone lesions on PSMA PET/CT. The search query constructed based on key words (“prostate”, “PSMA”, “PET”, “equivocal”, and “bone”) and their synonyms was as follows: Prostate AND ((PSMA OR prostate-specific membrane antigen) AND (“positron emission tomography” OR PET)) AND (“PSMA-RADS” OR ((unspecific OR nonspecific OR equivocal OR indeterminate) AND (bone OR skeletal OR osseous))). The study selection process was performed by two reviewers (S.W. and H.A.V.) in consensus.
Inclusion and exclusion criteria
Studies were included if they were relevant to the Patient, Intervention, Comparator, Outcome framework [18]: (1) “patients” with prostate cancer (regardless of clinical setting), (2) PSMA PET/CT as “intervention” (regardless of type of PSMA-targeted radioligand) and interpretation with or without using PSMA-RADS, (3) No “comparator”, and (4) proportion of patients with equivocal bone lesions and their malignancy rates as “outcome”. We excluded studies if they met any of the following criteria: (1) publication types other than original articles or conference abstracts (e.g., review articles, editorials, and case reports), (2) cohort of < 10 patients, (3) insufficient information in the study to extract proportion relevant to the research question (i.e., prevalence of equivocal bone lesions and their malignancy rates), and (4) overlapping cohorts. When two or more studies were based on overlapping (or identical) cohorts, the one with more comprehensive and/or updated data was selected.
Data extraction and quality assessment
The following characteristics of the studies were tabulated using a standardized form: (1) study – author name(s), publication year, patient enrollment period, institution, country, design (prospective vs. retrospective); (2) clinical – age, clinical setting (e.g., primary staging, biochemical recurrence), Gleason score, prostate-specific antigen (PSA) level, reference standard for determining if equivocal bone lesions were malignant or not; and (3) PET – vendor, model, type of radioligand, injected dose, uptake time, acquisition time, definition of equivocal bone lesion (e.g., based on PSMA-RADS vs. other in-house criteria), number of patients with equivocal bone lesions, number of equivocal bone lesions, and number of equivocal bone lesions that were deemed malignant.
Assessments of the quality of the studies and their risk of bias were done based on Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) [19]. All 4 domains (patient selection, index test, reference standard, and flow and timing) were assessed for studies evaluating the malignancy rates. However, only two domains (patient selection and index test) were assessed for studies that evaluated the prevalence of equivocal bone lesions. As evaluating the prevalence of equivocal bone lesions does not directly involve correlating them to a “reference standard”, the other two domains (reference standard and flow and timing) were not relevant for assessing quality and bias specifically for these studies. Both the data extraction and quality assessment were first done independently by two authors (S.W. and D.F.) and discrepancies were resolved by consensus with a third author (H.A.V.).
Data synthesis and analysis
The primary outcomes of this meta-analysis were: (1) the prevalence of equivocal bone lesions defined as the proportion of patients that had one or more equivocal bone lesions among all patients that underwent PSMA PET/CT and (2) the malignancy rate of equivocal bone lesions defined as the proportion of lesions that were deemed to be metastasis based on the reference standard among all evaluated equivocal bone lesions. The secondary outcomes were to explore heterogeneity by performing subgroup analyses, most importantly stratified to the type of PSMA-targeted radioligand ([18 F]PSMA-1007 vs. others [[68Ga]Ga-PSMA-11 or [18 F]DCFPyL], [18 F]PSMA-1007 vs. [68Ga]Ga-PSMA-11, and [18 F]PSMA-1007 vs. [18 F]DCFPyL]), but also by additionally relevant subgroups such as clinical setting (e.g., primary staging, biochemical recurrence, etc.) or definition of equivocal bone lesions (e.g., based on PSMA-RADS).
Pooling of the proportions and calculation of their 95% confidence intervals (CI) were done using a random-effects model with the “meta” package in statistical software R (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria) [20]. Heterogeneity was assessed with the Higgins I2 test [21]. Heterogeneity was explored by comparing pooled proportions of the subgroups [22]. Publication bias was assessed with the Egger test and visualized using the funnel plot [23]. P values < 0.05 were considered statistically significant.
Results
Literature search
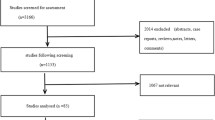
Three-hundred and twenty-two articles were initially identified from the systematic database search. After removal of 96 duplicates and exclusion of 168 papers by screening of titles and abstracts, 44 articles were considered potentially eligible. Upon full-text review, 19 of these studies were excluded for the following reasons (Supplementary Table 1): study does not deal with equivocal bone lesions (n = 3); equivocal bone lesions and equivocal soft tissue lesions were not separately documented (n = 6); overlapping patient cohort (n = 8); and topic deals with equivocal bone lesions on PSMA PET/CT but details of neither their prevalence nor malignancy rates were provided (n = 2). Ultimately, 25 studies (4484 patients) were included in the systematic review and meta-analysis (24–48). Flowchart for study selection is shown in Fig. 1.
