Abstract
Lassa fever is a zoonotic viral illness that is endemic in West Africa. The disease has been a subject of intensive research in the mathematics and non-mathematics fields after the first case was confirmed in Nigeria in 1969. Treatment is inevitable after the full incubation of a disease but there may not be a total compliance to treatment guidelines due to factors like poverty and ignorance especially in poor communities. These factors can seriously affect the dynamics of Lassa fever but have not been paid attentions to in the literature. Based on this, a stage of infection when the disease has been fully incubated is considered and a new mathematical model is designed to examine the effect of treatment compliance on the dynamics and control of Lassa fever. The model validity was examined and established using ample mathematics theorems. The equilibria and a threshold for disease eradication were derived. The stability was analyzed and the necessary and sufficient conditions for the equilibria of the model to be stable both locally and globally were derived. Further, sensitivity analysis was carried out to investigate the relative contributions of various parameters to Lassa fever spread and management. Numerical simulation was later conducted via a logical parameter values from the literature to visualize the effect of parameters perturbations on the dynamics of the disease. Results from the study revealed that Lassa fever eradication is a function of total compliance to treatment procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lassa fever is a deadly viral zoonotic illness instigated by Lassa virus. It is transmitted through direct contact especially between infected animals or humans and susceptible humans [14]. The disease is rampant during dry seasons due to excessive dust from dead hosts [7]. At present, there are no approved vaccines for Lassa fever [10]. However, several attempts have been made to develop reliable vaccines for Lassa virus [9, 17, 18, 29].
Lassa fever is endemic in West Africa and there have been frequent emergence and re-emergence of the disease in Mali, Sierra-Leone, Ghana, Liberia, Benin, Guinea and Nigeria [12]. Reliable data attributed about 5000 mortalities and 300,000 new infections to Lassa fever across West African countries each year [27]. The first case of Lassa fever was confirmed in the Nigerian town called Lassa in 1969 and the disease has been occurring from time to time in the country since then [6]. Within the first two months in 2020, the case fatality ratio of Lassa fever in Nigeria is about 41.9% with 72 mortalities from 172 confirmed cases [10].
The primary hosts of Lassa virus are rodents that belong to the genus Mastomys usually regarded as “multimammate rats” [8]. Mastomys rodents infected with the Lassa virus never become sick, but can spread the virus to other primates and man through their excreta [28].
Many scholars have contributed and dedicated much time to the study of Lassa fever and several models have been developed to study the transmission dynamics of Lassa fever theoretically [1, 5, 11, 13, 22,23,24,25]. Lassa fever can be attributed to many factors but existing studies have been motivated by factors like isolation, re-infection and latency [3, 15, 21, 28]. The spread of Lassa fever and effect of available intervention strategies have also been a subject of intense theoretical study [2, 4, 16, 19, 20, 26, 30].
When an infection is fully incubated, treatment becomes imperative. However, the rate of compliance to treatment guidelines might not be 100% due to many factors like ignorance and poverty especially in poor resource setting communities. Attentions have not been paid to these factors in the literature but can seriously affect the dynamics of Lassa fever. On this ground, a stage of infection when the disease has been fully incubated is considered and a new mathematical model is designed to examine the effect of treatment compliance on the dynamics and control of Lassa fever.
2 Model formulation
The compartments of the model are classified into seven namely susceptible humans \((S_h)\), infected humans \((I_h)\), non-treatment compliant humans \((NT_h)\), treatment compliant humans \((T_h)\), recovered humans \((R_h)\), susceptible rodents \((S_r)\) and infected rodents \((I_r)\). The model is formulated based on the following assumptions:
-
1.
A poor resource setting community is considered and susceptible humans are not protected against infections.
-
2.
When an infection is fully developed, treatment becomes inevitable therefore asymptomatic stage is neglected.
-
3.
Individuals who are treatment compliant do follow treatment guidelines strictly. They do not interact with susceptible humans or rodents and do not spread the disease.
-
4.
Non-treatment compliant individuals are negligent and do mix with the susceptible humans and rodents and do spread the disease.
-
5.
Recovery is not permanent and there is tendency for reinfection.
-
6.
Lassa fever deaths only occur for infectious humans \((I_h)\) while individuals under treatment \((T_h)\) and \((NT_h)\) do not die of Lassa fever infections.
-
7.
Infectious humans \((I_h)\) and non-treatment compliant humans \((NT_h)\) do not spread infection to susceptible rodents.
Following these assumptions, the flow between the compartments is displayed in Fig. 1.
The definitions for the model parameters are presented in Table 1.
