Abstract
Purpose
Cryotherapy is an increasingly popular recovery strategy used in an attempt to attenuate the negative impact of strenuous physical activity on subsequent exercise. Therefore, this study aimed to assess the effects of whole body cryotherapy (WBC) and cold water immersion (CWI) on markers of recovery following a marathon.
Methods
Thirty-one endurance trained males completed a marathon. Participants were randomly assigned to a CWI, WBC or placebo group. Perceptions of muscle soreness, training stress and markers of muscle function were recorded before the marathon and at 24 and 48 h post exercise. Blood samples were taken at baseline, post intervention and 24 and 48 h post intervention to assess inflammation and muscle damage.
Results
WBC had a harmful effect on muscle function compared to CWI post marathon. WBC positively influenced perceptions of training stress compared to CWI. With the exception of C-reactive protein (CRP) at 24 and 48 h, neither cryotherapy intervention positively influenced blood borne markers of inflammation or structural damage compared to placebo.
Conclusion
The findings show WBC has a negative impact on muscle function, perceptions of soreness and a number of blood parameters compared to CWI, contradicting the suggestion that WBC may be a superior recovery strategy. Further, cryotherapy is no more effective than a placebo intervention at improving functional recovery or perceptions of training stress following a marathon. These findings lend further evidence to suggest that treatment belief and the placebo effect may be largely responsible for the beneficial effects of cryotherapy on recovery following a marathon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Across both recreational and elite level sport, athletes regularly train or even compete multiple times a week. It is well documented that novel or exhaustive exercise, whether mechanical or metabolic in nature, can result in exercise-induced muscle damage (EIMD) (Belcastro et al. 1998) and inflammation (Pyne 1993). These physiological stresses can manifest as reduced performance potential, likely to result from increased muscle soreness (Cheung et al. 2003) and decreased muscle function (Byrne and Eston 2002), as well as increased stiffness and swelling that can last for a number of days following the initial insult (Armstrong 1984).
Where performance is a crucial consideration, the optimisation of recovery in between exercise bouts to minimise any negative impact on subsequent performance is vital (Barnett 2006). Cryotherapy, either in the form of cold water immersion (CWI) or whole body cryotherapy (WBC), is becoming an increasingly popular recovery strategy employed by athletes (Hohenauer et al. 2015). Changes in physiological mechanisms resulting from a decrease in muscle and/or skin temperature include the following: reduced inflammation, analgesia, reductions in cardiovascular strain, decreased blood flow, reduced tissue metabolism, increased removal of muscle metabolites as well as neuromuscular, cardiovascular and hormonal changes (Ihsan et al. 2016; Leeder et al. 2012). It is likely that any performance effects resulting from a cryotherapy intervention could be attributed to one, or a combination, of these physiological phenomena.
Despite the growing body of literature, there is still a lack of clarity regarding the efficacy of CWI and WBC as recovery strategies. This may be due in part to the fact that the type and magnitude of physiological stress experienced following a bout of exercise is heavily dependent on the specific nature and duration of the exercise. Evidence suggests that CWI can attenuate soreness (Bleakley et al. 2012; Hohenauer et al. 2015; Leeder et al. 2012) following a variety of exercise stressors but the effect on muscle function remains less clear (Bleakley et al. 2012; Hohenauer et al. 2015). However, there is conflicting evidence to demonstrate that CWI has no effect on soreness (Leeder et al. 2015) following repeated sprints. Similarly, WBC has been shown to attenuate soreness following metabolic and mechanical stress (Hausswirth et al. 2011), but recent evidence suggests that ambiguity remains in the literature (Costello et al. 2015). However, there is little evidence to support improvements in functional recovery (Bleakley et al. 2014). It is likely that the equivocal results are due to differences in temperature, timing of application, type of exercise stress and training status of participants (Minett and Costello 2015).
The rise in popularity of WBC as an alternative to CWI may be explained in part by the capacity to attain far lower exposure temperatures, possibly offering enhanced benefits to recovery. It has been proposed that cryotherapy has the potential to limit inflammation by decreasing peripheral blood flow and, therefore, limiting migration of inflammatory cytokines to areas of structural damage (Mawhinney et al. 2017). However, although WBC produces greater temperature gradients for tissue cooling, the relatively poor thermal conductivity of air compared to water limits the potential for significant subcutaneous and core body cooling (Bleakley et al. 2014). This is supported by both Costello et al. (2014) and Mawhinney et al. (2017) who demonstrated that CWI exposure elicits greater reductions in skin and tissue temperature than WBC. Despite a growing body of literature, there are still relatively few studies that directly compare WBC and CWI (Abaïdia et al. 2016; Mawhinney et al. 2017). Further research is required to afford researchers better understanding of the circumstances under which either treatment is, or is not effective, and whether one intervention can offer any substantial benefit over the other.
To date only one study has directly compared the efficacy of CWI and WBC on functional recovery. Abaïdia et al. (2016) compared the effects of CWI (10 min at 10 °C) and WBC (3 min at − 110°) on recovery following eccentric single-leg hamstring exercise. Their results demonstrated that there was a very likely moderate effect in favour of CWI for recovery of single and double leg CMJ compared to WBC 72 h post exercise. Further, CWI elicited a moderate reduction in perceived soreness and a moderate increase in perception of recovery at 24 and 48 h post, respectively. The authors concluded that CWI was more effective than WBC in enhancing recovery of CMJ 72 h after exercise. However, the exercise stress utilised in this study lacks ecological validity, and there was no control group for comparison.
