Abstract
Background
Perinatal growth abnormalities program susceptibility to childhood obesity, which is further exaggerated by maternal overweight and obesity (MO) during pregnancy. Exercise is highly accessible, but reports about the benefits of maternal exercise on fetal growth and childhood obesity outcomes are inconsistent, reducing the incentives for pregnant women to participate in exercise to improve children’s perinatal growth.
Objective
This systematic review and meta-analysis aims to establish evidence-based efficacy of exercise in mothers with normal weight (MNW) and MO during pregnancy in reducing the risks of perinatal growth abnormalities and childhood obesity. In addition, the impacts of exercise volume are also assessed.
Methods
The PubMed, ScienceDirect, Web of Science, and Cochrane Library databases were searched from inception to February 15, 2020. We included randomized controlled trials with exercise-only intervention or exercise with other confounders in pregnant MNW (body mass index, BMI 18.5–24.9 kg/m2) and MO (BMI ≥ 25 kg/m2), which were further subgrouped in the meta-analysis. Primary outcomes included birth weight, preterm birth, small for gestational age (SGA), large for gestational age (LGA), infant and childhood weight, and childhood obesity. A linear meta-regression analysis was also used to explore the effects of exercise volume on outcomes.
Results
99 studies were included in the meta-analysis (n = 596,876), and individual study quality ranged from fair to good according to the Newcastle–Ottawa scale assessment. Exercise only interventions in MNW reduced preterm birth by 15% (26 studies, n = 76,132; odds ratio [OR] 0.85; 95% CI 0.72, 1.01; I2 = 83.3%), SGA by 17% (33 studies, n = 92,351; OR 0.83; 95% CI 0.71, 0.98; I2 = 74.5%) and LGA by 17% (29 studies, n = 84,310; OR 0.83; 95% CI 0.74, 0.95; I2 = 60.4%). Exercise only interventions in MO reduced preterm birth by 33% (2 studies, n = 3,050; OR 0.67; 95% CI 0.70, 0.96; I2 = 0%), SGA by 27% (8 studies, n = 3,909; OR 0.73; 95% CI 0.50, 1.05; I2 = 40.4%) and LGA by 55% (9 studies, n = 81,581; OR 0.45; 95% CI 0.18, 1.11; I2 = 98.3%). Exercise only interventions in MNW reduced childhood obesity by 53% (3 studies, n = 6,920; OR 0.47; 95% CI 0.36, 0.63; I2 = 77.0%). However, no significant effect was observed in outcomes from exercise confounders in either MNW or MO. In the meta-regression, the volume of exercise-only intervention in MNW was negatively associated with birth weight, greatly driven by volumes more than 810 metabolic equivalents (MET)-min per week. Other outcomes were not associated with exercise volume.
Conclusions
This systematic review and meta-analysis suggests that exercise during pregnancy in both MNW and MO safely and effectively reduce the risks of preterm birth, SGA, and LGA. Furthermore, MNW exercise also reduces the risk of childhood obesity. Overall, regardless of prepregnancy BMI, maternal exercise during pregnancy provides an excellent opportunity to mitigate the high prevalence of adverse birth outcomes and childhood obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Maternal exercise during pregnancy effectively reduces the risks of preterm birth, SGA, LGA, and childhood obesity. |
Exercise volume over 810 metabolic equivalents per min per week contributes to lower birth weight but is not associated with preterm birth or SGA. |
Maternal exercise interventions can be used to reduce adverse birth outcomes and childhood obesity across different maternal body mass indices. |
1 Introduction
Globally, over 41 million children under age 5 are obese, and over 340 million children aged 5–19 years are either overweight or obese [1, 2]. The prevalence of child obesity has tripled since 1975 and is currently up to 18% [1, 2]. Childhood obesity is likely sustained into adolescence and adulthood, which predisposes children to the development of serious health complications at an early age, including type 2 diabetes mellitus, hypertension, and cardiovascular diseases [3]. The increase in childhood obesity is associated with multiple factors, including high energy food intake, a lack of physical activity, and imbalanced nutrition [4]. However, growing evidence suggests that early intrauterine life also plays a critical role in shaping the trajectory of child weight gain and fatness [5].
Gestational age and birth weight are critical variables in fetal development [6,7,8]. Abnormal early development programs long-term child health, including predisposition to obesity and type 2 diabetes mellitus. Adverse fetal development and birth outcomes, including preterm birth (defined as < 37 completed weeks of gestation), small birth weight for gestational age (SGA; birth weight < 10th percentile for age and sex), and large birth weight for gestational age (LGA; birth weight > 90th percentile for age and sex), account for 11.1, 15.5, and 13% of the world’s live births, respectively, and substantially increase the risks of child obesity by 59, 19, and 100%, respectively [6,7,8]. While abnormal prenatal growth is associated with multiple etiologies, prepregnancy maternal overweight and obesity (MO; body mass index BMI ≥ 25 kg/m2) is one of the main drivers of fetal growth restriction, preterm birth, and excessive growth [9]. Previous meta-analyses have revealed that prepregnancy MO increases the risk of preterm birth by 50%, SGA by 70%, and LGA by 57%, contributing to a more than twofold increase in childhood obesity risk [10,11,12,13,14]. Currently, approximately one-third of women of childbearing age are obese in the United States, perpetuating “a vicious mother–child obesity cycle” [15,16,17].