The transfer diagram in Fig. 1 is translated into the following system of nonlinear first-order ordinary differential equations
The population of man and mastomys at time t is split to Eqs. (8) and (9) respectively
subject to initial conditions,
The growth for man and mastomys populations is described by
The model must satisfy positivity and boundedness properties before it can be valid. Besides, it must be mathematically and biologically well-posed. Each of the properties shall be verified one after the other to establish the validity of the model.
2.1 Positivity of solutions
The solutions of the model must be positive since the model monitors human and animal populations. We shall show that the solutions of the system are positive for all \(t>0.\)
Theorem 1
Given the positive initial variables \(S_{0\,h}>0, I_{0\,h}>0, NT_{0\,h}>0, T_{0\,h}>0, R_{0\,h}>0, S_{0r}>0, I_{0r}>0,\) the solutions \((S_h,I_h,NT_h,T_h,R_h,S_r,I_r)\) of the system are positive for all \(t>0.\)
Proof
Assuming \(\nabla _L=Sup~~t>0:S_h(t)>0, I_h(t)>0, NT_h(t)>0, T_h(t)>0, R_h(t)>0, S_r(t)>0, I_r(t)>0\) then \(\nabla _L>0.\) Supposing also that \(\nabla _L>\infty \) then \(S_h,I_h,NT_h,T_h,R_h,S_r,I_r\) becomes zero at \(\nabla _L.\) Therefore, from Eq. (1), it follows that
By the same procedure, it can be shown that \(I_h>0,\) \(NT_h>0,\) \(T_h>0,\) \(R_h>0,\) \(S_r>0,\) \(I_r>0,\) \(\forall ~~t>0\). \(\square \)
2.2 Boundedness of solutions
Theorem 2
The solutions \((S_h,I_h,NT_h,T_h,R_h,S_r,I_r)\) of the model are bounded.
Proof
From Eq. (11)
With no infection,
Integrating inequality (17),
When \(t=0,\)
Hence,
Also, from Eq. (12),
With no infection,
Integrating inequality (19),
When \(t=0,\)
Hence,
Following Birkhoff and Rota’s theorem [31, 45, 46], Eqs. (18) and (20) becomes
Paticularly, \(N_H(t)\le \dfrac{\beta _h}{d_1}\) and \(N_R(t)\le \dfrac{\varphi _r}{d_2}\) if \(N_H(0)\le \dfrac{\beta _h}{d_1}\) and \(N_R(0)\le \dfrac{\varphi _r}{d_2}\) respectively. Therefore, all the solutions of the model enter
where
Hence, all the solutions of the system with nonnegative initial conditions in \(\Omega \) remain in \(\Omega \) for all \(t>0.\) It therefore follows that \(\Omega \) is positively invariant. Hence, the disease dynamics governed by the system (1)–(7) can be considered in \(\Omega \) where the system is mathematically and epidemiologically well posed. \(\square \)
3 Qualitative analysis
3.1 Equilibria
The model equilibrium points are obtained to analyze the long-term dynamics of the disease. The system of Eqs. (1)–(7) is set to zero to obtain two equilibria. i.e.
The solutions \(S_h^{\circ }, I_h^{\circ }, NT_h^{\circ }, T_h^{\circ }, R_h^{\circ }, S_r^{\circ }\) and \(I_r^{\circ }\) satisfy Eq. (22) and clearly indicate that the equilibrium is not trivial and the populations do not go into extinction as long as human recruitment rate \(\beta _h\) and the mastomys recruitment rate \(\varphi _r\) are not zero. Hence, \(I_h=NT_h=T_h=R_h=0\) when Lassa virus is totally absent from the community and the system admits a steady state, \(H_{\circ },\) that represents the infection-free equilibrium (DFE). Therefore, the system has the DFE denoted by
However, if the community is invaded with Lassa virus, each of the variables become nonzero and the condition \(I_h=NT_h=T_h=R_h=0\) fails to hold. Assuming \(H^{*}\) defines the system’s steady state when the community is invaded with the virus with points \(S_h^{*}, I_h^{*}, NT_h^{*}, T_h^{*}, R_h^{*}, S_r^{*}, I_r^{*}\). Then, solving the system at the endemic equilibrium, the points are obtained as
3.2 Reproduction number
To predict the transmission potential of the virus when an infectious agent emerges in a population where every individual and mastomys are completely susceptible, we compute the reproduction number, \(R_{\circ }\), which is defined by Diekmann et al. [32] as the average number of secondary cases instigated by an infectious agent in a naive population throughout the period of his infectiousness. For our model, \(R_{\circ }\) is computed following the procedure in Driesche and Watmough [33] which quantifies Lassa fever spread in terms of infections from humans and rodents. Infections from humans include infections from both infected \(I_h\) and the non-treatment compliance \(NT_h\). Therefore, at the DFE, the rate of appearance of new infections and the transfer of humans and mastomys are partitioned into two Jacobian matrices F and V such that
The spectral radius of matrix \(FV^{-1}\) defines the \(R_{\circ }\) for the model. \(R_{\circ }\) for the model is made up of \(R_H\) and \(R_r\), the infection spread from humans and mastomys respectively. Therefore, \(R_{\circ }=\) max\((R_H,R_r)\) such that
While \(R_H\) governs the number of new cases of Lassa fever due to infections from infectious individuals who are yet to access treatment \((I_h)\) and those who are under treatment but who do not comply with treatment guidelines \((NT_h)\), \(R_r\) governs the average number of new cases of Lassa fever due to infections from infectious rodents \((I_r)\). The transmissibility of Lassa virus depends on the numerical values of \(R_H\) and \(R_r\). If \(R_H\) and \(R_r\) are less than one, the virus will not spread but if one or both \(R_H\) and \(R_r\) are greater than one, the virus will spread in the population.