Given the trend for increasing use of WBC despite equivocal research relating to both CWI and WBC, it is pertinent to make direct comparisons between the two modalities to investigate whether one method can offer a considerable advantage over the other. There are currently only a handful of studies directly comparing WBC and CWI and methodological differences make it difficult to draw cross study comparisons; the need for specificity in post-exercise recovery strategies has previously been highlighted by Minett and Costello (2015) and Stephens et al. (2016).
Long duration endurance exercise such as marathon running results in alterations of a number of physiological and perceptual parameters including muscle soreness, muscle function, muscle damage (CK) and inflammation (CRP) (Hill et al. 2014; Shanely et al. 2013). Whilst a number of studies have utilised CWI as a means of rapid cooling in the treatment of exertional heatstroke following marathon performance (Casa et al. 2007; Mcdermott et al. 2009), there appears to be little evidence evaluating the efficacy of CWI or WBC on recovery following a marathon.
Therefore, the aim of this study was to investigate the effects of WBC and CWI on both physiological and perceptual parameters of recovery in trained runners following the completion of a marathon run.
Materials and methods
Participants
31 healthy male volunteers participated in this study (Table 1). Participants were trained endurance runners and had an expected completion time of 4.5 h or less for a marathon. All participants were non-smokers with no history of recent illness or other disease. In the 5 days prior to the run and for the duration of the study, participants were asked to abstain from therapeutic treatments including massage and anti-inflammatory drugs (NSAID), as well as any nutritional supplements. Participants were instructed to refrain from strenuous exercise (other than the marathon itself) for at least 2 days before each testing session.
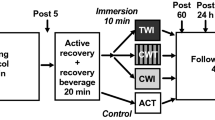
Study design
All procedures were granted ethical clearance by the Institutional committee according to the Helsinki declaration prior to testing. Participants received both verbal and written information about the purpose and potential risks of all study procedures. Participants gave their written informed consent and completed a comprehensive health questionnaire, before being randomly assigned into the placebo (n = 10), CWI (n = 11) or WBC (n = 10) intervention group. On the first testing day and prior to the marathon run participants were familiarised with all testing procedures before baseline measures of all dependent variables (DVs) were recorded. Participants began their allocated treatment intervention within 15 min of cessation of exercise and then provided a further blood sample for analysis. Repeat measurements of all DVs were recorded at 24 and 48 h following completion of the run. Data collection took place over a number of months and environmental conditions were recorded for each marathon day. Participant characteristics, completion times and environmental conditions are included in Table 1.
Exercise protocol
Participants completed the marathon in North London and were asked to pace the run as if it were a competitive race. The route was predominantly grass and unpaved footpaths, with some short concrete sections. Participants completed 9 laps of a 4.7-km loop which was marshalled at the start/finish point. Participants were allowed to consume fluids, electrolytes and/or food ad libitum during the run but were asked to avoid consuming any supplements containing BCAAs, protein, antioxidants or caffeine. By using an outdoor route and self-selected pace, it was hoped that the run would more closely mimic a real-world scenario than a treadmill-based protocol.
Dependent variables
Peak torque and isometric contractions
Peak knee extensor torque and maximal voluntary isometric contraction (MVIC) were measured on the self-reported dominant limb using an isokinetic dynamometer (Biodex 3, Biodex Medical Systems, Shirley, NY, USA). Following a standardised warm-up, participants performed three maximal efforts at 60° s− 1. Participants were encouraged to work as fast and as hard as possible against the resistance of the dynamometer arm throughout the full range of motion. MVIC was measured at a knee angle of 90° in accordance with previous studies (de Ruiter et al. 2003). Participants completed three maximal 5-s efforts. Peak values were used for analysis.
Drop jump (DJ)
Participants dropped from a platform at a height of 30 cm onto a portable jump mat (Kinematic Measurement System, KMS, Fitness Technology, Australia) and then jumped vertically for maximum height as quickly as possible. Emphasis was placed on minimum ground contact time, whilst maintaining maximum jump height. Participants kept their hands on their hips for the duration of the movement and performed three maximal jumps at each testing point. Reactive strength index (RSI) for each effort was calculated by dividing vertical displacement (jump height) in metres, by ground contact time in seconds (Flanagan and Comyns 2008) and peak RSI values were used for analysis.
Perceived soreness
Participants indicated their perceived levels of muscle soreness of the lower limbs during a body weight squat (approx. knee angle of 90°) using a 0 (no soreness on movement) to 10 (muscles too sore to move) likert scale. This method has been used successfully in previous studies to monitor changes in perceptions of pain following exercise (Vaile et al. 2007).
Daily analysis of the lifestyle demands of athletes (DALDA)
The questionnaire comprises two sections: part A identifies potential sources of stress (including home life, work, sleep and sports training), whilst part B allows individuals to rate stress reactions symptoms as worse than normal, normal, or better than normal. Several studies have successfully utilised the DALDA questionnaire to monitor fatigue and recovery (Hogarth et al. 2015; Robson-Ansley et al. 2009). Only results for part ‘B’ are reported here, after Halson et al. (2002) and Coutts et al. (2007) stated that there were only significant changes in the second part of the questionnaire following a period of intensified training.
