Abstract
Background
Studies investigating the effects of common recovery modalities following acute strenuous exercise have reported mixed results.
Objectives
This systematic review with meta-analysis and meta-regression compared the effects of cold-water immersion (CWI) against other common recovery modalities on recovery of athletic performance, perceptual outcomes, and creatine kinase (CK) following acute strenuous exercise in physically active populations.
Study Design
Systematic review, meta-analysis, and meta-regression.
Methods
The MEDLINE, SPORTDiscus, Scopus, Web of Science, Cochrane Library, EmCare, and Embase databases were searched up until September 2022. Studies were included if they were peer reviewed, published in English, included participants who were involved in sport or deemed physically active, compared CWI with other recovery modalities following an acute bout of strenuous exercise, and included measures of performance, perceptual measures of recovery, or CK.
Results
Twenty-eight studies were meta-analysed. CWI was superior to other recovery methods for recovering from muscle soreness, and similar to other methods for recovery of muscular power and flexibility. CWI was more effective than active recovery, contrast water therapy and warm-water immersion for most recovery outcomes. Air cryotherapy was significantly more effective than CWI for the promotion of recovery of muscular strength and the immediate recovery of muscular power (1-h post-exercise). Meta-regression revealed that water temperature and exposure duration were rarely exposure moderators.
Conclusion
CWI is effective for promoting recovery from acute strenuous exercise in physically active populations compared with other common recovery methods.
Protocol Registration
Open Science Framework: https://doi.org/10.17605/OSF.IO/NGP7C
Similar content being viewed by others
Avoid common mistakes on your manuscript.
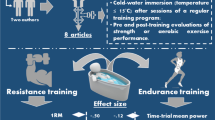
Cold-water immersion (CWI) was more effective than active recovery, contrast water therapy and warm-water immersion for most outcomes, including reducing muscle soreness and improving muscular power. |
Water temperature and exposure duration were rarely impactful effect moderators; however there was a dose–response effect of a lower temperature and shorter duration positively influencing the recovery of muscular power after CWI 24-h post-exercise when compared with active recovery. |
Air cryotherapy was more effective than CWI for immediately recovering muscular power (1-h post-exercise) and for recovering muscular strength. |
1 Introduction
High training and competition loads may induce acute physiological fatigue from which recovery is required to maximise athletic performance in training and competition [1]. As a result, numerous methods to accelerate recovery following training or exercise are commonly utilised with the aim of enhancing the effects of recovery to optimise future performance.
Common recovery methods include water immersion, cold air exposure, massage, and active recovery. Water immersion submerges the body (entire or partial) in cold water (8–20 °C, cold-water immersion [CWI]) [2,3,4,5], warm water (24–38 °C, warm-water immersion [WWI]) [3, 5, 6] or a combination of cold and warm temperatures (contrast water therapy [CWT]) [7,8,9] for durations ranging from 5 to 30 min [5, 10,11,12]. Cold air exposure (air cryotherapy) exposes athletes (either whole body or partial body) to air temperatures ranging from − 85 to − 140 °C for short durations (2.5–3 min) [2, 13,14,15,16,17]. Massage is manual manipulation of specific areas of the body using rubbing, stroking, and kneading techniques [18, 19]. Active recovery is the performance of low-intensity aerobic exercise following strenuous exercise [11, 20]. The mechanisms by which these recovery methods are proposed to accelerate recovery differ but are similarly associated with alterations in post-exercise swelling and oedema [21].
While there have been many reviews examining the effects of various recovery methods on a range of perceptual, physiological and performance outcomes, these reviews have typically only compared one recovery method with passive recovery (i.e., no specific recovery intervention) [22,23,24,25,26]. These reviews have also arbitrarily pooled crossover and parallel studies with no consideration for the statistical differences between study methodologies [22,23,24,25,26]. For example, many crossover studies only report mean values for each treatment group, with no consideration for within-participant differences; this oversight reduces the precision of the results [27]. In addition, reviews that have compared more than one recovery modality have typically only analysed one or two outcome variables (i.e., delayed-onset muscle soreness [DOMS] or physiological markers of muscle damage such as creatine kinase [CK]) [24, 28], and there have been limited comparisons of the effects of multiple recovery modalities on exercise performance [29,30,31]. Furthermore, the limited number of reviews that have compared multiple recovery modalities on subsequent exercise performance did not report heterogeneity or did not attempt to reduce heterogeneity through subgroup analysis [29,30,31]. These reviews also made recommendations based purely on the effect size of the outcome measure. However, when making recommendations in relation to efficacy of recovery protocols, the evidence presented should incorporate measures of heterogeneity, number of participants evaluated, and level of bias as recommended by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method for grading evidence quality and strength of recommendations [32]. This level of scientific rigour has not been applied in previous reviews.
CWI is one of the most commonly used recovery methods by physically active individuals [21]. Therefore, this review applied GRADE criteria to compare the effects of CWI with other commonly used recovery methods on perceptual, physiological and exercise performance outcomes following strenuous exercise in physically active participants. Additionally, this review compared the time course of recovery and evaluated dose–response effects. Identifying protocols that aid recovery following strenuous exercise will inform appropriate prescription of recovery modalities for physically active individuals.
2 Methods
2.1 Design
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for the reporting of systematic reviews and meta-analyses [33]. This review was prospectively registered with Open Science Framework (10.17605/OSF.IO/NGP7C). No amendments to the protocol occurred after registration, and no protocol was prepared.
