Abstract
At the present time, there is a dilemma concerning the best management of the neck in patients presenting with early head and neck squamous cell carcinoma (HNSCC). Occult cervical metastasis is found in up to a quarter of HNSCC patients with radiologically N0 necks, and for this reason, conventional treatment includes elective neck dissection (END) alongside tumour excision. Sentinel node biopsy (SNB) offers an alternative accurate and minimally invasive method of staging the neck, which has been safely applied to oral cancer. SNB is a patient-specific procedure which has an enhanced recovery compared to END but is currently not widely offered to patients. There are exciting developments in the technology supporting SNB, improving the accuracy and ease of the procedure and opening up the technique to new tumour types. We describe our experiences in using a novel intraoperative navigation device for sentinel node retrieval and review other advances in SNB practice which have the potential to change the standard management for patients with early HNSCC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In head and neck cancer, knowledge of the lymph nodes status is relevant for prognostic and therapeutic reasons as dissemination is almost exclusively via the lymphatic system to the cervical lymph nodes [1, 2]. Despite intensive preoperative radiological staging, occult metastases are subsequently found in up to 25 % of patients with an apparently N0 neck [3]. In order to protect the minority with occult metastasis, current policy recommends that all patients undergo elective neck dissection, despite the fact that this surgery is unnecessary in most cases.
This situation is changing as promising results have been reported in detecting early tumour metastases in head and neck (HN) cancer using sentinel lymph node (SLN) biopsy [4]. This diagnostic technique is now the standard of care in patients with melanoma and breast cancer, as an alternative to elective node dissection. In the head and neck, validation studies have shown that functional lymphatic mapping can be reliably undertaken by injecting radiolabeled colloid (most commonly technitium-99m) around the periphery of a primary tumour [5, 6]. The radiotracer (99mTc nanocolloid) drains through the lymphatic fluid channels, collecting in the SLN and creating hotspots (areas of increased tracer localization) allowing detection by nuclear medicine imaging techniques (lymphoscintigraphy, SPECT/CT) [7].
Conventional planar lymphoscintigraphy (dynamic and static) gamma imaging is widely available, but there are a number of limitations to its usefulness in the head and neck. The size and static nature of the imaging equipment leave it necessarily based in the outpatient department. The result is that only superficial lesions readily accessible for injection are eligible for SNB, precluding deeper tumours (e.g. tongue base, larynx) that have to be injected under GA. Furthermore, planar two-dimensional imaging lacks a three-dimensional (3D) representation of the hotspot within the neck, and the imaging process does not allow depth measurement or facilitate surgical navigation to the SLN [8]. Another difficulty arises when the sentinel node is located close to the injection site. In such circumstances, the SLN is enveloped within the general radiation blush and the subsequent images do not reliably delineate the SN from the main tumour [8]. There is therefore a need to optimize detection, localization and resection of SLNs, in this anatomically challenging region.
This has been partially achieved by integrating single-photon emission computed tomography (SPECT) with computed tomography (CT) [7]. One of the first studies reported a series of HN cancer patients in which SPECT/CT identified more SLNs than conventional images in 15 out of 32 patients (47 %) [9]. Subsequent studies have shown similar findings [10, 11]. The anatomical detail provided also allows exclusion of artefacts such as skin contamination and swallowed radiotracer [10]. The disadvantages of SPECT/CT are the additional dose of radiation required for the CT component of the investigation, and once again the equipment is firmly based in the outpatient department so excluding deep tumours from this diagnostic test.
Recently, intraoperative freehand SPECT (fhSPECT) imaging has been proposed as a new method to improve SLN identification. Rather than mounting the detector inside a rotating gantry, as in SPECT/CT imaging, the handheld gamma probe is linked to a conventional 3D navigation system. This allows intraoperative three-dimensional tracking of radiation hotspots that can be superimposed on a real-time image of the patient. Algorithmic processing of data collected by a tracked gamma probe combined with radiation counts per second is computed to show the depth and position of the hotspot within the region of interest.