Blood sampling
Approximately 8 ml of blood was collected with stasis from the antecubital vein into plasma separation vacutainers (containing EDTA) for the purpose of assessing muscle damage and inflammation. Whole blood samples were assessed using a blood counter (AcT diff 5 CP, Beckman Coulter, High Wycombe, UK) to correct values for changes in plasma volume. The manufacturer reports the coefficients of variation for this system as < 2% for haematocrit and < 1% for haemoglobin within the reportable range. Blood was then centrifuged at 3000 rpm for 8 min before being aliquoted and stored at − 80 °C for later analysis. Blood markers included creatine kinase-M (CK-M), C reactive protein (CRP), interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α) and were all determined in duplicate.
CK-M
Plasma CK-M concentrations were measured by simple step enzyme-linked immunosorbent assay (ELISA, Abcam, Cambridge, UK). The reported assay ranges are 54.3–268.9 U/L, the minimum detection concentration (MDC) is 0.014 U/L and the human serum intra- and inter-assay CV are 3 and 9%, respectively.
CRP
Plasma CRP concentration was determined using a quantitative sandwich (QS) enzyme-linked immunoassay (ELISA) technique (Quantikine, R&D Systems Europe Ltd., Abingdon, UK). The limit of quantification (LOQ), defined as the lowest concentration that could be distinguished from 0 was 7.8 pg/ml with an intra and inter-assay CV of 6.6 and 8.3%, respectively.
IL-6
Plasma IL-6 concentration was determined by a QS-ELISA (Quantikine, R&D Systems Europe Ltd., Abingdon, UK). The limit of quantification (LOQ), defined as the lowest concentration that could be distinguished from 0, was 0.38 pg/ml. The serum intra- and inter-assay precision, determined by CV, was 3.8 and 8.3%, respectively.
TNF-α
Plasma TNF-α concentration was measured by QS-ELISA (Quantikine, R&D Systems Europe Ltd., Abingdon, UK). The limit of quantification (LOQ), defined as the lowest concentration that could be distinguished from 0 was 0.52 pg/ml. The serum intra- and inter-assay precision, determined by CV was 4.9 and 9.9%, respectively.
Correction for haemoconcentration
Bouts of acute exercise, such as long-distance running, have been shown to produce a transient fluid shift out of the intravascular space, resulting in haemoconcentration (Kargotich et al. 1998). To minimise any confounding effects of the dynamic nature of plasma volume (Alis et al. 2015), changes in plasma volume were assessed using the following equation: ΔPV (%) 100 × ((HBpre/HBpost) × (100 − HTCpost)/(100 − HTCpre) − 1), where PV is plasma volume, HB is haemoglobin and HTC is haematocrit. All blood markers were then adjusted using the following equation: (Biomarker)c = (Biomarker)u × (1 + ΔPV (%) /100), where c is corrected and u is uncorrected.
Interventions
Whole body cryotherapy
The WBC group was exposed to two cold treatments in a cryotherapy chamber (CryoClinics, London, UK). Participants (up to 2 at a time) spent 3 min in the chamber set to − 85 °C ± 5 °C followed by a 15-min warming period in an ambient room before entering the chamber for a further 4-min bout at − 85 °C ± 5 °C. During exposure participants were asked to walk around slowly while flexing and extending their elbows and fingers, and wore a pair of shorts, gloves, dry socks and shoes, a hat covering the ears and a mask to protect the nose and mouth. Before entering the chamber participants were asked to remove glasses, contact lenses and any jewellery or piercings.
Cold water immersion
Immediately after cessation of exercise participants sat in a mobile ice bath (iSprint Twin, iCool, Cranlea, UK) ensuring their lower limbs and iliac crest were fully immersed. Participants remained in the ice bath filled with water cooled to 8 degrees (± 0.5°) for 10 min. The ice bath was connected to a chiller unit (MiCool, iCool, Cranlea, UK) so that water temperature could be monitored and maintained within the desired parameters for the duration of the treatment. During exposure participants wore shorts and immediately after they were asked to towel themselves dry and change into clean, dry clothing. This protocol is comparable to those utilised in other single exposure studies examining the effects of CWI on various measures of recovery (Bailey et al. 2007; Jakeman et al. 2009).
Placebo
As it was not possible to blind participants to their recovery intervention, a placebo, rather than a control group, was used. The phytochemicals found in Tart Montmorency cherry juice have previously been shown to reduce inflammation and improve muscle recovery following a marathon (Bell et al. 2014). Therefore, the placebo group was informed that they were taking a tart cherry juice supplement for 5 days before the run, the day of the run and for 2 days after (8 days in total). Participants consumed 2 × 30 ml per day of a fruit flavored drink which did not contain any antioxidants or phytonutrients. Participants were asked to rest quietly for 10 min following completion of the run. It was hoped that the use of a placebo (sham) group would minimise associated placebo effects (i.e. effects of the treatment that were not related to the treatment itself) (McClung and Collins 2007).