2.2 Search Strategy and Selection Criteria
The MEDLINE, SPORTDiscus, Scopus, Web of Science, Cochrane Library, EmCare, and Embase databases were searched from inception until 12 September 2022 using the following search strategy, which was adapted for each database:
athlet* or sport* or exerci* or football* or soccer or hockey or basketball* or netball* or volleyball* or "track and field" or cycli* or running or runner* or swim* or handball or softball* or tennis or baseball or cross country or cricket or surf* or skiing or golf or hurdling or bicycling or boxing or gymnast* or martial arts or racquet sports or badminton or jogg* or walk* or weight lifting or lift* weights or weight?lift* or wrestling or resistance train* or endurance train* or interval train* or climb* or strength* train* or strength* program and (cold* or ice* or low* temp*) adj3 (bath* or hydrotherap* or immers* or submers* or submerg*)
Database search results were exported to Endnote© (version 20; Thomson-Reuters, Toronto, CA, USA) and then uploaded to Covidence© Systematic Review software (Veritas Health Innovations, Melbourne, VIC, Australia). All duplicates were removed before two reviewers independently screened titles and abstracts for eligibility (EM, SS). Full texts were obtained for the remaining articles and independently assessed for eligibility by two reviewers (EM, SS). Results from each reviewer were compared after each stage and any discrepancies were resolved by an independent reviewer (JB). Reference lists of all eligible studies and any previous systematic reviews were checked to identify any additional eligible studies that were not identified by the primary search.
Inclusion criteria were (1) peer-reviewed randomised controlled trials published in the English language; (2) participants were competing at any level of sporting competition or deemed physically active; (3) protocols that used CWI following an acute bout of strenuous exercise (defined by the authors as exercise that would induce muscle damage) with further immersions permitted to be completed on subsequent days; (4) used varying recovery modalities as the comparator intervention; and (5) outcome measures included recovery of exercise performance (flexibility, muscular strength [including maximal voluntary contractions or 1RM testing], muscular power [including jump performance, anaerobic power performance of < 10 s or sprint performance]) or physiological (CK) and perceptual markers of recovery (DOMS, perceived recovery). Studies were excluded if they used combined treatments that may confound CWI results (e.g., combining CWI with compression garments, CWI with active recovery, CWI with nutritional supplements), or utilised training interventions involving more than one session of exercise. Data published as theses or conference abstracts were excluded.
2.3 Risk of Bias
An assessment of methodological quality for the selected studies was undertaken using the Randomised Controlled Trial (RCT) checklist from the Scottish Intercollegiate Guidelines Network (SIGN) [34]. The SIGN RCT checklist was developed to ensure a balance between methodological quality and practicality of use for authors and was used in this review as it is specific to the design of the studies included. Before commencing assessment, definitions provided by SIGN were clarified by the review team. Two reviewers appraised each study based on these appraisal definitions (EM, SS). Any discrepancies were resolved by an independent reviewer (JDB). A grade of ‘yes’, ‘no’, ‘can’t say’ or ‘not applicable’ was issued for each appraisal item. ‘Yes’ and ‘not applicable’ answers were indicative of a lower risk of bias, therefore the total frequency of ‘yes’ and ‘not applicable’ was tallied to indicate overall methodological quality. Quality of the studies was labelled as ‘high quality’, ‘acceptable’, ‘low quality’ or ‘unacceptable’ [34].
2.4 Data Extraction
Data were extracted by one reviewer (EM) and entered in a standardised Microsoft Excel© spreadsheet (V2105, Microsoft Corporation, Redmond, WA, USA). These data were independently cross-checked by another reviewer (SS) and any discrepancies were resolved through discussion. Further information was sought from study authors if all information could not be obtained from the full-text article. The extracted information included publication details (author information, publication date, country of origin), study methodology (sample size, exercise intervention, study type, assessment measures, comparison intervention), participant information (age, sex, height, body mass, sport, training history), CWI protocol (temperature, duration, number of immersions, depth of immersion, body position during immersion, timing of immersion post-exercise), comparator recovery protocol (recovery method, type [if applicable], intensity [if applicable], temperature [if applicable], duration, body position during protocol [if applicable], multiple applications [if applicable]), and assessment measures (test, units, measurements at various timepoints, effect sizes, confidence intervals [CIs], p values).
2.5 Statistical Considerations
Standardised mean difference (SMD) with Hedges' g correction for positive bias was used to determine the effect sizes for comparing the effect of recovery modalities and facilitating data synthesis. For the purpose of this review, effect sizes were presented for each study and were considered trivial (SMD < 0.20), small (SMD 0.20–0.60), moderate (SMD 0.61–1.20), large (SMD 1.21–2.00) and very large (SMD > 2.00) [35]. Effect size precision was described using 95% CIs whenever sufficient information was provided by the study authors.
The metafor statistical package in R software (version 4.1.0, R Foundation for Statistical Computing, Vienna, Austria) was used to perform random-effects meta-analysis and meta-regression. Restricted maximum likelihood estimation was used for model fitting and the inverse variance method was used to weight the study effects. Separate analyses were performed for each recovery timepoint (1 h, 24 h, 48 h, 72 h, 96 h). The primary comparison was CWI compared with all other recovery methods. Subgroup analyses were undertaken for CWI compared with each specific type of other recovery method where possible. Water temperature and exposure duration were explored as potential continuous moderator variables. A unique identification number was assigned to each study and included as a random factor in the meta-analysis. Outcomes from studies that reported multiple CWI versus other recovery methods comparisons were assigned to the same study identification number due to the lack of independence of those observations.
Crossover studies were combined with parallel studies using the approach described by Elbourne and colleagues [27]. This approach required crossover studies to report a CI, standard error, or p value from a paired t test in addition to mean treatment effects or mean and standard deviation for each condition. Crossover studies that did not provide this information were still included if values could be estimated using information available from other included studies that considered the same outcomes and comparison conditions, as described by Elbourne and colleagues [27]. The most conservative estimate was used in all cases where estimation was required.
I2 statistics were used to explore statistical heterogeneity within each meta-analysis and indicated the consistency of effect sizes between the included studies [36]. Statistical heterogeneity was considered low (I2 < 25%), moderate (I2 = 25–49%) or high (I2 > 50%) [36]. The GRADE system was used to rate the overall quality of evidence synthesis as high, moderate, low, or very low [32]. Specifically, the quality rating was downgraded one level from high for each of the following limitations: total number of unique participants < 100 (imprecision), high statistical heterogeneity and more than 50% of the studies in the meta-analysis deemed to be low quality.