Encouraging results have been reported in patients with melanoma and breast cancer [12, 13] as well as initial reports in tumours of the head and neck (HN) region [8, 11, 14–18]. Once validated, this system has the potential to replace conventional outpatient-based SPECT-CT imaging with an intraoperative technique and open new applications for sentinel node biopsy in previously inaccessible tumours. This advantage is not limited to the head and neck but could be applied to any solid tumour that spreads via the lymphatics.
Here, we review the application of this system for patients with early-stage HN squamous cell carcinoma (SCC), based on the current literature and on our own experience. This information is organized into three sections: (1) navigation radio-guided surgery using fhSPECT: surgical set-up and review of head and neck literature; (2) recommended protocol and initial results; and (3) emerging developments for SLN biopsy in HN cancer.
Navigation radio-guided surgery using fhSPECT: surgical set-up and review of head and neck literature
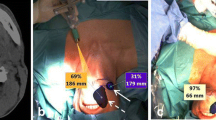
First introduced in 2007 [19], fhSPECT has evolved in the past seven years from a prototype to a commercial product, the declipseSPECT cart system (SurgicEye GmbH, Munich, Germany, Fig. 1).
The major advantage of fhSPECT over regular SPECT/CT is its ability to provide real-time 3D tracking of radioactivity hotspots in the operation room. The concept behind fhSPECT is to combine a tracking system (which is aware of the position of the patient) with a gamma probe whose orientation is stereotactically tracked by two infrared cameras through disposable fiducial markers (Navigation I-Spheres, SurgicEye GmbH, Fig. 2), updating its relative position 20 times per second with accuracy below 0.2 mm [15]. The probe serves as a navigation pointer and enables the surgeon to measure the distance between the tip of the probe and the SLN. The area is scanned by moving the gamma probe methodically over the surgical field from several different angles after which an activity volume reconstruction algorithm is started [20]. The augmented reality reconstructed images are superimposed on a conventional video image of the patient almost immediately making this ideally suited for intraoperative use and enabling the surgeon to re-scan during the procedure without unduly lengthening the operation [8]. Furthermore, the reference target attached to the patient makes it possible to move the operating table during surgery without invalidating the acquired image [15]. It is also possible to import SPECT/CT images, which can be co-localized to the patient by the reference targets aiding preoperative planning, but it is not currently possible to merge the SPECT/CT and fhSPECT images.
This device was successfully evaluated in breast cancer and melanoma [12, 13]. A review of the literature on fhSPECT for visualization and localization of SLNs in patients with HN SCC revealed seven publications including case series and single case reports [8, 11, 14–18].
Bluemel et al. present a validation study recruiting 23 patients with T1–T2N0M0 oral cancer to undergo fhSPECT-guided SLN biopsy and concurrent elective neck dissection. They demonstrated a SLN detection rate of 98 %, and a sensitivity and negative predictive value of 100 % compared to the elective neck dissection specimen [8]. In the largest series to date, Heuveling et al. [18] present 66 cases of oral cavity cancer, in which fhSPECT was undertaken alongside traditional imaging techniques. Freehand SPECT detected 94 % of the sentinel nodes found by other methods and was felt by the surgeon to be of additional benefit in SLN localization in 24 % of operations particularly in patients where the injection site was close to the SLN. In preliminary work, Mandapathil et al. [15] highlighted the value of fhSPECT for SLN detection in previously inaccessible tumours of the head and neck (oropharyngeal, supraglottic, nasal cavity). Patients underwent intraoperative injection of radiotracer 30 min prior to tumour resection and SLN mapping by fhSPECT. The extent of levels resected during the selective neck dissection was planned in accordance with the intraoperative image (sentinel node-guided neck dissection). The excised sentinel lymph nodes were sent for histopathological evaluation, detecting metastasis in three of five patients. No further metastatic lymph nodes were detected in any other node harvested during the concurrent selective neck dissection.