3.3 Local stability of the equilibria, \(H_{\circ }\) and \(H^{*}\)
The DFE \(H_{\circ }\), the endemic equilibrium \(H^{*}\) and the \(R_{\circ }\) of the model have been derived. The stability of the equilibria \(H_{\circ }\) and \(H^{*}\) have to be investigated to examine the possibility of Lassa fever extinction or persistence in the population.
Theorem 3
The DFE \(H_{\circ }\) is locally asymptotically stable if \(R_H<1\) and \(R_r<1\) but unstable if one or both \(R_H\) and \(R_r\) are greater than one
Proof
The Jacobian of the model evaluated at DFE \(H_{\circ }\) is obtained as
Solving \(|J(H_{\circ })-\lambda I|=0\) yields
or
From Eq. (35),
\(\lambda _5\) can be further expressed as
Hence, \(\lambda _5<0\) if \(R_r<1\). Suppose the matrix in Eq. (36) is considered and suppose it is M. The trace and determinant of M are respectively
Therefore, \(Det(M)>0\) if \(R_H<1\). Also in Eq. (37), following the definition of \(R_H\), if \(R_H<1\) then \(\alpha _1\rightarrow 0\) and \(Tr(M)<0\). Hence, all the roots of \(|J(H_{\circ })-\lambda I|=0\) are negative and the DFE \(H_{\circ }\) is locally asymptotically stable if \(R_H<1\) and \(R_r<1\). \(\square \)
The investigation of the local stability of the endemic equilibrium \(H^{*}\) shall follow after the necessary and sufficient conditions for local asymptotic stability of the DFE \(H_{\circ }\) have been derived.
Theorem 4
The endemic equilibrium \(H^{*}\) exists and is locally asymptotically stable if \(R_{\circ }>1\) otherwise \(H^{*}\) is locally asymptotically unstable (i.e. if \(R_{\circ }<1\))
Proof
Linearization approach will prove difficult to establish the local stability of \(H^{*}\) due to the dimension of the system. Therefore, Theorem 4.1 (iv) in Castillo-Chavez and Song [34] shall be employed to examine the local stability of \(H^{*}\) of the model. The Jacobian of the system evaluated at DFE \(H_{\circ }\) with \(\alpha _3=\alpha _3^{*}\) chosen as the bifurcation parameter is derived as
where
\(\alpha _3=\alpha _3^{*}\) is chosen as the bifurcation parameter to examine whether or not poor compliance with treatment guidelines can trigger Lassa fever outbreak. The right-eigen vectors of \(J^{*}=J(H_{\circ })|_{\alpha _3=\alpha _3^{*}}\) in terms of \(w_2\) where \(w_2=w_2>0\) consists of
Also, \(J^{*}=J(H_{\circ })|_{\alpha _3=\alpha _3^{*}}\) has the left eigen-vectors satisfying the condition \(\mathbf {v.w}=1\) such that \(v_1=v_3=v_4=v_5=v_6=v_7=0\) but \(v_2=v_2>0\). The stability of \(H^{*}\) is governed by the signs of the coefficients of \('a'\) and \('b'\) whose formulae are respectively defined in Theorem 4.1 (iv) in [34] as
Therefore, the values of a and b are determined after a few algebraic manipulations as
Since \(b>0\) and following the same Theorem 4.1 (iv) in [34], there exists an endemic equilibrium \(H^{*}\) that is locally asymptotically stable if \(a<0\). \(\square \)
Having derived the necessary and sufficient conditions for the existence of local stability of each equilibrium of the model, the investigation of the global stability the model equilibria shall follow.