Characteristics of included studies
The characteristics of the included studies are summarized in Tables 1, 2 and 3. Among all 25 studies, 8 (32%) evaluated the prevalence of equivocal bone lesions only [28, 30,31,32, 35, 40, 42, 48], 3 (12%) the malignancy rate of equivocal bone lesions only [34, 45, 47], and the remaining 14 (56%) both the prevalence and the malignancy rates of equivocal bone lesions [24,25,26,27, 29, 33, 36,37,38,39, 41, 43, 44, 46]. Most studies were retrospective in design (n = 22, 88%) [24,25,26, 28,29,30,31,32,33,34,35, 37,38,39, 41,42,43,44,45,46,47,48] and performed at single centers (n = 23, 92%) [24,25,26,27,28, 30,31,32,33, 35,36,37,38,39,40,41,42,43,44,45,46,47,48]. PSMA PET was performed for primary staging in 6 studies (24%) [27, 33, 35, 37, 43, 48], biochemical recurrence in all or most of the patients in 6 (24%) [25, 26, 30, 34, 41, 45], and a mixed cohort in 9 (36%) [24, 28, 29, 32, 38,39,40, 46, 47]. In the remaining, one study (4%) specifically included patients with non-detectable PSA levels after RP and the remaining 3 (12%) did not report cohort characteristics [36]. [18F]PSMA-1007 was used in 13 studies (52%) [24,25,26,27,28,29,30, 32, 35,36,37, 44, 45], [68Ga]Ga-PSMA-11 in 6 (24%) [31, 34, 39, 42, 43, 48], [18F]DCFPyL in 3 (12%) [35, 38, 47], and a combination of [18F]PSMA-1007 and non-[18F]PSMA-1007 radioligands in 3 (12%) [33, 41, 46]. In 9 studies (36%), PSMA PET/CT was interpreted and equivocal bone lesions were accordingly defined per PSMA-RADS version 1.0 [24, 26, 33,34,35, 38, 43, 46, 47]. In the 16 remaining studies, various definitions were used (or details were not provided), most common being PSMA uptake without a morphologic correlate on CT (n = 5, 20%) [27, 32, 36, 37, 41]. Most of the studies that evaluated the malignancy rate of equivocal bone lesions (13/17 [76.5%]) used the best value comparator (BVC; a combination of imaging, clinical, histopathologic, or biochemical evaluations such as response to therapy) as the reference standard for determining whether the equivocal bone lesion was malignant or not [24, 25, 27, 29, 33, 34, 37,38,39, 41, 43, 46, 47]. The other studies used the following reference standards: biopsy (n = 1, 5.9%) [45], magnetic resonance imaging (MRI) (n = 2, 11.8%) [26, 44], and PSA kinetics (n = 1, 5.9%) [36].
Quality and risk of bias
The overall quality of the included studies was considered moderate to good, with 13 (76.5%) of the 17 studies evaluating malignancy rate of equivocal bone lesions satisfying 4 or more of the 7 QUADAS-2 domains and 5 (62.5%) of the 8 studies only evaluating the prevalence of equivocal bone lesions satisfying 3 or more of the 4 evaluated QUADAS-2 domains. The detailed breakdown of QUADAS-2 assessments are provided in Fig. 2.
Grouped bar charts summarizing risk of bias (left) and concern for applicability (right) in 25 included studies according to the QUADAS-2 tool. Individual study results are shown at the bottom. For 8 studies that only reported the prevalence of equivocal bone lesions on PSMA PET/CT (but not their malignancy rates), a modified approach using only 2 of the 4 domains (patient selection and index test) were assessed as the other 2 domains (reference standard and flow and timing) were not relevant
For patient selection, 2 studies were considered at high risk for bias and had high concern for applicability: in one study, PSMA PET/CT was performed in patients that had undetectable PSA after prostatectomy [36] and another study only evaluated patients who were referred for PSMA PET-guided biopsies [45]. Another study had an unclear risk of bias as it was not explicitly mentioned whether patient enrollment was consecutive or not [37].
Regarding the index test, in the aforementioned study where patients had undetectable PSA after prostatectomy, readers were not blinded and were aware of this unique clinical setting, resulting in a high risk of bias [36]. In another study, PSMA PET was interpreted alone – specifically without correlation with CT – which not only negatively influences the diagnostic accuracy of PSMA PET (high risk of bias), but also is not applicable to current day practice where they are interpreted simultaneously (high concern for applicability) [39]. Most other studies (n = 12) were at unclear risk of bias as it was not explicitly stated whether PSMA PET/CT was interpreted blinded to the outcome.
In the reference standard domain, only one study had low risk of bias, as it exclusively was based on histopathological assessment of PSMA-PET guided biopsy [45]. However, this was considered to have high concern for applicability, as it would not be feasible to biopsy all equivocal bone lesions in daily practice. Three studies had high risk of bias as they were based on only MRI correlation (n = 2) [26, 44] or only PSA kinetics (n = 1) [36]. All 13 other studies were considered at unclear risk as they were based on the BVC.