Preliminary studies and cases illustrated above suggest that fhSPECT offers a comparable utility to SPECT/CT allowing intraoperative navigation for sentinel node detection although further work is required to establish conclusive assertions as to the false-negative rate, sensitivity and specificity of the system and therefore if sentinel node mapping by fhSPECT can be safely moved to a purely intraoperative technique.
Recommended protocol in head and neck tumours and initial results
Our recommendation is that patients undergo routine preoperative scanning until the sensitivity and specificity of the technique has been proven. The procedure commences with a peritumoural injection of 0.1–0.2 mls radiolabeled colloid (10–40 MBq 99mTc-nanocoll, dependent on 1- or 2-day protocol) at 4 equidistant points around the periphery of the tumour. Immediate fhSPECT scan is performed up to 10 min after injection in the nuclear medicine department, and experience shows that a radioactivity hotspot is usually detected within this time frame. Without further delay, planar imaging is obtained (40 min–2 h) followed by a SPECT-CT.
The patient and the SurgicEye system are then transferred to the operating theatre. The surgical procedure involves an fhSPECT pre-excision scan and a post-excision scan, with an optional interim scan [8]. For optimal image acquisition, the patient should not move during the scanning process and metal surgical instruments within the scanned area should be removed. However, the software has an autocorrect feature that will remove artefacts caused by such anomalies. In order to validate the intraoperative technique, SLN retrieval is firstly directed by fhSPECT and results of the planar and SPECT/CT imaging are only revealed once the surgeon has completed fhSPECT SLN retrieval. Any additional nodes shown on the SPECT/CT and planar imaging are then also excised.
To date, 39 patients with oral cavity tumours and six patients with salivary gland tumours have been recruited to this protocol with a 93 % detection of positive sentinel nodes compared to 100 % for both planar lymphoscintigraphy and SPECT/CT. Our final results will not be available until 2016, but some illustrative cases are discussed below (Figs. 3, 4, 5, 6).
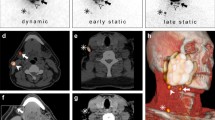
A 80-year-old female non-smoker presents with squamous cell carcinoma of the anterior alveolus (a). Preoperative staging T1N0M0 and wide excision plus SNB are recommended by MDT. One-day SNB protocol using 20 MBq is undertaken with planar lymphoscintigraphy (b) and SPECT/CT (c) at 2 h. No sentinel nodes are found in preoperative imaging. Intraoperative pre-excision fhSPECT imaging is undertaken at 3 h post-injection (d) clearly showing a submental SN. Sentinel node was successfully retrieved and proved negative for metastasis. a SCC lingual lower alveolus. b Static LSG with injection site masking. c SPECT/CT showing signal from injection site only. d Intraoperative fhSPECT showing injection site and SN
A 60-year-old female presents 9-mm low-grade mucoepidermoid carcinoma in tail of the left parotid gland. Two-day SNB protocol is undertaken alongside local excision of tumour. 80-MBq nanocolloid in four doses is delivered peritumourally under USS guidance. Planar and SPECT/CT imaging (a) does not identify SN. At surgery injection site and intraparotid SN is identified by fhSPECT (c, d). Tumour is completely excised, and SN is negative for metastasis. a Preoperative lymphoscintigraphy and SPECT/CT. b Rendered SPECT/CT. c 3D view fhSPECT scan. d Intraoperative fhSPECT showing injection site (lower) and SN (upper)
A 24-year-old male non-smoker presents with SCC affecting the left ventral tongue (a). Preoperative staging T1N0M0 and wide excision plus SNB are recommended by MDT. One-day SNB protocol using 20 MBq is undertaken with immediate fhSPECT scan (b) and planar lymphoscintigraphy (c) both show injection site plus bilateral sentinel nodes. In this case, SPECT/CT could not be performed due to equipment maintenance. Biopsy was undertaken and proved positive for metastasis on the left side. Completion bilateral neck dissection was performed with no further positive nodes detected. a SCC left ventral tongue. b fhSPECT scan immediately post-injection. c Planar LSG