Statistical analysis
Confidence intervals (CI) and magnitude-based inferences were calculated for each dependent variable using methods described by Batterham and Hopkins (2006). The smallest practically worthwhile effect for muscle function variables was the smallest standardised (Cohen) change in the mean: 0.2 times the between-subject SD for baseline values of all participants (Batterham and Hopkins 2006). The smallest worthwhile change for muscle soreness and DALDA was a change in raw values of 1.0, and for blood parameters a factor of 1.1 was used (Hopkins 2015). In order to account for large inter-individual differences in blood parameters, baseline values were used as a covariate. Qualitative descriptors relate to the likelihood of positive, trivial or negative outcomes. Clinical inferences were based on threshold chances of harm and benefit of 0.5 and 25%, respectively. In cases where the inference was unclear, a beneficial inference was reported where the odds ratio of benefit/harm was greater than 66. In order to overcome heteroscedastic error, the analysis of dependent variables was conducted on log-transformed data (Nevill and Lane 2007), except in the cases of muscle soreness and DALDA. Interval scaling makes it inappropriate to log-transform data for these variables (Nevill and Lane 2007) so analysis was conducted on raw values. Each dependent variable was analysed using a published spreadsheet by Hopkins (2015). Changes are reported as percentages for function variables, raw changes for perceptual variables and factor changes for blood markers.
Results
The outcomes for changes over time as well as group comparisons for all parameters can be seen in Tables 2 and 3. The marathon resulted in decreases in muscle function, increases in circulating CK, increases in perceptions of soreness and alterations in a number of blood borne markers of inflammation.
Muscle function
A summary of the statistical analyses for the effect of each intervention on markers of muscle function can be seen in Table 2.
Peak torque knee extension
At baseline, the peak torque knee extension values were 178.24 ± 28.41, 195.33 ± 29.92 and 203.72 ± 39.47 Nm for placebo, CWI and WBC, respectively. Peak torque decreased in all groups at both time points post marathon. WBC was harmful at all time points in comparison to both CWI and placebo (Fig. 1).
MVIC
At baseline, MVIC values were 197.85 ± 51.15, 221.81 ± 37.48 and 228.60 ± 54.68 N for placebo, CWI and WBC, respectively. Changes in MVIC were unclear or trivial in the CWI and placebo groups, whilst there were harmful changes in the WBC group. WBC was harmful compared to both CWI and placebo at all time points.
Reactive strength index
At baseline, RSI values were 0.88 ± 0.21, 0.89 ± 0.30 and 1.03 ± 0.29 m s− 1 for placebo, CWI and WBC, respectively. RSI decreased in all groups from baseline to 24 h, with unclear or trivial changes from baseline to 48 h. WBC was harmful compared to CWI and placebo at all time points.
Perceptual responses
A summary of the statistical analyses for the effect of each intervention on perceptual responses can be seen in Table 2.
Perceived muscle soreness
At baseline, soreness values were 1 ± 2, 1 ± 1 and 1 ± 1 (VAS 0–10) for placebo, CWI and WBC, respectively (Fig. 2). Perceptions of soreness increased in all groups from baseline to 24 h, and in the placebo group from baseline to 48 h. WBC was possibly beneficial compared to placebo at 48 h, whilst all other group comparisons were trivial or unclear.
DALDA
At baseline, DALDA values were 4 ± 3, 1 ± 2 and 4 ± 4 scores marked as worse than normal for placebo, CWI and WBC, respectively. The number of scores marked ‘worse than normal’ increased in the CWI and placebo group at 24 and 48 h, whereas the change was unclear at 24 h and beneficial at 48 h for WBC. At all time points, WBC was beneficial compared to CWI and unclear compared to placebo. CWI was harmful compared to the placebo at all time points.
Blood markers
A summary of the statistical analyses for the effect of each intervention on blood markers can be seen in Table 3.
CK
At baseline, CK values were 31.2 ± 18.0, 25.0 ± 14.5 and 44.2 ± 70.4 U L− 1 for placebo, CWI and WBC. respectively. CK increased in all groups at both time points post marathon. WBC was harmful compared to CWI, and both cryotherapy interventions were harmful compared to placebo at 24 h. All group comparisons from baseline to 48 h were unclear.
CRP
At baseline, CRP values were 1625 ± 3838, 586 ± 378 and 553 ± 573 ng mL− 1 for placebo, CWI and WBC, respectively. CRP increased in all groups from baseline to 24 h and in the CWI and placebo groups from baseline to 48 h. WBC was harmful compared to CWI and placebo at 24 h, but beneficial in the same comparisons at 48 h. CWI was beneficial compared to placebo at both time points.
IL-6
At baseline, IL-6 values were 42.60 ± 104.35, 75.93 ± 157.27 and 17.81 ± 33.51 pg/ml for placebo, CWI and WBC, respectively. IL-6 increased in all groups immediately post marathon and remained elevated in the WBC group only at 24 h post. From baseline to post, WBC was beneficial compared to CWI, CWI was harmful compared to placebo and the comparison between WBC and placebo was unclear. At 24 h WBC was harmful compared to CWI and placebo, whilst the comparison between CWI and placebo was unclear.
TNF-α
At baseline, TNF-α values were 52.5 ± 118.0, 327.6 ± 928.1 and 58.9 ± 119.4 pg/ml for placebo, CWI and WBC, respectively. TNF-α increased in all groups following the marathon. Comparisons between WBC and CWI were unclear at all time points, WBC was harmful compared to placebo immediately post and CWI was harmful compared to placebo at all time points.