3 Results
3.1 Search Results
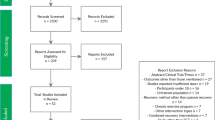
The database searches identified 6255 potential studies. Following the removal of duplicates and ineligible articles, 28 studies were included in the pooled meta-analyses, while 26 were included in the stratified meta-analyses. Two studies were included in the pooled meta-analysis only, as there were insufficient comparators in their recovery or outcome subgroup to be included in the stratified meta-analyses [37, 38]. Eleven studies were unable to be included in either meta-analysis due to a lack of comparators [39,40,41,42,43,44,45,46,47,48,49]. A complete overview of articles included in the review can be found in Table 1. A complete overview of the screening process can be found in Fig. 1.
3.2 Risk of Bias
Only one (3%) study was classified as being of high quality, 24 (86%) were classified as being of acceptable quality, and three (11%) studies were classified as being of low quality. The most common issues identified from the risk-of-bias analysis was that concealment of the treatment from the researchers was rarely completed, with only one study concealing treatment [51]. Randomisation of treatment groups was also poor for most studies, with only two studies adequately randomising participants [15, 51]. Individual results of the risk of bias separated by category can be found in Online Supplement 1.
3.3 Meta-Analysis of All Recovery Methods Compared with Cold-Water Immersion (CWI)
3.3.1 Pooled Effects on DOMS
CWI had a limited effect on the recovery of DOMS compared with the other recovery methods. At 1 h, there was a non-significant trivial effect in favour of CWI (GRADE = high) (Table 2). At 24 h (Fig. 2) and 48 h, there were small significant effects in favour of CWI (24 h GRADE = moderate; 48 h GRADE = high) (Table 2). At 72 h and 96 h, there were small and trivial non-significant effects, respectively, in favour of other recovery methods (GRADE = moderate) (Table 2).
Forest plot illustrating the influence of CWI compared to other recovery methods 24 hours post exercise on muscle soreness (stratified by recovery method). CI confidence interval, CWI cold water immersion, deg degrees, Ecc eccentric, Ex Mode exercise modality, HIT high intensity training, min minutes, SMD standardised mean difference
3.3.2 Pooled Effects on Muscular Power
CWI had no effect on the recovery of power performance compared with other recovery methods. At 1 h and 24 h (Fig. 3), there were small and trivial non-significant effects, respectively, in favour of CWI (GRADE = moderate) (Table 2); at 48 h, there was a non-significant trivial effect that did not favour any recovery method (GRADE = moderate) (Table 2); and at 72 h, there was a trivial non-significant effect in favour of other recovery methods (GRADE = low) (Table 2).
Forest plot illustrating the influence of CWI compared to other recovery methods 24 hours post exercise on muscular power (stratified by recovery method). CI confidence interval, CWI cold water immersion, deg degrees, Ecc eccentric, Ex Mode exercise modality, HIT high intensity training, LED light emitting diode, min minutes, SMD standardised mean difference
3.3.3 Pooled Effects on Strength
CWI had no effect on the recovery of strength compared with other recovery methods. There were small to large non-significant effects in favour of other recovery methods at all timepoints (1 h, 24 h, 48 h, GRADE = moderate; 72 h, GRADE = low) (Table 2, Fig. 4).
Forest plot illustrating the influence of CWI compared to other recovery methods 24 hours post exercise on muscular strength (stratified by recovery method). CI confidence interval, CWI cold water immersion, deg degrees, Ecc eccentric, Ex Mode exercise modality, min minutes, SMD standardised mean difference
3.3.4 Pooled Effects on Perceived Recovery
CWI had no effect on perceptions of recovery compared with other recovery methods. At 24 h, there was a trivial non-significant effect in favour of CWI (GRADE = high) (Table 2, Fig. 5). At 48 h, there was a non-significant null effect that did not favour any recovery method (GRADE = moderate) (Table 2).
Forest plot illustrating the influence of CWI compared to other recovery methods 24 hours post exercise on perceived recovery (stratified by recovery method). CI confidence interval, CWI cold water immersion, deg degrees, Ecc eccentric, Ex Mode exercise modality, min minutes, SMD standardised mean difference
3.3.5 Pooled Effects on Flexibility
CWI had no effect on the recovery of flexibility compared with other recovery methods. There was moderate to very large non-significant effects in favour of CWI at all timepoints (GRADE = low) (Table 2, Fig. 6).
Forest plot illustrating the influence of CWI compared to other recovery methods 24 hours post exercise on flexibility (stratified by recovery method). CI confidence interval, CWI cold water immersion, deg degrees Ecc eccentric, Ex Mode exercise modality, HIT high intensity training, min minutes, SMD standardised mean difference
3.3.6 Pooled Effects on Creatine Kinase
CWI had a limited effect on reducing CK levels in the blood compared with other recovery methods. At 1 h, there was a small non-significant effect in favour of other methods (GRADE = low) (Table 2). At 24 h, there was a small effect with a minor degree of uncertainty in favour of CWI, as indicated by the 95% CI (effect size = − 0.58 (− 1.17, 0.01); p = 0.06; GRADE = high) (Table 2, Fig. 7) and a small non-significant effect in favour of CWI at 48 h (GRADE = moderate) (Table 2).
Forest plot illustrating the influence of CWI compared to other recovery methods 24 hours post exercise on creatine kinase (stratified by recovery method). CI confidence interval, CWI cold water immersion, deg degrees, Ecc eccentric, Ex Mode exercise modality, LED light emitting diode, min minutes, SMD standardised mean difference
3.3.7 Meta-Regression Outcome
The meta-regression run in conjunction with the pooled meta-analysis found that water temperature and exposure duration did not significantly moderate effects at any timepoint for any outcome measure.
3.4 Meta-Analysis Stratified by Recovery Intervention
3.4.1 CWI Compared with Active Recovery
CWI had limited effect on DOMS compared with active recovery. At 1 h, there was a small significant effect in favour of CWI (GRADE = moderate) (Table 3), and at 24 h (Fig. 2) and 48 h, there were trivial non-significant effects in favour of CWI (GRADE at both timepoints = moderate) (Table 3). Water temperature and exposure duration did not significantly moderate effects at any timepoint.