Finally, in the flow and timing domain, those 13 studies (at unclear risk in the reference standard domain) were considered to be at high risk of bias since individual patients in the study had different reference standard by definition (i.e., BVC). Two additional studies were at unclear risk of bias as the interval between PSMA PET/CT and the reference standard was not explicit [26, 44].
Prevalence of equivocal bone lesions
Twenty-two studies reported the prevalence of equivocal bone lesions on PSMA PET/CT. The prevalence in each study ranged from 0 to 78% and the pooled prevalence across all 22 studies was 20% (95% CI: 12–31%) (Fig. 3). Substantial heterogeneity was present (I2 = 95.8%). There was no significant publication bias according to the Egger’s test (p = 0.21) and funnel plot (Supplementary Fig. 1A). At subgroup analyses, type of PSMA-targeted radioligand was associated with heterogeneity (p < 0.01), but not the clinical setting (p = 0.32) or how equivocal bone lesions were defined (p = 0.42) (Table 4). Specifically, studies that used [18F]PSMA-1007 (n = 14) were associated with a greater prevalence compared to those that used other radioligands ([68Ga]Ga-PSMA-11 and [18F]DCFPyL, n = 10): 36% (95% CI 26–48%) vs. 8% (95% CI 4–14%), respectively (Fig. 4). Subgroup analysis comparing [18F]PSMA-1007 with each of the radioligands are shown in Supplementary Fig. 2.
Forest plots showing pooled prevalence of equivocal bone lesions stratified to subgroups based on type of PSMA-targeted radioligand. Studies that used [18F]PSMA-1007 (n = 14) had a greater pooled prevalence compared to those that used other radioligands ([68Ga]Ga-PSMA-11 and [18F]DCFPyL, n = 10, p < 0.01)
Malignancy rate of equivocal bone lesions
Seventeen studies reported the malignancy rate of equivocal bone lesions on PSMA PET/CT. The prevalence in each study ranged from 0 to 77% and the pooled prevalence across all 17 studies was 14% (95% CI: 7–25%) (Fig. 5). Substantial heterogeneity was present (I2 = 90.1%). There was no significant publication bias according to the Egger’s test (p = 0.52) and funnel plot (Supplementary Fig. 1B). At subgroup analyses, type of PSMA-targeted radioligand was associated with heterogeneity (p = 0.01), but not the clinical setting (p = 0.32) or how equivocal bone lesions were defined (p = 0.60) (Table 4). Specifically, studies that used [18F]PSMA-1007 (n = 12) were associated with a lower malignancy rate compared to those that used other radioligands ([68Ga]Ga-PSMA-11 and [18F]DCFPyL, n = 8): 8% (95% CI 3–19%) vs. 29% (95% CI 17–44%), respectively (Fig. 6). Subgroup analysis comparing [18F]PSMA-1007 with each of the radioligands are shown in Supplementary Fig. 3.
Forest plots showing pooled malignancy rate of equivocal bone lesions stratified to subgroups based on type of PSMA-targeted radioligand. Studies that used [18F]PSMA-1007 (n = 12) had a lower pooled malignancy rate compared to those that used other radioligands ([68Ga]Ga-PSMA-11 and [18F]DCFPyL, n = 8, p = 0.01)
Discussion
In this systematic review and meta-analysis, we investigated the prevalence of equivocal bone lesions on PSMA PET/CT in patients with prostate cancer and how often they are malignant. Overall, equivocal bone lesions were often encountered, approximately in 1 of 5 patients (pooled prevalence = 20%, 95% CI: 12–31%). However, the rate of malignancy was low with approximately 1 in 7 equivocal bone lesions deemed to be metastatic disease (pooled malignancy rate = 14%, 95% CI: 7–25%). The results of our study substantiate on a larger scale, the trends we have been observing in smaller individual reports. Although PSMA PET/CT has revolutionized how we manage patients with prostate cancer, our study highlights that we need to keep in mind this important pitfall of equivocal bone lesions. There needs to be increased awareness to all stakeholders involved, including expert and community nuclear medicine physicians and radiologists who interpret PSMA PET/CT, referring physicians, and patients and caregivers in order to further optimize clinical recommendation based on the most accurate or reliable information which is clinically available [49].