3.4 Global stability of the equilibria, \(H_{\circ }\) and \(H^{*}\)
Theorem 5
The DFE \(H_{\circ }\) is globally asymptotically stable (GAS) if \(R_{\circ }<1\) but unstable if \(R_{\circ }>1\).
Proof
The comparison theorem in [35] which has been used in some existing Lassa fever model [7] shall be used to investigate the existence of global stability for the DFE \(H_{\circ }\) of our proposed Lassa fever model. By definition, the DFE \(H_{\circ }\) is GAS if all the eigenvalues of \(|(F-V)-\lambda I|=0\) are negative. From Eq. (30),
where
Equation (52) can be solved by using the principle \(a=0\) or \(b=0\) if \(a \times b=0\). Now, from Eq. (52),
provided that \(R_r<1\). Or,
On substituting for \(k_1\) and \(k_2\) and following Rourth Hurtwitz stability criteria [36, 47], the two roots in Eq. (53) are negative if
and,
From inequality (55),
Inequalities (54) and (56) are true provided that \(R_H<1\). The validity of inequality (56) is clear whenever \(R_H<1\). Inequality (54) would also be true because the transmission parameter \(\alpha _1\rightarrow 0\) when \(R_H<1\). Hence, all the eigenvalues of Eq. (51) are negative if \(R_H<1\) and \(R_r<1.\) Therefore, it is sufficient to conclude that the global stability of the DFE \(H_{\circ }\) of the proposed model is a function of the numerical values of \(R_H<1\) and \(R_r<1.\) \(\square \)
Having examined the global stability the DFE \(H_{\circ }\) of the model, we proceeded to investigate the global stability of the endemic equilibrium \(H^{*}\).
Theorem 6
The endemic equilibrium \(H^{*}\) of the model is globally asymptotically stable if \(R_{\circ }>1.\)
Proof
Suppose L is a nonlinear Lyapunov function. The endemic equilibrium \(H^{*}\) is globally asymptotically stable if \(\dfrac{dL}{dt}\le 0\) [37, 38]. Define L as
Simplifying Eq. (59) in terms of \( S_h^{*} \& S_h, I_h^{*} \& I_h, NT_h^{*} \& NT_h, T_h^{*} \& T_h, R_h^{*} \& R_h, S_r^{*} \& S_r\) and \( I_r^{*} \& I_r\) then
Thus, \(\dfrac{dL}{dt}\le 0\). Also, \(\dfrac{dL}{dt}=0\) provided that \(S_h=S_h^{*}, I_h=I_h^{*}, NT_h=NT_h^{*}, T_h=T_h^{*}, R_h=R_h^{*}, S_r=S_r^{*}, I_r=I_r^{*}\). The only largest invariant set in \(\left\{ (S_h, I_h, NT_h, T_h, R_h, S_r, I_r)\in \Omega :\dfrac{dL}{dt}=0 \right\} \) is \(H^{*}\). Therefore, \(H^{*}\) is GAS in the interior \(\Omega \) by LaSalle’s invariance principle [39, 40]. \(\square \)
3.5 Sensitivity analysis
The contribution of the model parameters to increase or decrease in the reproduction numbers \(R_H\) and \( R_r\) can be investigated by the sensitivity index formula which has been widely employed in the literature [7, 15, 41].This will enable the policy makers to identify the parameters to be targeted for intervention to control the spread of Lassa fever. The indices for \(\beta _h, \alpha _1, \alpha _3, \eta , \gamma _1, d_1, \tau \) and \(\vartheta \) in terms of \(R_H\) are determined one after the other as follows:
Also, the indices for \(\varphi _r, \epsilon \) and \(d_2\) in terms of \(R_r\) are determined as
where \(m_1=\tau \gamma _1+2d_1\gamma _1+\vartheta \gamma _1+2d_1\tau +3d_1^2+2d_1\vartheta ,~~ m_2=(d_1(\tau \gamma _1+d_1\gamma _1+\vartheta \gamma _1+d_1\tau +d_1^2+d_1\vartheta ))^2, ~~m_3=\gamma _1+d_1,~~ m_4=\tau +d_1+\vartheta .\)
4 Numerical simulation and discussion
To support the claims and illustrate what was obtained analytically in Sect. 3, some numerical simulations are performed to visualize the effect of parameter perturbations on the structure of the model. The parameter for human mortality rate, \(d_1\), is the only parameter whose value is estimated. The values for other parameters are searched from the literature and where they are not available, a logical set of values are used. The life expectancy in Nigeria in 2021 was about 60.87 years [42]. The natural death rate is generally the inverse of the life expectancy. Therefore, the natural death rate for human \(d_1=\dfrac{1}{60.87}\) year\(^{-1}\) \(=\dfrac{52}{60.87}\) week\(^{-1}\). The model parameters have been defined in Table 1 but the initial values assigned to each parameter to conduct the simulation is stated in Table 2 with the unit of measurement in a year.