CWI had no effect on promoting recovery of power performance compared with active recovery. At 24 h, there was a trivial non-significant effect in favour of CWI (GRADE = low) (Fig. 3, Table 3). There were significant moderating effects at 24 h of both water temperature and exposure duration, whereby for every 1-min increase in duration, the effect size decreased by 0.07 (− 0.12, − 0.01; p = 0.01) and for every 1° increase in temperature, the effect size decreased by 0.06 (− 0.10, − 0.01; p = 0.007). At 48 h, there was a trivial non-significant effect in favour of active recovery (GRADE = moderate) (Table 3).
CWI had no effect on feelings of perceived recovery compared with active recovery. At 24 h, there was a trivial non-significant effect in favour of CWI (GRADE = moderate) (Fig. 5, Table 3). Water temperature and exposure duration did not significantly moderate effects at any timepoint.
3.4.2 CWI Compared with Contrast Water Therapy
CWI had limited effect on DOMS compared with CWT. At 1 h and 24 h (Fig. 2), there were trivial to small non-significant effects in favour of CWI (GRADE at both timepoints = moderate) (Table 3), and at 48 h, there was a small significant effect in favour of CWI (GRADE = low) (Table 3). Water temperature and exposure duration did not significantly moderate effects at any timepoint.
CWI had no effect on promoting recovery of power performance compared with CWT. At 1 h, 24 h (Fig. 3) and 48 h, there were moderate non-significant effects in favour of CWI (1 h GRADE = low; GRADE for 24 h and 48 h timepoints = moderate) (Table 3). Water temperature and exposure duration did not significantly moderate effects at any timepoint.
CWI had no effect on promoting recovery of flexibility compared with CWT. At 1 h, 24 h (Fig. 6) and 48 h, there were moderate to very large non-significant effects in favour of CWI (GRADE for all timepoints = low) (Table 3). Water temperature and exposure duration did not significantly moderate effects at any timepoint.
3.4.3 CWI Compared with Warm-Water Immersion
CWI had no effect on DOMS compared with WWI. At 1 h, 24 h (Fig. 2) and 48 h, there were trivial to moderate non-significant effects in favour of CWI (1 h GRADE = moderate; 24 h and 48 h GRADE = low) (Table 3). Water temperature and exposure duration did not significantly moderate effects at any timepoint.
CWI had no effect on promoting recovery of power performance compared with WWI. At 24 h, there was a small non-significant effect in favour of WWI (GRADE = low) (Fig. 3, Table 3), and at 48 h, there was a trivial non-significant effect in favour of CWI (GRADE = low) (Table 3). Water temperature and exposure duration did not significantly moderate effects at either timepoint.
CWI had no effect on promoting recovery of strength performance compared with WWI. At 1 h, there was a small non-significant effect in favour of WWI (GRADE = low) (Table 3), and at 24 h (Fig. 4) and 48 h, there were small to trivial non-significant effects in favour of CWI (GRADE = low) (Table 3). Water temperature and exposure duration did not significantly moderate effects at any timepoint.
CWI had no effect on reducing CK concentration compared with WWI. At 1 h, there was a small non-significant effect in favour of WWI (GRADE = low) (Table 3). Water temperature and exposure duration did not significantly moderate effects.
3.4.4 CWI Compared with Air Cryotherapy
CWI had no effect on DOMS compared with air cryotherapy. At 24 h, there was a trivial non-significant effect in favour of CWI (GRADE = high) (Fig. 2, Table 3), and at 48 h and 72 h, there were trivial to small non-significant effects in favour of air cryotherapy (48 h GRADE = moderate; 72 h GRADE = low) (Table 3). Water temperature and exposure duration did not significantly moderate effects at any timepoint.
CWI had no effect on promoting recovery of power performance compared with air cryotherapy. At 1 h, there was a moderate significant effect in favour of air cryotherapy (GRADE = moderate) (Table 3). At 24 h (Fig. 3), 48 h and 72 h, there were trivial non-significant effects in favour of CWI (GRADE for 24 h and 48 h = moderate; GRADE for 72 h = low) (Table 3). Water temperature and exposure duration did not significantly moderate effects at any timepoint.
CWI had no effect on promoting recovery of strength performance compared with air cryotherapy. At 1 h, 24 h (Fig. 4) and 48 h, there were large to very large non-significant effects in favour of air cryotherapy (GRADE at all time points = low) (Table 3). Water temperature and exposure duration did not significantly moderate effects at any timepoint.
3.4.5 CWI Compared with Massage
CWI had no effect on promoting recovery of power performance compared with massage. At 24 h, there was a trivial non-significant effect in favour of CWI (GRADE = low) (Fig. 3, Table 3). Water temperature and exposure duration did not significantly moderate effects.
4 Discussion
The aim of this review was to examine the efficacy of CWI for promoting recovery of performance, perceptual and physiological outcomes compared with commonly used recovery modalities following strenuous exercise. A second aim was to evaluate dose–response effects of water temperature and/or duration of exposure during CWI. The majority of findings favoured CWI compared with other recovery modalities, but few results reached statistical significance. CWI was more effective than other recovery modalities for improving DOMS at 1 h, 24 h and 48 h post-exercise. There was a dose–response effect of a lower temperature and shorter duration positively influencing the recovery of muscular power after CWI 24 h post-exercise when compared with active recovery, with shorter and colder exposures facilitating greater recovery. However, air cryotherapy was more effective than CWI in the recovery of muscular power performance 1 h post-exercise.
This is the first review to compare CWI with other recovery modalities and their effects on physiological, perceptual, and physical performance measures at specific time points following differing exercise interventions in physically active populations. Despite a more specific search strategy than previous reviews, this review identified a greater number of studies for inclusion in the analysis. This is also the first review to use meta-regression to evaluate dose–response effects of water temperature and/or exposure durations on outcome measures. Furthermore, this is the first review to account for methodological variations within parallel and crossover study designs to increase the precision of the results reported.
4.1 CWI as a Recovery Method
Pooled effects comparing CWI with all other recovery methods examined showed that CWI was as effective as, and sometimes superior to, other recovery methods for the recovery of many performance outcomes (Fig. 8). Coaches and athletes should strongly consider its use as part of their recovery process during competitive phases. However, it should be acknowledged that CWI may blunt training adaptations during preparation phases, particularly for resistance-based training programmes due to attenuated changes in the muscle [1, 57].