Type of radioligand was a significant factor of heterogeneity between the included studies. Specifically, the prevalence of equivocal bone lesions was higher and their malignancy rate was lower in studies using [18F]PSMA-1007 (pooled estimate = 36%, 95% CI 26–48%) and 8%, 95% CI 3–19%, respectively) compared with other radioligands (pooled estimate = 8%, 95% CI 4–14% and 29%, 95% CI 17–44%, respectively). The reason for this difference between type of PSMA-targeted radioligand is not yet clearly understood. It has been suggested that the lower positron energy (and greater spatial resolution) and the longer half-life (and superior signal-to-background ratio) of [18F] compared to [68Ga] may partly explain this [29]; however, [18F]DCFPyL also is synthesized with [18F] and therefore this explanation cannot be considered valid. Others have suggested that it may be related to the higher binding affinity to receptors and internalization rate of [18F]PSMA-1007 [50, 51]. Regardless, the difference between [18F]PSMA-1007 and other radioligands has important clinical implications. [18F]PSMA-1007 has been considered advantageous for delineating the primary tumors and local recurrence after radical treatment and for radiotherapy planning (e.g., dose painting) due to its lack of urinary excretion compared with other radioligands [52,53,54]. Therefore, these characteristics related to radioligand type should be taken into consideration when choosing which one to use, and how to interpret them (in addition to jurisdictional constraints and access issues such as [18F]PSMA-1007 not yet approved in the United States and whether there is a cyclotron on site). Moreover, additional training may be needed before using [18F]PSMA-1007 to avoid overcalling these equivocal bone lesions.
Other factors such as clinical setting or how equivocal bone lesions were defined were not a substantial source of heterogeneity (p = 0.32–0.60). As bone metastases can virtually occur in any clinical setting, across the broad categories of primary staging, biochemical recurrence, and even during systemic treatment for known metastatic disease, the risk stratification itself (e.g., PSA level, PSA kinetics, or Gleason grade group) may be more important in determining whether the equivocal bone lesion is metastatic or not. For example, while the overall pooled malignancy rate was 14% (95% CI: 7–25%), few of the studies at the extreme ends warrant mention to illustrate this point. For example, two studies in which PSMA PET/CT was performed in predominantly high-risk patients – Paone et al. [37] (high risk in 58.8% [47/80], median PSA 23.7 ng/ml) and Simsek et al. [43] (high risk in 81.2% [289/356], median PSA 16.4 ng/mL) had very high malignancy rates of 76.9% (70/91) and 70.0% (7/10), respectively, substantially higher than the overall pooled malignancy rate of 14%. These data support tailoring the workup of equivocal bone lesions on PSMA PET/CT depending on the pretest likelihood of bone metastasis. They can be considered metastasis and managed accordingly in patients at high risk for metastasis (or with co-existing additional sites of definite metastasis elsewhere); on the contrary, in a patient at low risk for metastasis (especially who is a candidate for locoregional definitive treatment), higher thresholds should be applied for calling equivocal bone lesions as metastases so that the patient will not be denied potentially curative intent treatment. If risk is truly indeterminate, additional imaging, biopsy, or follow-up to confirm their significance may still be required. Further investigation is needed to see whether quantitative analysis of PSMA PET (e.g., maximum standardized uptake value [SUVmax] such as cutoff of > 7.2 suggested by Arnfield et al. [24]), secondary PSMA uptake patterns (e.g., focality and changes in late-phase acquisition [55]), more in-depth morphological analysis of CT correlates, or using additional tools such as genomic classifiers (e.g., Decipher test) will help identify metastasis or not in such scenarios [56, 57].
Although the definition of equivocal bone lesions (or how PSMA PET/CT was interpreted) was not a substantial factor of heterogeneity, this issue deserves more in-depth discussion. Nine (36.0%) of the studies used PSMA-RADS version 1.0, while various other definitions were used (or details not provided) in the remaining studies. No studies up to now have evaluated the recently updated PSMA-RADS version 2.0 [13]; however, only minimal impact on the prevalence and malignancy rate of equivocal bone lesions is to be expected considering the content of the modifications. It is unclear whether the conduct of using PSMA-RADS (or any other standardized reporting system) will in itself improve assessment of bone lesion. However, lessons can be learned from the history of the debate between Prostate Imaging Reporting and Data System (PI-RADS) versus Likert scale for interpreting MRI. While diagnostic performance was not significantly different (at least in high-volume tertiary centers), PI-RADS eventually became the standard for prostate MRI interpretation because it provided clear structure, standardization, and definition allowing the potential for decreasing inter-reader variability [58, 59]. PSMA-RADS could be used as a platform for research or utilized as part of clinical trials across different institutions and settings to help establish the clinical significance and most optimal way to manage equivocal bone lesions on PSMA PET/CT. Such structured reporting may also improve inter-reader agreement for PSMA PET/CT interpretation [60].