A 52-year-old female ex-smoker presents with SCC of left lateral border of the tongue (a). Preoperative staging T2N0M0 and wide excision plus SNB are recommended by MDT. One-day SNB protocol using 30 MBq is undertaken with planar lymphoscintigraphy (b) and SPECT/CT at 2 h showing two SN on the left side. Intraoperative fhSPECT imaging was suboptimal (c). Two SN were removed, from left level IIa (positive for metastasis) and III. Subsequent completion neck dissection found two further positive nodes, and the patient was recommended adjuvant radiotherapy. After completion of treatment, she developed a metastatic node in the right retropharynx. a T2N0M0 SCC Left tongue. b Planar LSG showing two SN. c Intraoperative fhSPECT showing two dominant SN and further scattered areas of uptake
Emerging developments for SLN biopsy in HN cancer
Aside from fhSPECT navigation, there are other possibilities for intraoperative sentinel node imaging such as portable gamma cameras [21–24] and fusion ultrasound/SPECT [25]. Alongside the development of sentinel node detection equipment, there are concurrent advances in the tracers used during the biopsy procedure. The development of hybrid tracers, a combination of a radiotracer and near infrared (NIR) fluorescent dye, allows dual labelling of the lymph node, aiding detection and reducing the chances of false-negative node sampling. This is particularly pertinent in tumours of the floor of the mouth where some investigators have reported increased false-negative rate, up to 28 %, due to the proximity of the injection site to the draining lymph nodes [5, 6]. Studies have shown the reliability of dual tracers such as ICG-99mTc-nanocolloid [26] (a combination of indocyanine green (ICG) and (99m) Tc-nanocolloid) showing 100 % concordance in sentinel node identification with standard tracer. This is in contrast to traditional optical tracer, where blue dye is injected separately and flows independent to the radiotracer occasionally resulting in flow to different lymph nodes. A further advantage of the combined tracer is that the optical signal can be detected through overlying tissue plus the hybrid tracer structure aids its retention within the node. Early investigations with other hybrid tracers such as nanocolloidal albumin-IRDye800cw [27] show promising signal intensity, but many of the brightest fluorescent tracers have yet to be proven safe for use in humans. Herein lies the future of tracer molecules if the approval process can be afforded.
A new radiopharmaceutical (single modality) in the form of 99mTc-tilmanocept (Lymphoseek®) [28] has recently been tested in oral cancer showing an impressive FNR of 2.56 % in cohort of 83 patients. This differs from other colloidal tracers by specifically targeting CD-206 mannose receptors on lymphatic macrophages and dendritic cells rather than relying solely on non-specific physical properties to facilitate lymphatic flow and trapping within the node. Lymphoseek® [28] appears to be held up for a prolonged period (well over 24 h) and with a small molecular weight it may be an advantage at sites where there is sluggish lymphatic flow.
Further, parallel and complimentary developments in both nuclear medicine (PET/CT, PET/MR, portable gamma imaging) and surgical technologies (augmented reality, robotic surgery) are enabling a new era of interspeciality collaboration. This expanding field has been termed guided intraoperative scintigraphic tumour targeting (GOSTT) [29, 30] and in the future should allow patients to benefit from seamless integration between diagnostic imaging and minimally invasive tumour excision.