Following the parameter values in Table 2, the sensitivity indices of the model parameters derived in Eqs. (61)–(71) in relation to the transmission parameters \(R_H\) are \(R_r\) are computed and the results are presented in Table 3.
In Table 3, natural death rates parameters \(d_1\) and \(d_2\), effective contact rate parameter \(\epsilon \) between infectious and susceptible rodents, mortality rate due to infection parameter \(\vartheta \) and progression rate parameter \(\tau \) into treatment compartment are less significant to the disease dynamics and are excluded in the the discussion of result for Table 3. Other key parameters, \(\beta _h, \alpha _1, \alpha _3, \eta , \gamma _1\) and \(\varphi _r\), that have major influence on the dynamics of the disease are discussed. Parameters with positive indices contribute to the spread of Lassa fever while those with negative indices inhibit Lassa fever transmission.
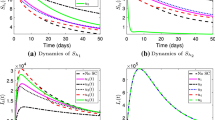
Based on the sensitivity analysis results in Table 3, \(\beta _h, \alpha _3, \eta , \varphi _r\) and \(\gamma _1\) are more crucial to Lassa fever dynamics considering the magnitude of their values. It is revealed that \((\beta _h)\), the human recruitment rate and \((\varphi _r)\), the recruitment rate for rodents have the highest sensitivity indices of 1.000. The interpretation of the value is that Lassa fever transmission (i.e., \(\mathcal {R}_0\)) approximately reduces by 10% if any of \((\beta _h)\) or \((\varphi _r)\) is reduced by 10%. This shows that any intervention that can limit the influx of Lassa fever carriers into the human population and at the same time reduce the rodent population is crucial to the eradication of Lassa fever spread. Also, the sensitivity indices for \(\alpha _3\), the effective contact rate between susceptible and infectious individuals and \(\eta \), the proportion of infected individuals who comply with treatment guidelines revealed an important information about Lassa fever transmission and management. The indices indicate that a 100% compliance with Lassa fever treatment guidelines \((\eta )\) could avert the spread of the disease in the population by 87%, whereas disregard for treatment procedures could instigate a 87% spread of the disease in the susceptible individuals’ population \((\alpha _3)\). Again, the effect of compliance with treatment guidelines is also revealed by the sensitivity index of \(\gamma _1\) which indicates that Lassa fever spread could be reduced by about 70% if \(\gamma _1\) is increased by 100%. That is, Lassa fever could be minimized if infectious individuals who are not compliant with treatment guidelines turn over a new leaf. Therefore, any strategy that aims to eliminate Lassa fever in the endemic areas has to take compliance with treatment procedure with all seriousness. The effect of treatment compliance on the transmission dynamics of Lassa fever is later examined quantitatively by varying the value of the treatment compliant parameter \((\eta )\) as well as the value of the parameter for spreading rate of Lassa virus from non-treatment compliant humans to the susceptible individuals \((\alpha _3)\). The results of the analysis are displayed in Fig. 2 using the initial values \(S_h=0.97, I_h=0.016, NT_h=0.0005, T_h=0.01, R_h=0.002, S_r=0.00097, I_r=0.0001\).
The impact of compliance and non-compliance with treatment guidelines on the spread of Lassa fever can be visualized in Fig. 2 especially in terms of the population of infectious individuals who are yet to be receiving treatment \(I_h(t)\). Emphasis is placed on these individuals because infectious humans who are yet to be receiving treatment have major contributions to Lassa fever escalation and their populations could be influenced by the level of compliance with treatment procedures of the infectious individuals who are under treatment. In Fig. 2a, with high rate of compliance with treatment guidelines, the population of the infectious individuals falls continuously and tends to zero after fifth year. It is shown in Fig. 2a that high rate of compliance with treatment guidelines lapses the period for new cases to attain the peak (delay instant transmission of the virus) and also elongates time to reach stability. The reproduction numbers from human-to-human \(R_H\) and rodent-to-human \(R_r\) are both less than unity, 0.852 and 0.544 respectively when there is high rate of compliance with the treatment guidelines which makes the DFE to be stable both locally and globally. Therefore, Lassa fever outbreak can be brought under control with strong adherence to Lassa fever treatment procedures.