There were a few limitations in this systematic review and meta-analysis. First, there was substantial heterogeneity between the included studies. Nevertheless, we were able to demonstrate that at least some of this heterogeneity arises from the differences in type of PSMA-targeted radioligand ([18F]PSMA-1007 vs. others [[68Ga]Ga-PSMA-11 or [18F]DCFPyL]). Secondly, most (13/17 [76.5%]) of the included studies used BVC as the reference standard for determining whether the equivocal bone lesion was malignant or not. Although we acknowledged that this is a potential source for unclear risk of bias in the QUADAS-2 assessments, it should be emphasized that BVC is not perfect. As a matter of fact, using BVC could lead to both false positive and negative conclusions. Nonetheless, biopsy (used as the sole reference standard in 1 study [45]) cannot be used for all patients nor would that be ethically justified. Furthermore, interpretation of bone biopsies can be challenging especially when lesions are sclerotic, which is often the case in the setting of patients with prostate cancer, with approximately 1 in 4 biopsies yielding nondiagnostic results [61]. BVC is commonly used in clinical practice to decide whether equivocal bone lesions are metastasis or not. Therefore, the pooled estimates derived from this meta-analysis probably reflect real world evidence. Finally, we were unable to directly analyze the relationship between the location of the equivocal bone lesion and malignancy rate. Yet, it is well known from extensive literature that the likelihood of metastasis is different between locations – not only between axial and non-axial bones but even within axial bones (e.g., ribs vs. vertebrae or pelvic bones) [62, 63]. Although further investigation will be needed in this specific context, we can speculate that equivocal bone lesions on PSMA PET/CT will less likely be metastasis in the ribs, especially when in isolation without additional sites of disease in the pelvis or vertebrae.
In conclusion, equivocal bone lesions are often encountered on PSMA PET/CT but exhibit a low malignancy rate. Compared to other radioligands, [18F]PSMA-1007 requires special attention as it is associated with a higher frequency and lower rate of metastasis.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- BVC:
-
best value comparator
- MRI:
-
magnetic resonance imaging
- PET/CT:
-
positron emission tomography/computed tomography
- PSA:
-
prostate-specific antigen
- PSMA:
-
Prostate-specific membrane antigen
- PSMA-RADS:
-
PSMA Reporting and Data System
- QUADAS-2:
-
Quality Assessment of Diagnostic Accuracy Studies-2
References
Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP (1998) Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer 82(11):2256–2261
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P et al (2020) Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 395(10231):1208–1216
Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY et al (2019) Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate Cancer: a prospective single-arm clinical trial. JAMA Oncol 5(6):856–863
Hope TA, Eiber M, Armstrong WR, Juarez R, Murthy V, Lawhn-Heath C et al (2021) Diagnostic accuracy of 68Ga-PSMA-11 PET for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: a multicenter prospective phase 3 imaging trial. JAMA Oncol 7(11):1635–1642
Pienta KJ, Gorin MA, Rowe SP, Carroll PR, Pouliot F, Probst S et al (2021) A phase 2/3 prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in prostate Cancer patients (OSPREY). J Urol 206(1):52–61
Surasi DS, Eiber M, Maurer T, Preston MA, Helfand BT, Josephson D et al (2023) Diagnostic performance and safety of Positron Emission Tomography with 18F-rhPSMA-7.3 in patients with newly diagnosed unfavourable Intermediate- to very-high-risk prostate Cancer: results from a phase 3, prospective, Multicentre Study (LIGHTHOUSE). Eur Urol 84(4):361–370
Han S, Woo S, Kim YJ, Suh CH (2018) Impact of 68Ga-PSMA PET on the management of patients with prostate Cancer: a systematic review and Meta-analysis. Eur Urol 74(2):179–190
Mottet N, Conford P, van den Bergh RCN, Briers E, Eberli D, De Meerleer G et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer [Internet]. [cited 2023 Nov 22]. http://uroweb.org/guidelines/compilations-of-all-guidelines/
Lowrance W, Dreicer R, Jarrard DF, Scarpato KR, Kim SK, Kirkby E et al (2023) Updates to advanced prostate Cancer: AUA/SUO Guideline (2023). J Urol 209(6):1082–1090
de Galiza Barbosa F, Queiroz MA, Nunes RF, Costa LB, Zaniboni EC, Marin JFG et al (2020) Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging 20(1):23