Conclusion
The initial evaluation of fhSPECT for precise localization of radioactivity hotspots in the HN region has shown its value; if these promising results can be confirmed in larger patient cohorts, navigation-guided SLN biopsy can be safely introduced for the management of the N0 neck in patients with HN cancer and extended to other solid tumours. Considering up to 75 % of patients with early-stage oral cancer are currently over treated with elective neck dissection, then SLN biopsy is a diagnostic technique that may be practice-changing, allowing many more patients with clinically N0 necks to benefit from a tailored surgical approach. Intraoperative sentinel node imaging techniques described here should provide precise information on the localization of the SLN, reducing operating time and the false-negative rate of this procedure. This in turn will enhance patient post-operative recovery and most importantly long-term quality of life for patients diagnosed with early cancer [14].
References
Thiele OC et al (2012) The role of elective supraomohyoidal neck dissection in the treatment of early, node-negative oral squamous cell carcinoma (OSCC): a retrospective analysis of 122 cases. J Craniomaxillofac Surg 40(1):67–70
O’Connor R et al (2013) The relative cost of sentinel lymph node biopsy in early oral cancer. J Craniomaxillofac Surg 41(8):721–727
Psychogios G et al (2013) Incidence of occult cervical metastasis in head and neck carcinomas: development over time. J Surg Oncol 107(4):384–387
Ferris RL, Kraus DH (2012) Sentinel lymph node biopsy versus selective neck dissection for detection of metastatic oral squamous cell carcinoma. Clin Exp Metastasis 29(7):693–698
Civantos FJ et al (2010) Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: results of a prospective multi-institutional trial. J Clin Oncol 28(8):1395–1400
Ross GL et al (2004) Sentinel node biopsy in head and neck cancer: preliminary results of a multicenter trial. Ann Surg Oncol 11(7):690–696
Alkureishi LW et al (2009) Joint practice guidelines for radionuclide lymphoscintigraphy for sentinel node localization in oral/oropharyngeal squamous cell carcinoma. Ann Surg Oncol 16(11):3190–3210
Bluemel C, Herrmann K, Kübler A, Buck AK, Geissinger E, Wild V, Hartmann S, Lapa C, Linz C, Müller-Richter U (2014) Intraoperative 3-D imaging improves sentinel lymph node biopsy in oral cancer. Eur J Nucl Med Mol Imaging 41(12):2257–2264. doi:10.1007/s00259-014-2870-z
Bilde A et al (2006) The role of SPECT-CT in the lymphoscintigraphic identification of sentinel nodes in patients with oral cancer. Acta Otolaryngol 126(10):1096–1103
Haerle SK et al (2009) Is there an additional value of SPECT/CT over planar lymphoscintigraphy for sentinel node mapping in oral/oropharyngeal squamous cell carcinoma? Ann Surg Oncol 16(11):3118–3124
Schilling C, Gnanasegaran G, McGurk M (2014) Three-dimensional imaging and navigated sentinel node biopsy for primary parotid malignancy: new application in parotid cancer management. Head Neck 36(9):E91–E93
Bluemel C et al (2013) Freehand SPECT for image-guided sentinel lymph node biopsy in breast cancer. Eur J Nucl Med Mol Imaging 40(11):1656–1661
Mihaljevic AL et al (2014) Transferring innovative freehand SPECT to the operating room: first experiences with sentinel lymph node biopsy in malignant melanoma. Eur J Surg Oncol 40(1):42–48
Heuveling DA et al (2012) Sentinel node biopsy using 3D lymphatic mapping by freehand SPECT in early stage oral cancer: a new technique. Clin Otolaryngol 37(1):89–90
Mandapathil M et al (2014) Freehand SPECT for sentinel lymph node detection in patients with head and neck cancer: first experiences. Acta Otolaryngol 134(1):100–104