On the other hand, the effect of disregard for compliance with the treatment procedures on the transmission of Lassa fever is illustrated in Fig. 2b in terms of the population of infectious individuals who are yet to be receiving treatment \(I_h(t)\). In Fig. 2b, with low rate of compliance with treatment guidelines signifying by the value of \(\eta \) (i.e., 0.1) which instigated \(\alpha _3=0.3, \tau =0.3, \gamma _2=0.005\), the population of the infectious individuals rises continuously. The rising nature of the curve in Fig. 2b pushes the disease to the stable endemic state as the reproduction numbers \(R_H\) and \(R_r\) equal 7.883 and 0.544 respectively. In this scenario, disregard for compliance with the Lassa fever treatment procedures alone accounts for the endemicity of the disease (i.e., \(R_H=7.883\)). It is therefore evident that total compliance with Lassa fever treatment guidelines is key to the eradication of the disease in the endemic areas.
5 Conclusion
In this work, a deterministic model was formulated via a system of first-order differential equations to examine the effect of treatment compliance on the dynamics and control of Lassa fever. The human and rodent populations were considered and model validity was established. The threshold quantities \(R_H\) and \(R_r\), that governed disease spread or elimination, were also derived. The necessary and sufficient conditions for the existence of both local and global stability of the DFE and the endemic equilibrium in relation to the threshold quantities \(R_H\) and \(R_r\) were also derived. Sensitivity analysis was further carried out for the threshold quantities \(R_H\) and \(R_r\) to quantify the effect of each model parameter on the spread and management of Lassa fever. The result revealed that the most sensitive parameters on the threshold quantities \(R_H\) and \(R_r\) were the human recruitment rate \((\beta _h)\), the recruitment rate for rodents \((\varphi _r)\), the effective contact rate between susceptible and infectious humans \((\alpha _3)\) and the proportion of infected individuals who comply with treatment guidelines \((\eta )\). Based on this result, simulations were conducted to investigate the impact of the sensitive parameters on the dynamical spread of the disease in terms of the population of the infectious individuals. In general, the outcomes of this work indicate that any interventions that ensure strict compliance with Lassa fever treatment guidelines and at the same time, reduce the population of rodents, will revolutionize the eradication of Lassa virus approaches in the endemic regions. A good instance of such interventions is to make disregard for Lassa fever treatment guidelines an offense punishable under the law. Also, rodent population may be reduced by a means of sanitation as well as application of any of the available rodent killer such as traps and rodenticides.
Data availability
No data associated in the manuscript.
References
Abdulhami, A., Hussaini, N.: Effects of quarantine on transmission dynamics of Lassa fever. Bayero J. Pure Appl. Sci. 11(2), 397–407 (2018)
Abdullahi, I.N., Anka, A.U., Ghamba, Peter Elisha, P. E., Onukegbe, N. B., Amadu, D. O., Salami, M. O.: Need for preventive and control measures for Lassa fever through the One Health strategic approach. Proc. Singap. Healthc. 29(3), 190–193 (2020). https://doi.org/10.1177/20101058209326
Adewale, S.O., Olopade, I.A., Ajao, S.O., Adeniran, G.A., Oyedemi, O.T.: Mathematical analysis of Lassa fever model with isolation. Asian J. Nat. Appl. Sci. 5(3), 49–57 (2016)
Ajayi, N.A., Nwigwe, C.G., Azuogu, B.N., Onyire, B.N., Nwonwu, E.U., Ogbonnaya, L.U., Onwe, F.I., Ekaete, T., Gunther, S., Ukwaja, K.N.: Containing a Lassa fever epidemic in a resource-limited setting: outbreak description and lessons learned from Abakaliki, Nigeria (January - March 2012). Int. J. Infect. Dis. 17, 1011–1016 (2012)