Rauscher I, Krönke M, König M, Gafita A, Maurer T, Horn T et al (2020) Matched-pair comparison of (68)Ga-PSMA-11 PET/CT and (18)F-PSMA-1007 PET/CT: frequency of pitfalls and detection efficacy in biochemical recurrence after radical prostatectomy. J Nucl Med 61(1):51–57
Rowe SP, Pienta KJ, Pomper MG, Gorin MA, PSMA-RADS Version (2018) 1.0: a step towards standardizing the interpretation and reporting of PSMA-targeted PET imaging studies. Eur Urol 73(4):485–487
Werner RA, Hartrampf PE, Fendler WP, Serfling SE, Derlin T, Higuchi T et al (2023) Eur Urol 84(5):491–502Prostate-specific Membrane Antigen Reporting and Data System Version 2.0
Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M et al (2018) Prostate Cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-Ligand PET/CT. J Nucl Med 59(3):469–478
Ceci F, Oprea-Lager DE, Emmett L, Adam JA, Bomanji J, Czernin J et al (2021) E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol Imaging 48(5):1626–1638
McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, and the, Group PRISMA-DTA et al (2018) Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. ;319(4):388–96
Woo S, Freedman D, Becker AS, Leithner D, Mayerhoefer M, Arita Y et al Equivocal bone lesions on PSMA PET/CT: How common are they and how often are they metastases? A systematic review and meta-analysis. PROSPERO 2023 CRD42023486697 [Internet]. [cited 2023 Dec 10]. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023486697
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372:n160
Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
Barker TH, Migliavaca CB, Stein C, Colpani V, Falavigna M, Aromataris E et al (2021) Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol 21(1):189
Lee J, Kim KW, Choi SH, Huh J, Park SH (2015) Systematic review and Meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical Researchers-Part II. Statistical methods of Meta-Analysis. Korean J Radiol 16(6):1188–1196
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2021) Introduction to Meta-Analysis. Wiley, p 547
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Arnfield EG, Thomas PA, Roberts MJ, Pelecanos AM, Ramsay SC, Lin CY et al (2021) Clinical insignificance of [(18)F]PSMA-1007 avid non-specific bone lesions: a retrospective evaluation. Eur J Nucl Med Mol Imaging 48(13):4495–4507
Bohil A, Nagabhushan S, Vinjamuri S (2021) F18 PSMA 1007 PET/CT experience with equivocal lesions in prostate cancer: has the time come for PSMA-RADS? J Nucl Med 62(Supplement 1):1343
Dietlein F, Kobe C, Hohberg M, Zlatopolskiy BD, Krapf P, Endepols H et al (2020) Intraindividual comparison of (18)F-PSMA-1007 with Renally Excreted PSMA ligands for PSMA PET Imaging in patients with relapsed prostate Cancer. J Nucl Med 61(5):729–734
Ettala O, Anttinen M, Tommila T, Malaspina S, Kemppainen J, Seppanen M et al (2023) How should lesions without anatomical correspondence in 18F-PSMA-1007 PET/CT be interpreted-a PROSTAGE follow-up study. Eur Urol 83:S158–S158
Foley R, Redman S, Graham R, Little D (2021) Initial experience of [18F] PSMA PET/CT Imaging in a District General Hospital. Nucl Med Commun 42(10):1175–1176
Grünig H, Maurer A, Thali Y, Kovacs Z, Strobel K, Burger IA et al (2021) Focal unspecific bone uptake on [(18)F]-PSMA-1007 PET: a multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur J Nucl Med Mol Imaging 48(13):4483–4494
Hoberück S, Löck S, Borkowetz A, Sommer U, Winzer R, Zöphel K et al (2021) Intraindividual comparison of [(68) Ga]-Ga-PSMA-11 and [(18)F]-F-PSMA-1007 in prostate cancer patients: a retrospective single-center analysis. EJNMMI Res 11(1):109
Janssen JC, Meißner S, Woythal N, Prasad V, Brenner W, Diederichs G et al (2018) Comparison of hybrid (68)Ga-PSMA-PET/CT and (99m)Tc-DPD-SPECT/CT for the detection of bone metastases in prostate cancer patients: additional value of morphologic information from low dose CT. Eur Radiol 28(2):610–619
Knappe L, Rominger A, Afshar-Oromieh A, Alberts I (2022) Follow-up of presumably unspecific bone uptakes in F18PSMA-1007-PET/CT. Eur J Nucl Med Mol Imaging 49(SUPPL 1):S500–S500
Kuten J, Dekalo S, Mintz I, Yossepowitch O, Mano R, Even-Sapir E (2021) The significance of equivocal bone findings in staging PSMA imaging in the preoperative setting: validation of the PSMA-RADS version 1.0. EJNMMI Res 11(1):3
Letang A, Crombé A, Rousseau C, Sargos P, Merlin C, Cantarel C et al (2022) Bone uptake in prostate Cancer patients: Diagnostic performances of PSMA-RADS v1.0, Clinical, Biological, and 68 Ga-PSMA-11 PET features to Predict Metastasis after biochemical recurrence. Clin Nucl Med 47(8):e529–e539