Where there are large differences in baseline values between groups (CRP, IL-6 and TNF-α), this is attributed to one or two individuals who had values substantially greater than the normal range. However, as results are analysed as the difference between groups in change over time, these participants were not removed from the analysis.
Discussion
The present study examined the efficacy of a single bout of whole body cryotherapy (WBC) or cold water immersion (CWI) on markers of recovery in trained endurance athletes following a marathon run. The marathon led to modest alterations in muscle function, perceptions of muscle soreness and stress response symptoms, as well as increases in markers of muscle damage and inflammation. In terms of comparison between the different interventions, WBC was harmful for recovery of muscle function compared to CWI, with both cryotherapy groups being detrimental in comparison to the placebo. WBC was beneficial for limiting stress response symptoms and muscle soreness in comparison to CWI and the placebo, respectively. Both cryotherapy interventions lead to greater increases in CK than the placebo, with WBC harmful compared to CWI at 24 h. There was little evidence of the cryotherapy interventions limiting inflammation based on IL-6 and TNF-α, and in some cases lead to greater increases. However, cryotherapy leads to the attenuation of increases in CRP.
WBC was detrimental to recovery of peak torque compared to both placebo and CWI at 24 and 48 h; there were no differences between CWI and placebo. These results suggest that CWI may offer benefits compared to WBC for the recovery of peak torque, but that it is no more effective than a placebo intervention. In the case of MVIC and RSI, WBC leads to greater decrements than both CWI and placebo, and CWI was also less effective than the placebo for recovery. The novel finding that cryotherapy is harmful for recovery in comparison to a placebo could be linked to an enhanced inflammatory response evidenced by an increase in pro-inflammatory markers (Hausswirth et al. 2011). Machado and colleagues (2016) have previously suggested that any CWI exposure below 10 °C could be classed as ‘severe’ cold, with the potential to cause adverse effects that are interpreted by the body as noxious stimuli. Although muscle temperature was not recorded in the present study, it is possible that both an 8 °C CWI exposure and a -85 °C WBC exposure resulted in substantial reductions of muscle temperature that elicited a stress response in the body (Machado et al. 2016). This may explain the harmful effects of cryotherapy in this case.
The present study’s results contrast with those from White, Rhind and Wells (2014) who reported that CWI (10 min or 30 min at 10 °C) facilitated restoration of muscle performance in a stretch–shortening cycle following high-intensity sprint exercise. This study suggests that immersion at 8 °C negatively impacts upon functional recovery after endurance exercise. The use of a placebo group in place of a control could explain the findings; research suggests that many of the hypothesised physiological benefits surrounding CWI are at least partly placebo related (Broatch et al. 2014). Broatch and colleagues (2014) found that CWI was no more effective than a placebo immersion protocol at improving muscle recovery following high-intensity exercise. They suggested that effective deception of participants is critical when using a placebo and that treatment belief is a powerful element (Beedie et al. 2017). Anecdotal evidence from the present study suggests that the placebo was administered effectively and that participants believed in its efficacy.
Differences in muscle soreness between WBC and CWI were either trivial or unclear. These results are in contrast to Abaïdia and colleagues (2016) who found that CWI resulted in lower soreness scores 48 h post eccentric exercise in comparison to WBC. These differences may be explained by the warmer WBC protocol utilised in the present study (− 85° versus − 110 °C), potentially indicating that warmer WBC temperatures are more effective at alleviating perceptions of soreness after exercise, compared to ‘extreme cold’ exposures (Machado et al. 2016). Alternatively, the use of a novel high-intensity eccentric biased exercise protocol may have produced greater structural damage, secondary inflammation and stimulation of pain receptors than that seen in the present study (Clarkson and Hubal 2002; Scholz and Woolf 2002). The finding that CWI was not effective at reducing soreness compared to the placebo is in contrast to the majority of previous research on the topic. As already discussed, an immersion temperature of 8 °C may have been suboptimal; exposure temperatures of between 10 and 15 °C are more commonly cited in the literature (Machado et al. 2016) and appear to be effective at reducing muscle soreness post exercise. Second, few studies investigating the influence of CWI on recovery have effectively blinded participants to their intervention group. The use of a placebo in the current study may negate some of the positive expectance effects attributable to the placebo effect (Broatch et al. 2014; McClung and Collins 2007) and, therefore, offer a more robust examination of the effectiveness of CWI and WBC interventions used post exercise.
Cryotherapy had a negative effect on structural muscle damage assessed via circulating CK. Between baseline and 24 h there was a harmful effect of WBC compared to CWI, and both cryotherapy treatments were harmful compared to the placebo. A recent study from Abaïdia and colleagues (2016) demonstrated a very likely large effect for CK in favour of CWI compared to WBC 24 h post exercise, supporting the present study’s indication that CWI may offer additional benefits over WBC for the attenuation of CK 24 h post exercise. Cryotherapy leads to greater increases in leukocytes following the marathon (unreported data). It is plausible that leucocytosis lead to an increased breakdown of the sarcolemma that increased the efflux of CK in the cryotherapy groups in comparison to the placebo.