Strength outcomes showed that there was no difference between CWI and other recovery methods. This finding is consistent with a previous meta-analysis comparing CWI with passive recovery, which found that CWI was ineffective for the recovery of strength performance [58]. Recovery methods that promote cooling may not be effective for the recovery of strength, as cooling the neuromuscular system may inhibit isometric strength (a measure most studies in this review used to indicate strength performance). However, dynamic power and strength performance (eccentric and concentric movements) are improved due to reduced neuromuscular fatigue [59].
CWI was more effective at reducing DOMS 24 and 48 h post-exercise compared with other methods. This may be due to the hydrostatic pressure of the water reducing swelling and inflammation, and colder temperatures having an analgesic effect [1, 21]. CWI was potentially more effective at reducing CK concentrations 24 h post-exercise when compared with other recovery modalities. Vasoconstriction of the blood vessels induced by the colder temperatures of CWI may contribute to this accelerated clearance [60]. The cooler temperatures also slow the delivery of inflammatory markers, which may reduce secondary tissue damage and lower inflammation [61].
4.2 Stratified Effects of CWI Compared with Other Recovery Methods
Low GRADE scores across many of the subgroup analyses indicate the limited amount of evidence available to substantiate results with a combination of low study numbers and subsequently low participant numbers, as well as high heterogeneity. An overview of the stratified results can be found in Fig. 8. More research is needed to be able to confirm the effects of each recovery method. Another consideration for recovery studies is the effect that belief in the method may have on the outcome measures. To account for placebo effects, researchers should include an additional group that receives a placebo condition that participants are led to believe is as effective as the intervention groups. Belief could also be quantified and used as a randomisation factor in parallel studies or as a covariate in crossover studies. The placebo effect and belief of recovery effects have been demonstrated successfully where participants receiving a sham recovery method recovered better than participants receiving accepted recovery methods [12].
4.2.1 The Effects of CWI Compared with Active Recovery
Active recovery is thought to accelerate the body’s return to homeostasis following strenuous exercise through the enhanced removal of blood lactate, restoring muscular energy supplies and reducing the severity of muscular injury and soreness [62]. However, CWI was found to be more effective at reducing DOMS 1 h post-exercise compared with active recovery. This could be due to the hydrostatic pressure during water immersion reducing swelling in the periphery, which may in turn reduce muscular pain. The analgesic effect of the cold temperature also lowers the activation threshold of tissue nociceptors and slows the conduction of nerve pain signals [63].
CWI had a limited effect on muscular power. However, meta-regression showed that decreasing the temperature and duration of CWI exposure was associated with greater recovery of muscular power compared with active recovery. This may be attributable to colder water temperatures reducing musculo-tendinous stiffness, which promotes performance of movements that utilise the stretch–shortening cycle [64].
4.2.2 CWI Compared with Contrast Water Therapy
CWT has been proposed to positively influence recovery due to the ‘pumping’ mechanism created by alternating between cold (promoting vasoconstriction) and hot (promoting vasodilation) temperatures [65,66,67]. The pumping mechanism may assist in the reduction of oedema, spasm and inflammation, as well as improve range of motion [65,66,67]. However, the present pooled recovery modality results for both muscular power and flexibility showed moderate to large effects across multiple time points in favour of CWI. These findings suggest that constant cold temperatures may be more effective at influencing recovery of performance than intermittent cold and hot temperatures, although these effects were not statistically significant.
For flexibility (only two to three studies included in each analysis), the 95% CIs were extremely wide, indicating that more RCTs comparing CWI and CWT are required. Muscular power analyses had more studies included (5–7 studies) and produced narrower 95% CIs with less overlap in favour of CWT. More studies would allow for the consideration of the moderating variables to indicate whether CWI is truly more effective than CWT.
CWI was also found to better influence recovery from muscle soreness over multiple timepoints compared with CWT, indicating that constant cold temperatures are more effective at reducing the oedema and inflammation that causes pain [21] than intermittent cold and hot temperatures [67].
4.2.3 CWI Compared with Warm-Water Immersion
WWI has been found to increase blood flow to the deep muscles (through vasodilation), which improves oxygen flow to these areas and may promote healing of the tissues post-exercise [68].
CWI was found to be more effective in reducing the effects of DOMS compared with WWI. The analgesic effect of the cold temperatures is likely to contribute to this; however it might also be attributable to a placebo effect due to athletes believing CWI will be more likely to improve recovery [69]. The null effects of the recovery of strength and power performance are in line with previous research where neither cold nor warm temperatures were more effective at recovering physical performance [70, 71]. The results of the present analysis suggested that WWI may be more effective at removing CK from the blood 1 h post-exercise compared with CWI. The warmer temperatures and vasodilation of the vessels may be more effective at clearing the metabolic byproducts than colder temperatures and vasoconstriction.
4.2.4 CWI Compared with Air Cryotherapy
Air cryotherapy was found to be more effective than CWI in some areas of performance recovery, but CWI was more effective for the recovery of DOMS within the first 24 h post-exercise. This suggests that the hydrostatic effect of CWI may be more important for recovery of DOMS than the impact of cold temperature.
Air cryotherapy is conducted at extreme temperatures (− 85 to − 110 °C) for short time periods (2.5–3 min exposure) and showed non-significant moderate to very large effects on the recovery of both strength and power. It is possible that the extreme cold conditions are beneficial to muscular performance; however previous studies have found that air cryotherapy increases muscular stiffness as well as stiffness in the connective tissues, which leads to increased risk of muscle damage [72] and decreased strength performance [64]. Therefore, caution should be used when considering the application of air cryotherapy for recovery of strength and power.