Mihatsch PW, Beissert M, Pomper MG, Bley TA, Seitz AK, Kübler H et al (2022) Changing threshold-based segmentation has no relevant Impact on Semi-quantification in the context of structured reporting for PSMA-PET/CT. Cancers (Basel). ;14(2)
Orevi M, Ben-Haim S, Abourbeh G, Chicheportiche A, Mishani E, Yutkin V et al (2022) False positive findings of [(18)F]PSMA-1007 PET/CT in patients after radical prostatectomy with undetectable serum PSA levels. Front Surg 9:943760
Paone G, Cuzzocrea M, Treglia G, Ruberto-Macchi T, Raditckova-Sarnelli M, Ceriani L et al (2022) Diagnostic accuracy of [F-18] PSMA-1007 PET/CT in intermediate and high risk prostate cancer primary staging: a single center retrospective analysis of semiquantitative PET/CT parameters, bio-distribution and clinical significance of potential equivocal non-specific findings (NS-F). Eur J Nucl Med Mol Imaging 49(SUPPL 1):S485–S485
Phelps TE, Harmon SA, Mena E, Lindenberg L, Shih JH, Citrin DE et al (2023) Predicting outcomes of Indeterminate Bone lesions on (18)F-DCFPyL PSMA PET/CT scans in the setting of high-risk primary or recurrent prostate Cancer. J Nucl Med 64(3):395–401
Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M et al (2016) Comparison of bone scintigraphy and (68)Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging 43(12):2114–2121
Rowe SP, Li X, Trock BJ, Werner RA, Frey S, DiGianvittorio M et al (2020) Prospective Comparison of PET Imaging with PSMA-Targeted (18)F-DCFPyL versus na(18)F for bone lesion detection in patients with metastatic prostate Cancer. J Nucl Med 61(2):183–188
Seifert R, Telli T, Opitz M, Barbato F, Berliner C, Nader M et al (2023) Unspecific (18)F-PSMA-1007 bone uptake evaluated through PSMA-11 PET, bone scanning, and MRI triple validation in patients with biochemical recurrence of prostate Cancer. J Nucl Med 64(5):738–743
Shanmugasundaram R, Roberts M, Wong V, Arianayagam M, Canagasingham B, Ferguson R et al (2020) Optimal detection of bone metastasis in primary staging of prostate cancer: direct comparison of 68-Gallium prostate specific membrane Antigen (PSMA) with bone scan in 532 patients. BJU Int 125:54–54
Simsek DH, Sanli Y, Engin MN, Erdem S, Sanli O (2021) Detection of metastases in newly diagnosed prostate cancer by using 68Ga-PSMA PET/CT and its relationship with modified D’Amico risk classification. Eur J Nucl Med Mol Imaging 48(5):1639–1649
Spurr M, Kimura M, Kulshresthra R, Kabala J, Challapalli A, Sakthithasan M (2022) Equivocal Radiotracer Uptake on 18F-PSMA-1007 PET/CT in patients with prostate Cancer. Intern Med J 52:41–42
Vollnberg B, Alberts I, Genitsch V, Rominger A, Afshar-Oromieh A (2022) Assessment of malignancy and PSMA expression of uncertain bone foci in [(18)F]PSMA-1007 PET/CT for prostate cancer-a single-centre experience of PET-guided biopsies. Eur J Nucl Med Mol Imaging 49(11):3910–3916
Wondergem M, van der Zant FM, Broos WAM, Knol RJJ (2021) Matched-pair comparison of (18)F-DCFPyL PET/CT and (18)F-PSMA-1007 PET/CT in 240 prostate Cancer patients: Interreader Agreement and Lesion Detection Rate of suspected lesions. J Nucl Med 62(10):1422–1429
Yin Y, Werner RA, Higuchi T, Lapa C, Pienta KJ, Pomper MG et al (2019) Follow-up of lesions with equivocal Radiotracer Uptake on PSMA-Targeted PET in patients with prostate Cancer: predictive values of the PSMA-RADS-3A and PSMA-RADS-3B categories. J Nucl Med 60(4):511–516
Zacho HD, Ravn S, Afshar-Oromieh A, Fledelius J, Ejlersen JA, Petersen LJ (2020) Added value of (68)Ga-PSMA PET/CT for the detection of bone metastases in patients with newly diagnosed prostate cancer and a previous (99m)tc bone scintigraphy. EJNMMI Res 10(1):31
Schöder H, Hope TA, Knopp M, Kelly WK, Michalski JM, Lerner SP et al (2022) Considerations on integrating prostate-specific membrane Antigen Positron Emission Tomography Imaging into clinical prostate Cancer trials by national clinical trials Network Cooperative groups. J Clin Oncol 40(13):1500–1505
Cardinale J, Schäfer M, Benešová M, Bauder-Wüst U, Leotta K, Eder M et al (2017) Preclinical evaluation of 18F-PSMA-1007, a New Prostate-Specific Membrane Antigen Ligand for prostate Cancer imaging. J Nucl Med 58(3):425–431
Eder M, Schäfer M, Bauder-Wüst U, Hull WE, Wängler C, Mier W et al (2012) 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem 23(4):688–697
Rahbar K, Weckesser M, Ahmadzadehfar H, Schäfers M, Stegger L, Bögemann M (2018) Advantage of 18F-PSMA-1007 over 68Ga-PSMA-11 PET imaging for differentiation of local recurrence vs. urinary tracer excretion. Eur J Nucl Med Mol Imaging 45(6):1076–1077
Exterkate L, Hermsen R, Küsters-Vandevelde HVN, Prette JF, Baas DJH, Somford DM et al (2023) Head-to-Head comparison of 18F-PSMA-1007 Positron Emission Tomography/Computed tomography and Multiparametric Magnetic Resonance Imaging with whole-mount histopathology as reference in localisation and staging of primary prostate Cancer. Eur Urol Oncol 6(6):574–581