Cryotherapy is proposed to potentiate anti-inflammatory actions by decreasing peripheral blood flow (Mawhinney et al. 2017). The results from the present study do not support this hypothesis, despite WBC demonstrating a beneficial effect on IL-6 compared to CWI from baseline to post, when compared to the placebo, CWI and WBC were harmful and unclear, respectively. Similarly, WBC and CWI were harmful for changes in TNF-α with no clear differences between the cryotherapy groups. As previously stated, Machado et al. (2016) suggest that ‘severe cold’ immersion protocols (5–10°) can actually negatively impact upon recovery, by eliciting a cold related stress response. This in turn may increase markers of inflammation. However, CRP was the only marker where cryotherapy treatment had a positive influence compared to placebo. The seemingly equivocal results could be explained in part by different time courses of the markers; IL-6 tends to peak immediately post exercise (Bernecker et al. 2013; Clifford et al. 2016; Mündermann et al. 2016), whereas CRP normally continues to increase until 24 h post exercise (Howatson et al. 2010). As such, cryotherapy applied post exercise may have been unable to attenuate increases in IL-6 but could positively impact upon the recovery of CRP.
Potential limitations of the current study should also be addressed. The cryotherapy chamber utilised during data collection was located a short distance off site, and as such, there may have been slight inconsistencies in the timing of the post exercise blood sample for participants in the WBC group compared to CWI and placebo. It is possible that the delay in sampling could have resulted in inflated pro-inflammatory values in the WBC group for the post exercise samples. However, the factor-fold increases in IL-6 and TNF-α were still greater in the CWI group compared to WBC immediately post. Second, the WBC treatment temperature in the present study (− 85°) was warmer than normally reported in the literature (− 110 to − 140 °C), dictated by the minimum operating temperature of the cryotherapy chamber. Therefore, although the results reported here add to the current body of literature, the results cannot be generalised to colder exposure temperatures.
Conclusion
In terms of comparison between cryotherapy modalities, with the exception of DALDA scores at 24 and 48 h, CRP at 48 h and IL-6 immediately following the marathon, WBC demonstrated an unclear, or negative impact on all markers at all time points compared to CWI. These findings contradict the widely held assumption that WBC can elicit enhanced recovery benefits when compared to more traditional cryotherapy applications such as CWI.
Second, with the exception of soreness at 48 h for WBC and CRP (24 and 48 h for CWI and 48 h for WBC) the implementation of a cryotherapy intervention resulted in unclear, trivial or harmful effects for every outcome measure when compared to the placebo intervention. This lends further weight to the suggestion that therapeutic effects attributed to cryotherapy protocols could be a product of the placebo effect. Therefore, this highlights the need for future research to implement effective placebo interventions in place of control groups, or to at least take into consideration a measure of treatment belief when comparing different intervention strategies.
It is hoped that the findings from this study will help inform practice of athletes, practitioners and coaches in relation to post exercise recovery strategies. Further research is warranted to investigate the impact of CWI versus WBC on recovery following different exercise stresses and to better understand the underlying physiological mechanisms.
Abbreviations
- CK:
-
Creatine kinase
- CMJ:
-
Counter movement jump
- CRP:
-
C-reactive protein
- CWI:
-
Cold water immersion
- DALDA:
-
Daily analysis of the lifestyle demands of athletes
- EIMD:
-
Exercise-induced muscle damage
- IL-6:
-
Interleukin-6
- MVIC:
-
Maximal voluntary isometric contraction
- RSI:
-
Reactive strength index
- TNF-α:
-
Tumour necrosis factor-α
- WBC:
-
Whole body cryotherapy
References
Abaïdia AE, Lamblin J, Delecroix B, Leduc C, McCall A, Nédélec M, Dupont G (2016) Recovery from exercise-induced muscle damage: cold water immersion versus whole body cryotherapy. Int J Sports Physiol Perform. https://doi.org/10.1123/ijspp.2015-0012
Alis R, Sanchis-Gomar F, Primo-Carrau C, Lozano-Calve S, Dipalo M, Aloe R, Lippi G (2015) Hemoconcentration induced by exercise : revisiting the Dill and Costill equation. Scand J Med Sci Sports 25(6):630–637. https://doi.org/10.1111/sms.12393
Armstrong RB (1984) Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc 16(6):529–538
Bailey DM, Erith SJ, Griffin PJ, Dowson A, Brewer DS, Gant N, Williams C (2007) Influence of cold-water immersion on indices of muscle damage following prolonged intermittent shuttle running. J Sports Sci 25(11):1163–1170. https://doi.org/10.1080/02640410600982659