Ayoade, A.A., Nyerere, N., Ibrahim, M.O.: An epidemic model for control and possible elimination of Lassa fever. Tamkang J. Math. (2023). https://doi.org/10.5556/j.tkjm.55.2024.5031
Akhmetzhanov, A.R., Asai, Y., Nishiura, H.: Quantifying the seasonal drivers of transmission for Lassa fever in Nigeria. Phil. Trans. R. Soc. B 374, 20180268 (2019)
Akinpelu, F.O., Akinwande, R.: Mathematical model for Lassa fever and sensitivity analysis. J. Sci. Eng. Res. 5(6), 1–9 (2018)
Yunus, A.O., Olayiwola, M.O., Omoloye, M.A., Oladapo, A.O.: A fractional order model of Lassa disease using the Laplace-Adomian Decomposition Method. Healthc. Anal. 3, 100167 (2023)
Branco, L.M., Grove, J.N., Geske, F.J.: Lassa virus-like particles displaying all major immuno-logical determinants as a vaccine candidate for Lassa hemorrhagic fever. Virol. J. 7, 279 (2019)
Collins, O.C., Okeke, J.E.: Analysis and control measures for Lassa fever model under socio-economic conditions. J. Phys. Conf. Ser. 1734, 012049 (2021). https://doi.org/10.1088/1742-6596/1734/1/012049
Tahmo, N.B., Wirsiy, F.S., Brett-Major, D.M.: Modeling the Lassa fever outbreak synchronously occurring with cholera and COVID- 19 outbreaks in Nigeria 2021: A threat to Global Health Security. PLOS Glob. Public Health 3(5), e0001814 (2023). https://doi.org/10.1371/journal.pgph.0001814
Favour, A.O., Anya, O.A.: Mathematical model for Lassa fever transmission and control. Math. Comput. Sci. 5(6), 110–118 (2020)
Iacono, G.L., Cunningham, A.A., Fichet-Calvet, E., Garry, R.F., Grant, D.S., Khan, S.H., Webb, C.T.: Using modeling to disentangle the relative contributions of zoonotic and anthroponotic transmission: the case of Lassa fever. PLoS Neglect. Trop. Dis. 9, e3398 (2015)
Ibrahim, M.A., Denes, A.: A mathematical model for Lassa fever transmission dynamics in seasonal environment with a view to the 2017–20 epidemic in Nigeria. Nonlinear Anal. Real World Appl. 60, 103310 (2021)
Idisi, O.I., Yusuf, T.T.: A mathematical model for Lassa fever transmission dynamics with impacts of control measures: analysis and simulation. Eur. J. Math. Stat. 2(2), 19–28 (2021)
Ilori, E.A., Furuse, Y., Ipadeola, O.B., Dan-Nwafor, C.C., Abubakar, A., Womi-Eteng, O.E., Ayodeji, O.: Epidemiologic and clinical features of Lassa fever outbreak in Nigeria, January 1-May 6, 2018. Emerg. Infect. Dis. 25, 1066–1074 (2018)
Lukashevich, I.S.: The search for animal models for Lassa fever vaccine development. Expert Rev. Vaccines 12, 71–86 (2013)
Lukashevich, I.S., Pushko, P.: Vaccine platforms to control Lassa fever. Expert Rev. Vaccines 15, 1135–1150 (2016)
Marien, J., Borremans, B., Kourouma, J., Baforday, J., Rieger, T., Gunther, S., Magassouba, N., Leirs, H., FichetCalvet, E.: Evaluation of rodent control to fight Lassa fever based on field data and mathematical modelling. Emerg. Microb. Infect. 8, 640–649 (2019)
Musa, S.S., Zhao, S., Gao, D., Lin, Q., Chowell, G., He, D.: Mechanistic modeling of the large-scale Lassa fever epidemics in Nigeria from 2016 to 2019. J. Theor. Biol. (2019). https://doi.org/10.1016/j.jtbi.2020.110209
Nwasuka, S.C., Nwachukwu, I.E., Nwachukwu, P.C.: Mathematical model of the transmission dynamics of Lassa fever with separation of infected individual and treatment as control measures. J Adv. Math. Comput. Sci. 32(6), 1–15 (2019)
Obabiyi, O.S., Onifade, A.A.: Mathematical model for Lassa fever transmission dynamics with variable human and reservoir population. Int. J. Differ. Equ. Appl. 16(1), 67–91 (2017)
Okoroiwu, H.U., Lopez-Munoz, F., Povedano-Montero, F.J.: Bibliometric analysis of global Lassa fever research (1970–2017): a 47-year study. BMC Infect. Dis. 18, 639–651 (2018)
Onah, I.S., Collins, O.C., Madueme, P.G.U., Mbah, G.C.E.: Dynamical system analysis and Optimal control measures of Lassa fever disease model. Int. J. Math. Math. Sci. 2020, 7923125 (2020). https://doi.org/10.1155/2020/7923125
Onuorah, M.O., Akinwande, N.I., Nasir, M.O., Ojo, M.S.: Sensitivity analysis of Lassa fever model. Int. J. Math. Stat. Stud. 4(1), 30–49 (2016)