Kerkmeijer LGW, Groen VH, Pos FJ, Haustermans K, Monninkhof EM, Smeenk RJ et al (2021) Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for patients with localized prostate Cancer: results from the FLAME Randomized Phase III Trial. J Clin Oncol 39(7):787–796
Bauckneht M, Miceli A, Signori A, Albano D, Capitanio S, Piva R et al (2023) Combined forced diuresis and late acquisition on [68Ga]Ga-PSMA-11 PET/CT for biochemical recurrent prostate cancer: a clinical practice-oriented study. Eur Radiol 33(5):3343–3353
Spratt DE, Liu VYT, Michalski J, Davicioni E, Berlin A, Simko JP et al (2023) Genomic classifier performance in intermediate-risk prostate Cancer: results from NRG Oncology/RTOG 0126 Randomized Phase 3 Trial. Int J Radiation Oncology*Biology*Physics 117(2):370–377
Chiu LW, Lawhn-Heath C, Behr SC, Juarez R, Perez PM, Lobach I et al (2020) Factors Predicting Metastatic Disease in (68)Ga-PSMA-11 PET-Positive osseous lesions in prostate Cancer. J Nucl Med 61(12):1779–1785
Rosenkrantz AB, Kim S, Lim RP, Hindman N, Deng FM, Babb JS et al (2013) Prostate cancer localization using multiparametric MR imaging: comparison of prostate imaging reporting and data system (PI-RADS) and likert scales. Radiology 269(2):482–492
Woo S, Editorial Comment (2023) PRECISE-The precisely right thing to Use when interpreting prostate MRI for active surveillance? AJR Am J Roentgenol 221(5):660
Grawe F, Blom F, Winkelmann M, Burgard C, Schmid-Tannwald C, Unterrainer LM et al (2023) Reliability and practicability of PSMA-RADS 1.0 for structured reporting of PSMA-PET/CT scans in prostate cancer patients. Eur Radiol
Suh CH, Yun SJ (2019) Diagnostic outcome of image-guided percutaneous core needle biopsy of sclerotic bone lesions: a Meta-analysis. AJR Am J Roentgenol 212(3):625–631
Chen MY, Franklin A, Yaxley J, Gianduzzo T, McBean R, Wong D et al (2020) Solitary rib lesions showing prostate-specific membrane antigen (PSMA) uptake in pre-treatment staging (68) Ga-PSMA-11 positron emission tomography scans for men with prostate cancer: benign or malignant? BJU Int 126(3):396–401
Woo S, Kim SY, Kim SH, Cho JY, JOURNAL CLUB (2016) Identification of bone metastasis with routine prostate MRI: a study of patients with newly diagnosed prostate Cancer. AJR Am J Roentgenol 206(6):1156–1163
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Study conceptualization: SW, HAV. Data acquisition: SW, DF, HAV. Data analysis: SW. Data interpretation: SW, ASB, DL, MEM, KPF, YA, SH, IAB, SST, DRW, MJZ, HAV. Drafting of manuscript: SW. Critical revision of manuscript: SW, ASB, DL, MEM, KPF, YA, SH, IAB, SST, DRW, MJZ, HAV. Supervision: HAV.
Corresponding author
Ethics declarations
Ethical approval
This study did not involve individual human participant data as it was a systematic review and meta-analysis using only study-level summary data provided openly in the literature. Nevertheless, it was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki, its subsequent amendments, or comparable ethical standards.
Informed consent
This study did not involve individual human participant data as it was a systematic review and meta-analysis using only study-level summary data provided openly in the literature. As such, the need for informed consent was not applicable.
Competing interests
MEM received honoraria for lectures from Siemens, GE, and BM but unrelated to the current work. KPF is a co-investigator on “Optimizing timing of rhPSMA-7.3 (18F), for assessing site(s) of recurrent disease following radical prostatectomy” (PI Herbert Lepor) but does not receive any salary support and was unrelated to the current work. IAB has received research support from GE Healthcare and Bayer, speaker honorarium from GE-Healthcare, Baer, Astellas, Janssen and Novartis, and institutionally compensated advisory role for Novartis, Merck & Cie and Ratio Radiotherapeutics but unrelated to the current work. DRW has received consulting fees from Leap Therapeutics, Foundation Medicine, Pfizer, Janssen, Sanofi, Lilly, Labcorp, Myovant, Bayer, AstraZeneca, Accutar and has received travel funding from Pfizer and Bayer but unrelated to the current work. The other authors have nothing to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Woo, S., Freedman, D., Becker, A.S. et al. Equivocal bone lesions on PSMA PET/CT: systematic review and meta-analysis on their prevalence and malignancy rate. Clin Transl Imaging (2024). https://doi.org/10.1007/s40336-024-00631-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40336-024-00631-6