Barnett A (2006) Using recovery modalities between training sessions in elite athletes: Does it help? Sports Med 36(9):781–796. https://doi.org/10.2165/00007256-200636090-00005
Batterham AM, Hopkins WG (2006) Making meaningful inferences about magnitudes. Int J Sports Physiol Perform 1(1):50–57
Beedie C, Whyte G, Lane AM, Cohen E, Raglin J, Hurst P, Coleman D, Foad A (2017) “Caution, this treatment is a placebo. It might work, but it might not”: why emerging mechanistic evidence for placebo effects does not legitimise complementary and alternative medicines in sport. Br J Sports Med. https://doi.org/10.1136/bjsports-2017-097747
Belcastro AN, Shewchuk LD, Raj DA (1998) Exercise-induced muscle injury: a calpain hypothesis. Mol Cell Biochem 179(1–2):135–145. https://doi.org/10.1023/A:1006816123601
Bell PG, McHugh MP, Stevenson E, Howatson G (2014) The role of cherries in exercise and health. Scand J Med Sci Sports 24(3):477–490. https://doi.org/10.1111/sms.12085
Bernecker C, Scherr J, Schinner S, Braun S, Scherbaum WA, Halle M (2013) Evidence for an exercise induced increase of TNFa and IL-6 in marathon runners. Scand J Med Sci Sports 23(2), 207–214. https://doi.org/10.1111/j.1600-0838.2011.01372.x
Bleakley C, Mcdonough S, Gardner E, Baxter GD, Ty J, Davison GW (2012) Cold water immersion cryotherapy for preventing and treating muscle soreness after exercise. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD008262.pub2.Copyright
Bleakley CM, Bieuzen F, Davison GW, Costello JT (2014) Whole-body cryotherapy: empirical evidence and theoretical perspectives. Open Access J Sports Med 5:25–36. https://doi.org/10.2147/OAJSM.S41655
Broatch JR, Petersen A, Bishop DJ (2014) Postexercise cold water immersion benefits are not greater than the placebo effect. Med Sci Sports Exerc 46(11):2139–2147. https://doi.org/10.1249/MSS.0000000000000348
Byrne C, Eston R (2002) The effect of exercise-induced muscle damage on isometric and dynamic knee extensor strength and vertical jump performance. J Sports Sci 20(5):417–425. https://doi.org/10.1080/026404102317366672
Casa DJ, Mcdermott BP, Lee EC, Yeargin SW, Lawrence E, Maresh CM (2007) Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev 35(3):141–149
Cheung K, Hume PA, Maxwell L (2003) Delayed onset muscle soreness: Treatment strategies and performance factors. Sports Med 33(2):145–164. https://doi.org/10.2165/00007256-200333020-00005
Clarkson PM, Hubal MJ (2002) Exercise-induced muscle damage in humans. Am J Phys Med Rehabilit Assoc Acad Phys 81(11 Suppl):S52–S69. https://doi.org/10.1097/01.PHM.0000029772.45258.43
Clifford T, Allerton DM, Brown MA, Harper L, Horsburgh S, Keane KM, Howatson G (2016) Minimal muscle damage after a marathon and no influence of beetroot juice on inflammation and recovery. Appl Physiol Nutr Metab 42(3):263–270
Costello JT, Donnelly AE, Karki A, Selfe J (2014) Effects of Whole Body cryotherapy and cold water immersion on knee skin temperature. Int J Sports Med 35(1):35–40. https://doi.org/10.1055/s-0033-1343410
Costello JT, Baker PR, Minett GM, Bieuzen F, Stewart IB, Bleakley C (2015) Whole-body cryotherapy (extreme cold air exposure) for preventing and treating muscle soreness after exercise in adults. Cochrane Database of Syst Rev https://doi.org/10.1002/14651858.CD010789
Coutts AJ, Slattery KM, Wallace LK (2007) Practical tests for monitoring performance, fatigue and recovery in triathletes. J Sci Med Sport 10(6):372–381. https://doi.org/10.1016/j.jsams.2007.02.007
de Ruiter CJ, van der Linden RM, van der Zijden MJA, Hollander AP, de Haan A (2003) Short-term effects of whole-body vibration on maximal voluntary isometric knee extensor force and rate of force rise. Eur J Appl Physiol 88(4–5):472–475. https://doi.org/10.1007/s00421-002-0723-0
Flanagan EP, Comyns TM (2008) The use of contact time and the reactive strength index to optimize fast stretch-shortening cycle training. Strength Cond J 30(5):32–38
Halson SL, Bridge MW, Meeusen R, Busschaert B, Gleeson M, Jones DA, Jeukendrup AE (2002) Time course of performance changes and fatigue markers during intensified training in trained cyclists. J Appl Physiol, 93(3), 947–956. https://doi.org/10.1152/japplphysiol.01164.2001
Hausswirth C, Louis J, Bieuzen F, Pournot H, Fournier J, Filliard J-R, Brisswalter J (2011) Effects of whole-body cryotherapy vs. far-infrared vs. passive modalities on recovery from exercise-induced muscle damage in highly-trained runners. PLoS one, 6(12), e27749. https://doi.org/10.1371/journal.pone.0027749
Hill J, Howatson G, van Someren KA, Walshe I, Pedlar C (2014) Influence of compression garments on recovery after marathon running. J Strength Cond Res 28(4):2228–2235
Hogarth LW, Burkett BJ, McKean MR (2015) Understanding the fatigue-recovery cycle in team sport athletes. J Sports Med Doping Stud 5(1):1000e143
Hohenauer E, Taeymans J, Baeyens J, Clarys P, Clijsen R (2015) The effect of post-exercise cryotherapy on recovery characteristics : a systematic review and meta-analysis. PLoS one, 10(9), 1–22 e0139028. https://doi.org/10.1371/journal.pone.0139028