4.2.5 CWI Compared with Massage
CWI and massage as recovery methods have rarely been compared using RCT study designs despite a large amount of anecdotal evidence involving massage in particular [73]. CWI was found to have a trivial effect on power recovery; however evidence was graded as low due to high heterogeneity and low study numbers. Massage is believed to have the same benefits to athletes that CWI has, and includes reducing swelling and pain in the muscle, as well as enhancing the clearance of metabolic byproducts [74]. However, despite the similar benefits, the mechanism of each modality differs. Massage relies on biomechanical mechanisms where pressure exerted on the tissues reduces passive and active stiffness and increases range of motion, which positively influences athletic performance [75]. Massage also increases blood flow to the skin and muscles and increases the release of relaxation hormones, which assists with decreasing pain and perceived fatigue [29, 75]. CWI uses the analgesic effects of the cold temperature to decrease pain [63], but the cold temperature also reduces musculo-tendinous stiffness, which positively influences athletic performance [64].
4.3 Limitations and Future Research
This review was influenced by some limitations. First, the low study numbers (with small sample sizes) and high heterogeneity has led to several low GRADE scores. Second, some studies were unable to be included in the meta-analyses due to differences in study designs, without adequate reporting of data that would allow pooling across designs. Finally, low study numbers also impacted the ability to distinguish between eccentric exercise recovery and high-intensity exercise recovery, and therefore specific recovery recommendations were unable to be discerned using these data. To improve this, more high-quality research (with larger sample sizes) is required comparing recovery modalities on athlete recovery.
5 Conclusion
As a recovery method, CWI is as effective as other recovery modalities for recovery following strenuous exercise in physically active individuals. CWI was more effective than active recovery, CWT and WWI for most outcomes, including reducing DOMS and improving muscular power. Air cryotherapy was more effective than CWI for immediately recovering muscular power (1 h post-exercise) and for recovering muscular strength.
References
Mujika I, Halson S, Burke LM, Balagué G, Farrow D. An integrated, multifactorial approach to periodization for optimal performance in individual and team sports. Int J Sports Physiol Perform. 2018;13(5):538–61.
Wilson LJ, Cockburn E, Paice K, Sinclair S, Faki T, Hills FA, et al. Recovery following a marathon: a comparison of cold water immersion, whole body cryotherapy and a placebo control. Eur J Appl Physiol. 2018;118(1):153–63.
Bouzid MA, Ghattassi K, Daab W, Zarzissi S, Bouchiba M, Masmoudi L, et al. Faster physical performance recovery with cold water immersion is not related to lower muscle damage level in professional soccer players. J Therm Biol. 2018;78:184–91.
Crowther F, Sealey R, Crowe M, Edwards A, Halson S. Influence of recovery strategies upon performance and perceptions following fatiguing exercise: a randomized controlled trial. BMC Sports Sci Med Rehab. 2017;9(1):25–33.
Hassan E. Thermal therapy and delayed onset muscle soreness. J Sports Med Phys Fit. 2011;51(2):249–54.
Ahokas EK, Ihalainen JK, Kyröläinen H, Mero AA. Effects of water immersion methods on postexercise recovery of physical and mental performance. J Strength Cond Res. 2019;33(6):1488–95.
Elias GP, Varley MC, Wyckelsma VL, McKenna MJ, Minahan CL, Aughey RJ. Effects of water immersion on posttraining recovery in Australian footballers. Int J Sports Physiol Perform. 2012;7(4):357–66.
Higgins TR, Climstein M, Cameron M. Evaluation of hydrotherapy, using passive tests and power tests, for recovery across a cyclic week of competitive rugby union. J Strength Cond Res. 2013;27(4):954–65.
Argus CK, Broatch JR, Petersen AC, Polman R, Bishop DJ, Halson S. Cold-water immersion and contrast water therapy: no improvement of short-term recovery after resistance training. Int J Sports Physiol Perform. 2017;12(7):886–92.
Ingram J, Dawson B, Goodman C, Wallman K, Beilby J. Effect of water immersion methods on post-exercise recovery from simulated team sport exercise. J Sci Med Sport. 2009;12(3):417–21.
Webb PN, Harris KN, Cronin BJ, Walker BC. The relative efficacy of three recovery modalities after professional rugby league matches. J Strength Cond Res. 2013;27(9):2449–55.
Broatch JR, Petersen A, Bishop DJ. Postexercise cold water immersion benefits are not greater than the placebo effect. Med Sci Sports Exerc. 2014;46(11):2139–47.
Hohenauer E, Costello J, Stoop R, Küng U, Clarys P, Deliens T, et al. Cold-water or partial-body cryotherapy? Comparison of physiological responses and recovery following muscle damage. Scand J Med Sci Sports. 2017;28(3):1252–62.
Hohenauer E, Costello JT, Deliens T, Clarys P, Stoop R, Clijsen R. Partial-body cryotherapy (− 135 °C) and cold-water immersion (10 °C) after muscle damage in females. Scand J Med Sci Sports. 2019;30(3):485–95.
Abaïdia A-E, Lamblin J, Delecroix B, Leduc C, McCall A, Nédélec M, et al. Recovery from exercise-induced muscle damage: cold-water immersion versus whole-body cryotherapy. Int J Sports Physiol Perform. 2017;12(3):402–9.
Rose CL, Caillaud C, Edwards KM, Siegler J, Graham K. Does whole body cryotherapy improve muscle recovery after damaging eccentric exercise? J Aust Strength Cond. 2014;22(5):48–51.
Wilson LJ, Dimitriou L, Hills FA, Gondek MB, Cockburn E. Whole body cryotherapy, cold water immersion, or a placebo following resistance exercise: a case of mind over matter? Eur J Appl Physiol. 2019;119(1):135–47.
Delextrat A, Calleja-González J, Hippocrate A, Clarke ND. Effects of sports massage and intermittent cold-water immersion on recovery from matches by basketball players. J Sports Sci. 2013;31(1):11–9.
Wiewelhove T, Schneider C, Döweling A, Hanakam F, Rasche C, Meyer T, et al. Effects of different recovery strategies following a half-marathon on fatigue markers in recreational runners. PLoS ONE. 2018;13(11):1–18.
Jones B, Lander J, Brubaker D. The effects of different recovery interventions following a repeated rugby union (sevens) game simulated protocol. J Aust Strength Cond. 2013;21(4):5–13.