Roberts, L.: Nigeria hit by unprecedented Lassa fever outbreak. Science 359, 1201–1202 (2018)
Sattler, R. A., SPaessler, S., Ly, H., Huang, C.: Animal models of Lassa fever. Pathogens 9, 197 (2020) https://doi.org/10.3390/pathogens9030197
Usman, S., Adamu, I.I.: Modelling the transmission dynamics of Lassa fever infection. Math. Theory Modell. 8(15), 42–63 (2018)
Warner, B.M., Safronetz, D., Stein, D.R.: Current research for a vaccine against Lassa hemorrhagic fever virus. Drug Des. Dev. Therapy 12, 2519–2527 (2018)
Zhao, S., Musa, S.S., Fu, H., He, D., Qin, J.: Large-scale Lassa fever outbreaks in Nigeria: quantifying the association between disease reproduction number and local rainfall. Epidemiol. Infect. 148, e4 (2020). https://doi.org/10.1017/S0950268819002267
Birkhorff, G., Rota, G.C.: Ordinary Differential Equations. Needham Heights, Ginn, Boston (1982)
Diekmann, O., Heesterbeek, J.A.P., Metz, J.A.J.: On the definition and computation of the basic reproduction ratio in models for infectious diseases in heterogeneous populations. J. Math. Biol. 28, 365–382 (1990)
Driessche, P.V.D., Wathmough, J.: Reproduction number and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180, 29–48 (2002)
Castillo-Chavez, C., Song, B.: Dynamical models of tuberculosis and their applications. Math. Biosci. Eng. 1, 361–404 (2004)
Lakshmikantham, V., Leela, S., Martynyuk, A.A.: Stability Analysis of Nonlinear Systems. Marcel Dekker Inc, New York and Bassel (1989)
Ayoade, A.A., Ibrahim, M.O.: Analysis of transmission dynamics and mitigation success of COVID-19 in Nigeria: An insight from a mathematical model. The Aligarh Bulletin of Mathematics 41(1), 81–106 (2022)
Adenuga, J.I., Ajide, K.B., Odeleye, A.T., Ayoade, A.A.: Abundant natural resources, ethnic diversity and inclusive growth in sub-Saharan Africa: a mathematical approach. Appl. Appl. Math. Int. J. (AAM) 16(2), 1221–1247 (2021)
Hugo, A., Simanjilo, E.: Analysis of an eco-epidemiological model under optimal control measures for infected prey. Appl. Appl. Math. Int. J. 14(1), 117–138 (2019)
LaSalle, J.P.: Stability theory for ordinary differential equations. J. Differ. Equ. 4, 57–65 (1968)
LaSalle, J.P., Lefschetz, S.: Stability by Liapunov’s Direct Method with Applications. Academic Press, New York (1961)
Ayoade, A.A., Ibrahim, M.O.: Modeling the dynamics and control of rabies in dog population within and around Lagos, Nigeria. Eur. Phys. J. Plus 138, 397 (2023)
Varrela, S.: Life expectancy at birth in Nigeria 2021, by genders. Available at https://www.statista.com/statistics/1122851/life-expectancy-in-nigeria-by-gender/ [Accessed 5 Feb 2021] (2021)
Ojo, M.M., Gbadamosi, B., Benson, T.O., Adebimpe, O., Georgina, A.L.: Modeling the dynamics of Lassa fever in Nigeria. J. Egypt. Math. Soc. 29, 1–19 (2021)
Nigeria Centre for Disease Control (NCDC). (2018). Nigeria Centre for Disease Control Handbook, Accessed July 29, 2020 from http://www.ncdc.gov.ng (2020)
Thota, S., Ayoade, A.A.: On dynamical analysis of prey-diseased predator model with refuge in prey. Appl. Math. Inf. Sci. 15(6), 717–721 (2021). https://doi.org/10.18576/amis/150605
Thota, S.: A three species ecological model with Holling Type-II functional response. Inf. Sci. Lett. 10(3), 439–444 (2021). https://doi.org/10.18576/isl/100307
Thota, S.: On an ecological model of mutualisim between two species with a mortal pVol. redator. Appl. Appl. Math. 15(2), 1309–1322 (2020)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Confict of interest
The authors declare no conflict of interest.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Ethical approval
No ethics approval was required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ayoade, A.A., Aliu, O. & Taiye, O. The effect of treatment compliance on the dynamics and control of Lassa fever: an insight from mathematical modeling. SeMA (2024). https://doi.org/10.1007/s40324-024-00353-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40324-024-00353-9