Hopkins WG (2015). Spreadsheets for analysis of controlled trials with adjustment for a predictor. Sportscience, (10), 46–50
Howatson G, McHugh MP, Hill JA, Brouner J, Jewell AP, Van Someren KA, Howatson SA (2010) Influence of tart cherry juice on indices of recovery following marathon running. Scand J Med Sci Sports, 20(6):843–852. https://doi.org/10.1111/j.1600-0838.2009.01005.x
Ihsan M, Watson G, Abbiss CR (2016) What are the physiological mechanisms for post-exercise cold water immersion in the recovery from prolonged endurance and intermittent exercise? Sports Med 46(8):1–15. https://doi.org/10.1007/s40279-016-0483-3
Jakeman JR, Macrae R, Eston R (2009) A single 10-min bout of cold-water immersion therapy after strenuous plyometric exercise has no beneficial effect on recovery from the symptoms of exercise-induced muscle damage. Ergonomics 52(4):456–460
Kargotich S, Goodman C, Keast D, Morton AR (1998) The influence of exercise-induced plasma volume changes on the interpretation of biochemical parameters used for monitoring exercise, training and sport. Sports Med 26(2):101–117
Leeder J, Gissane C, van Someren K, Gregson W, Howatson G (2012) Cold water immersion and recovery from strenuous exercise: a meta-analysis. Br J Sports Med 46(4):233–240. https://doi.org/10.1136/bjsports-2011-090061
Leeder J. D. C., Van Someren KA, Bell PG, Spence JR, Jewell P, Gaze D, Howatson G (2015) Effects of seated and standing cold water immersion on recovery from repeated sprinting. J Sport Sci. https://doi.org/10.1080/02640414.2014.996914
Machado AF, Ferreira PH, Micheletti JK, de Almeida AC, Lemes ÍR, Vanderlei FM, Pastre CM (2016) Can water temperature and immersion time influence the effect of cold water immersion on muscle soreness? A systematic review and meta-analysis. Sports Med 46(4):503–514. https://doi.org/10.1007/s40279-015-0431-7
Mawhinney C, Low DA, Jones H, Green DJ, Costello JT, Gregson W (2017) Water mediates greater reductions in limb blood flow than whole body cryotherapy. Med Sci Sports Exerc. https://doi.org/10.1249/MSS.0000000000001223
McClung M, Collins D (2007) Because I know it will!”: placebo effects of an ergogenic aid on athletic performance. J Sport Exerc Psychol 29(3):382–394
Mcdermott BP, Casa DJ, Connor FGO, Adams WB, Armstrong LE, Brennan AH, Lopez RM, Stearns RL, Troyanos C, Yeargin SW (2009) Cold-water dousing with ice massage to treat exertional heat stroke: a case series. Aviat Space Environ Med 80(8):720–722. https://doi.org/10.3357/ASEM.2498.2009
Minett GM, Costello JT (2015) Specificity and context in post-exercise recovery: it is not a one-size-fits-all approach. Front Physiol, 6:1–3. https://doi.org/10.3389/fphys.2015.00130
Mündermann A, Geurts J, Hügle T, Nickel T, Schmidt-Trucksäss A, Halle M, Hanssen H (2016) Marathon performance but not BMI affects post-marathon pro-inflammatory and cartilage biomarkers. J Sports Sci. https://doi.org/10.1080/02640414.2016.1184301
Nevill A, Lane A (2007) Why self-report “Likert” scale data should not be log-transformed. J Sports Sci, 25(1), 1–2. https://doi.org/10.1080/02640410601111183
Pyne DB (1993) Exercise-induced muscle damage and inflammation: a review. Aust J Sci Med Sport 26(3–4):49–58
Robson-Ansley PJ, Gleeson M, Ansley L (2009) Fatigue management in the preparation of Olympic athletes. J Sports Sci 27(13):1409–1420
Scholz J, Woolf CJ (2002) Can we conquer pain?. Nat Neurosci, 5(Supp), 1062–1067
Shanely RA, Nieman DC, Zwetsloot KA, Knab AM, Imagita H, Luo B, Zubeldia JM (2013) Evaluation of Rhodiola rosea supplementation on skeletal muscle damage and inflammation in runners following a competitive marathon. Brain Behav Immun 39:204–210 https://doi.org/10.1016/j.bbi.2013.09.005
Stephens JM, Halson S, Miller J, Slater GJ, Askew CD, Stephens JM, Askew CD (2016) Cold water immersion for athletic recovery: one size does not fit all. Int J Sports Physiol Perform. https://doi.org/10.1123/ijspp.2015-0012
Vaile JM, Gill ND, Blazevich AJ (2007) The effect of contrast water therapy on symptoms of delayed onset muscle soreness. J Strength Cond Res 21(3):697–702
White GGE, Rhind SSG, Wells GGD (2014) The effect of various cold-water immersion protocols on exercise-induced inflammatory response and functional recovery from high-intensity sprint exercise. Eur J Appl Physiol. https://doi.org/10.1007/s00421-014-2954-2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Funding
No funding was received for this work.
Additional information
Communicated by Narihiko Kondo.
Rights and permissions
About this article
Cite this article
Wilson, L.J., Cockburn, E., Paice, K. et al. Recovery following a marathon: a comparison of cold water immersion, whole body cryotherapy and a placebo control. Eur J Appl Physiol 118, 153–163 (2018). https://doi.org/10.1007/s00421-017-3757-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3757-z