Bleakley CM, Davison GW. What is the biochemical and physiological rationale for using cold-water immersion in sports recovery? A systematic review. Br J Sports Med. 2010;44(3):179–87.
Davis HL, Alabed S, Chico TJA. Effect of sports massage on performance and recovery: a systematic review and meta-analysis. BMJ Open Sport Exerc Med. 2020;6(1):1–9.
Leeder J, Gissane C, van Someren K, Gregson W, Howatson G. Cold water immersion and recovery from strenuous exercise: a meta-analysis. Br J Sports Med. 2011;46(4):233–40.
Hohenauer E, Taeymans J, Baeyens J-P, Clarys P, Clijsen R. The effect of post-exercise cryotherapy on recovery characteristics: a systematic review and meta-analysis. PLoS ONE. 2015;10(9):1–22.
Machado AF, Ferreira PH, Micheletti JK, de Almeida AC, Lemes ÍR, Vanderlei FM, et al. Can water temperature and immersion time influence the effect of cold water immersion on muscle soreness? A systematic review and meta-analysis. Sports Med. 2016;46(4):503–14.
Poppendieck W, Wegmann M, Ferrauti A, Kellmann M, Pfeiffer M, Meyer T. Massage and performance recovery: a meta-analytical review. Sports Med. 2016;46(2):183–204.
Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31(1):140–9.
Sánchez-Ureña B, Barrantes-Brais K, Ureña-Bonilla P, Calleja-González J, Ostojic S. Effect of water immersion on recovery from fatigue: a meta-analysis. Eur J Hum Mov. 2015;34:1–14.
Dupuy O, Douzi W, Theurot D, Bosquet L, Dugué B. An evidence-based approach for choosing post-exercise recovery techniques to reduce markers of muscle damage, soreness, fatigue and inflammation: a systematic review with meta-analysis. Front Physiol. 2018;9:1–15.
Higgins TR, Greene DA, Baker MK. Effects of cold water immersion and contrast water therapy for recovery from team sport: a systematic review and meta-analysis. J Strength Cond Res. 2017;31(5):1443–60.
Bieuzen F, Bleakley CM, Costello JT. Contrast water therapy and exercise induced muscle damage: a systematic review and meta-analysis. PLoS ONE. 2013;8(4):1–15.
GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490–7.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:71–9.
Scottish Intercollegiate Guidelines Network. Methodology checklist 2: randomised controlled trials. 2012 [cited 9 Nov 2020]. https://www.sign.ac.uk/media/1713/checklist_for_controlled_trials.doc. Accessed 9 Nov 2020
Hopkins W, Marshall S, Batterham A, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):3–12.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–60.
Adamczyk JG, Krasowska I, Boguszewski D, Reaburn P. The use of thermal imaging to assess the effectiveness of ice massage and cold-water immersion as methods for supporting post-exercise recovery. J Therm Biol. 2016;60:20–5.
Jajtner AR, Hoffman JR, Gonzalez AM, Worts PR, Fragala MS, Stout JR. Comparison of the effects of electrical stimulation and cold-water immersion on muscle soreness after resistance exercise. J Sport Rehab. 2015;24(2):99–108.
Chow G, Chung J, Fong S. Differential effects of post-exercise ice water immersion and room temperature water immersion on muscular performance, vertical jump, and agility in amateur rugby players: A randomized controlled trial. Sci Sports. 2018;33(6):271–9.
Roberts LA, Muthalib M, Stanley J, Lichtwark G, Nosaka K, Coombes JS, et al. Effects of cold water immersion and active recovery on hemodynamics and recovery of muscle strength following resistance exercise. Am J Physiol Regul Integr Comp Physiol. 2015;309(4):389–98.
Crowther FA, Sealey RM, Crowe MJ, Edwards AM, Halson SL. Effects of various recovery strategies on repeated bouts of simulated intermittent activity. J Strength Cond Res. 2019;33(7):1781–94.
Lane KN, Wenger H. Effect of selected recovery conditions on performance of repeated bouts of intermittent cycling separated by 24 hours. J Strength Cond Res. 2004;18(4):855–60.
Crampton D, Donne B, Warmington SA, Egaña M. Cycling time to failure is better maintained by cold than contrast or thermoneutral lower-body water immersion in normothermia. Eur J Appl Physiol. 2013;113(12):3059–67.
de Freitas VH, Ramos SP, Bara-Filho MG, Freitas DG, Coimbra DR, Cecchini R, et al. Effect of cold water immersion performed on successive days on physical performance, muscle damage, and inflammatory, hormonal, and oxidative stress markers in volleyball players. J Strength Cond Res. 2019;33(2):502–13.
Vaile J, Halson S, Gill N, Dawson B. Effect of hydrotherapy on recovery from fatigue. Int J Sports Med. 2008;29(7):539–44.
Bouchiba M, Bragazzi NL, Zarzissi S, Turki M, Zghal F, Grati MA, et al. Cold water immersion improves the recovery of both central and peripheral fatigue following simulated soccer match-play. Front Physiol. 2022;13:1–9.
Yarar H, Gök Ü, Dağtekin A, Saçan Y, Eroğlu H. The effects of different recovery methods on anaerobic performance in combat sports athletes. Acta Gymnica. 2021;51:1–6.
Khatami SM, Rashidi M, Heidarian MR. Effects of Yoga practice and immersion in cold water on blood lactic acid levels during recovery phase after Cunningham treadmill test in football players. Koomesh. 2021;23(5):607–16.
Baláš J, Kodejška J, Krupková D, Giles D. Males benefit more from cold water immersion during repeated handgrip contractions than females despite similar oxygen kinetics. J Physiol Sci. 2020;70(1):1–11.
Ascensão A, Leite M, Rebelo AN, Magalhäes S, Magalhäes J. Effects of cold water immersion on the recovery of physical performance and muscle damage following a one-off soccer match. J Sports Sci. 2011;29(3):217–25.
Dantas G, Barros A, Silva B, Belém L, Ferreira V, Fonseca A, et al. Cold-water immersion does not accelerate performance recovery after 10-km street run: randomized controlled clinical trial. Res Q Exerc Sport. 2020;91(2):228–38.