Bluemel C, Herrmann K, Müller-Richter U, Lapa C, Higuchi T, Wild V, Buck AK, Kübler A, Linz C (2014) Freehand SPECT-guided sentinel lymph node biopsy in early oral squamous cell carcinoma. Head Neck 36(11):E112–E116. doi:10.1002/hed.23596
Den Toom IJ, Heuveling DA, Flach GB, van Weert S, Karagozoglu KH, van Schie A, Bloemena E, Leemans CR, de Bree R (2015) Sentinel node biopsy for early-stage oral cavity cancer: the VU University Medical Center experience. Head Neck 37(4):573–578. doi:10.1002/hed.23632
Heuveling DA et al (2015) Evaluation of the use of freehand SPECT for sentinel node biopsy in early stage oral carcinoma. Oral Oncol 51(3):287–290
Wendler T et al (2007) Towards intra-operative 3D nuclear imaging: reconstruction of 3D radioactive distributions using tracked gamma probes. Med Image Comput Comput Assist Interv 10(Pt 2):909–917
Wendler T et al (2010) First demonstration of 3-D lymphatic mapping in breast cancer using freehand SPECT. Eur J Nucl Med Mol Imaging 37(8):1452–1461
Stoffels I et al (2012) Radio-guided surgery: advantages of a new portable gamma-camera (Sentinella) for intraoperative real time imaging and detection of sentinel lymph nodes in cutaneous malignancies. J Eur Acad Dermatol Venereol 26(3):308–313
Borbon-Arce M et al (2014) An innovative multimodality approach for sentinel node mapping and biopsy in head and neck malignancies. Rev Esp Med Nucl Imagen Mol 33(5):274–279
Mayoral M et al (2014) The added value of a portable gamma camera for intraoperative detection of sentinel lymph node in squamous cell carcinoma of the oral cavity: a case report. Rev Esp Med Nucl Imagen Mol 33(4):237–240
Hellingman D et al (2015) Detecting near-the-injection-site sentinel nodes in head and neck melanomas with a high-resolution portable gamma camera. Clin Nucl Med 40(1):e11–e16
Freesmeyer M et al (2014) Real-time ultrasound and freehand-SPECT. Experiences with sentinel lymph node mapping. Nuklearmedizin 53(6):259–264
Brouwer OR et al (2012) Comparing the hybrid fluorescent-radioactive tracer indocyanine green-99mTc-nanocolloid with 99mTc-nanocolloid for sentinel node identification: a validation study using lymphoscintigraphy and SPECT/CT. J Nucl Med 53(7):1034–1040
Heuveling DA et al (2012) Nanocolloidal albumin-IRDye 800CW: a near-infrared fluorescent tracer with optimal retention in the sentinel lymph node. Eur J Nucl Med Mol Imaging 39(7):1161–1168
Agrawal A, Civantos FJ, Brumund KT, Chepeha DB, Hall NC, Carroll WR, Smith RB, Zitsch RP, Lee WT, Shnayder Y, Cognetti DM, Pitman KT, King DW, Christman LA, Lai SY (2015) [99mTc]Tilmanocept accurately detects sentinel lymph nodes and predicts node pathology status in patients with oral squamous cell carcinoma of the head and neck: results of a phase III multi-institutional trial. Ann Surg Oncol. doi:10.1245/s10434-015-4382-x
Zaknun JJ et al (2012) Changing paradigms in radioguided surgery and intraoperative imaging: the GOSTT concept. Eur J Nucl Med Mol Imaging 39(1):1–3
Valdes Olmos RA et al (2014) The GOSTT concept and hybrid mixed/virtual/augmented reality environment radioguided surgery. Q J Nucl Med Mol Imaging 58(2):207–215
Conflict of interest
Dr. Clare Schilling, Dr. Andrea Corrado Profeta, Dr. Gopinanth Gnanasegaran and Professor Mark McGurk declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
All patients gave consent for images reproduced in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s40336-016-0208-5.
Rights and permissions
About this article
Cite this article
Schilling, C., Corrado, A., Gnanasegaran, G. et al. Role of intraoperative sentinel node imaging in head and neck cancer. Clin Transl Imaging 3, 217–223 (2015). https://doi.org/10.1007/s40336-015-0121-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-015-0121-3