Elias GP, Wyckelsma VL, Varley MC, McKenna MJ, Aughey RJ. Effectiveness of water immersion on postmatch recovery in elite professional footballers. Int J Sports Physiol Perform. 2013;8(3):243–53.
Getto CN, Golden G. Comparison of active recovery in water and cold-water immersion after exhaustive exercise. Athl Train Sports Health Care. 2013;5(4):169–76.
Hayter KJ, Doma K, Schumann M, Deakin GB. The comparison of cold-water immersion and cold air therapy on maximal cycling performance and recovery markers following strength exercises. PeerJ. 2016;4:1–17.
Higgins TR, Cameron ML, Climstein M. Acute response to hydrotherapy after a simulated game of rugby. J Strength Cond Res. 2013;27(10):2851–60.
Pournot H, Bieuzen F, Duffield R, Lepretre P-M, Cozzolino C, Hausswirth C. Short term effects of various water immersions on recovery from exhaustive intermittent exercise. Eur J Appl Physiol. 2011;111(7):1287–95.
Petersen AC, Fyfe JJ. Post-exercise cold water immersion effects on physiological adaptations to resistance training and the underlying mechanisms in skeletal muscle: a narrative review. Frontiers Sports Act Living. 2021;3:1–26.
Moore E, Fuller JT, Buckley JD, Saunders S, Halson SL, Broatch JR, et al. Impact of cold-water immersion compared with passive recovery following a single bout of strenuous exercise on athletic performance in physically active participants: a systematic review with meta-analysis and meta-regression. Sports Med. 2022;52:1667–88.
Halder A, Gao C. Muscle cooling and performance: a review. Eur J Sports Med. 2015;2(1):39–48.
Ihsan M, Watson G, Abbiss CR. What are the physiological mechanisms for post-exercise cold water immersion in the recovery from prolonged endurance and intermittent exercise? Sports Med. 2016;46(8):1095–109.
Schaser KD, Vollmar B, Menger MD, Schewior L, Kroppenstedt SN, Raschke M, et al. In vivo analysis of microcirculation following closed soft-tissue injury. J Orthop Res. 1999;17(5):678–85.
Andersson HM, Raastad T, Nilsson J, Paulsen G, Garthe I, Kadi F. Neuromuscular fatigue and recovery in elite female soccer: effects of active recovery. Med Sci Sports Exerc. 2008;40(2):372–80.
Algafly AA, George KP. The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br J Sports Med. 2007;41(6):365–9.
Wilson GJ, Murphy AJ, Pryor JF. Musculotendinous stiffness: its relationship to eccentric, isometric, and concentric performance. J Appl Physiol. 1994;76(6):2714–9.
Myrer JW, Draper DO, Durrant E. Contrast therapy and intramuscular temperature in the human leg. J Athl Train. 1994;29(4):318–22.
Gregson W, Black MA, Jones H, Milson J, Morton J, Dawson B, et al. Influence of cold water immersion on limb and cutaneous blood flow at rest. Am J Sports Med. 2011;39(6):1316–23.
Higgins D, Kaminski TW. Contrast therapy does not cause fluctuations in human gastrocnemius intramuscular temperature. J Athl Train. 1998;33(4):336–40.
Becker BE, Hildenbrand K, Whitcomb RK, Sanders JP. Biophysiologic effects of warm water immersion. Int J Aquat Res Educ. 2009;3(1):24–37.
Beedie CJ. Placebo effects in competitive sport: qualitative data. J Sports Sci Med. 2007;6(1):21–8.
Bailey D, Erith S, Griffin P, Dowson A, Brewer D, Gant N, et al. Influence of cold-water immersion on indices of muscle damage following prolonged intermittent shuttle running. J Sports Sci. 2007;25(11):1163–70.
Rowsell GJ, Coutts AJ, Reaburn P, Hill-Haas S. Effects of cold-water immersion on physical performance between successive matches in high-performance junior male soccer players. J Sports Sci. 2009;27(6):565–73.
Point M, Guilhem G, Hug F, Nordez A, Frey A, Lacourpaille L. Cryotherapy induces an increase in muscle stiffness. Scand J Med Sci Sports. 2018;28(1):260–6.
Hemmings BJ. Physiological, psychological and performance effects of massage therapy in sport: a review of the literature. Phys Ther Sport. 2001;2(4):165–70.
Best TM, Hunter R, Wilcox A, Haq F. Effectiveness of sports massage for recovery of skeletal muscle from strenuous exercise. Clin J Sport Med. 2008;18(5):446–60.
Weerapong P, Hume PA, Kolt GS. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005;35(3):235–56.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Emma Moore is supported by a Research Training Program (Domestic) Scholarship from the Australian Commonwealth Department of Education and Training. No other sources of funding were used to assist in the preparation of this article.
Conflicts of interest
Emma Moore, Joel T. Fuller, Sienna Saunders, Shona L. Halson, James R. Broatch and Clint R. Bellenger declare that they have no conflicts of interest. Jonathan D. Buckley is a recipient of a grant from the Norwood Football Club to evaluate the effects of CWI on recovery of athletic performance. Norwood Football Club had no involvement in the current manuscript.
Author contributions
Emma Moore, Jonathan D. Buckley, Shona L. Halson, James R. Broatch and Clint R. Bellenger contributed to the design of the review and completion of the search strategy. Emma Moore and Sienna Saunders completed data screening and data extraction. Joel T. Fuller was responsible for the meta-analysis. Emma Moore drafted the manuscript. All authors edited and revised the manuscript and approved the final version of the manuscript.
Data availability statement
The datasets generated and/or analysed during the current systematic review are available in the Online Supplementary Material 2–8, see ESM.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moore, E., Fuller, J.T., Bellenger, C.R. et al. Effects of Cold-Water Immersion Compared with Other Recovery Modalities on Athletic Performance Following Acute Strenuous Exercise in Physically Active Participants: A Systematic Review, Meta-Analysis, and Meta-Regression. Sports Med 53, 687–705 (2023). https://doi.org/10.1007/s40279-022-01800-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-022-01800-1