During the last few decades, a large volume of studies have shown that exercise during pregnancy benefits maternal health and postpartum recovery, and these studies have been systematically reviewed in previous meta-analyses [18,19,20,21,22,23]. A 2017 meta-analysis showed that exercise during pregnancy can effectively improve psychological well-being and reduce postpartum depressive symptoms [18]. A 2018 meta-analysis showed that exercise during pregnancy can also reduce the risk of cesarean section by 12% [19]. Similar beneficial effects have also been reported in other meta-analyses [20, 21, 24]. In addition, the role of exercise during pregnancy in controlling gestational weight gain and diabetes has also been evaluated in meta-analyses [19, 20, 22, 23]. Without considering maternal body weight as a subgroup factor, previous meta-analyses reported that exercise reduces gestational weight gain by 1.1 kg [20] and the risk of gestational diabetes by 41% [22]. After taking into account maternal body weight, exercise during pregnancy reduces the risk of gestational diabetes by 42% in normal-weight women [19], and the diabetic risk by 24% in overweight and obese women [23], showing that body weight during pregnancy is a critical factor in altering pathophysiological responses to exercise interventions.
In addition to improving maternal health, systematic reviews and meta-analyses also reveal that maternal exercise may benefit the intrauterine environment, which improves fetal development and birth outcomes, though data are limited and inconsistent [20, 25,26,27,28]. Several meta-analyses have shown that maternal exercise during pregnancy can reduce the risks of LGA [20] and preterm birth [25], and has no negative impact on SGA risk [20, 25, 26] or gestational length [20], showing that exercise during pregnancy is safe and beneficial for fetal development. However, other meta-analyses showed that maternal exercise is associated with SGA [27] and has no effect on reducing LGA risk [26] and preterm birth [28], leading to substantial confusion and reducing incentives to exercise during pregnancy [29,30,31]. Accordingly, the American College of Obstetricians and Gynecologists (ACOG) and the US Department of Health and Human Services (DHHS) recommended pregnant women without contraindications to perform moderate-intensity aerobic exercise for at least 20–30 min per day or 150 min per week [32, 33]. Despite that, only 9–15% of pregnant women meet the current exercise recommendations, and the number is much less for overweight and obese pregnant women [29,30,31].
There are various reasons for a lack of exercise during pregnancy, such as knowledge gaps, lack of time, energy, motivation, social support, accessibility of exercise options, and poor physical health [34]. In addition, studies have also reported that fear regarding baby health during and after exercise is one of the main reasons women are sedentary during pregnancy [34]. Although mounting evidence suggests that maternal exercise is safe and beneficial for fetal and child health, some inconsistent reports, showing negative impacts on fetal growth [35, 36], could potentially reduce the incentive to exercise during pregnancy. To alleviate public concerns and fully uncover the benefits of gestational exercise in child health, it is necessary to perform a systematic review and meta-analysis with better consideration of factors affecting exercise outcomes. Because maternal normal weight (MNW) and MO women differ in physiological and metabolic status during pregnancy [37], maternal body weight is a critical factor in how fetal development is altered due to maternal exercise [20, 25, 26, 38], which was not fully considered in previous meta-analyses [20, 25,26,27,28], potentially contributing to the inconsistent association between exercise and outcomes. In addition, maternal exercise is often intermingled with other lifestyle factors, including diet, smoking, alcohol drinking, and stress, which were also not well considered in previous studies [20, 25,26,27,28]. Failure to differentiate exercise impacts from confounders not only substantially introduces study variations, but also prevents revealing the full impacts of maternal exercise on child health [20, 25, 26, 38]. Furthermore, altering the intrauterine environment and fetal development may exert long-term impacts on child growth and health, which were not covered in previous meta-analyses [20, 25,26,27,28]. Considering the limitations of previous studies, the objective of this systematic review and meta-analysis was to synthesize existing evidence of the effects of exercise-only and exercise with confounders during pregnancy, in both MNW and MO, on child growth trajectory and obesity risks. In addition, the impacts of exercise volume (dose) were also assessed.
2 Methods
2.1 Search Strategy and Selection Criteria
This systematic review and meta-analysis was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement, and the checklist was completed (Electronic Supplementary Material Table S1) [39]. This meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020205031). Eligibility criteria followed the PICOS guideline (Population; Intervention; Comparison; Outcome; Study design) [33].
2.1.1 Population
Women who did not receive exercise interventions (control or usual care arms) versus those who received exercise interventions during pregnancy (> 16 years) were the population of interest in this meta-analysis. The included pregnant women had uncomplicated singleton pregnancies and no health issues (2 weeks before and during pregnancy) including (a) type 1 and 2 diabetes mellitus, (b) hypertension or heart issues, (c) chronic renal disease, (d) multiple pregnancy, (e) Rh sensitization, (f) corticosteroid medication, (g) cervical incompetence or cerclage history, (h) antibiotics or tocolytics, (i) sickle cell disease, (j) thalassemia, (k) hemoglobin C, (l) lung diseases, (m) hyperthyroidism, (n) polycystic ovarian syndrome with medication, (o) anemia, (p) eating disorder, and (q) psychosis. The included population also did not have exercise contraindications during or immediately following exercise, including uterine contraction, hypoxia, and other fetal distress, which were defined by the American College of Obstetricians and Gynecologists Committee Opinion and Society of Obstetricians and Gynecologists of Canada [33, 40]. Included studies included pregnant women with prepregnancy normal weight (MNW; BMI 18.5–24.9 kg/m2) and prepregnancy maternal overweight or obesity (MO; BMI ≥ 25 kg/m2). Data from maternal overweight (25 ≤ BMI < 30 kg/m2) and obesity (BMI ≥ 30 kg/m2) exercisers were analyzed together in the meta-analysis due to limited data and no separation in randomized trials.
2.1.2 Interventions
Exercise interventions included both quantified physical activity (skeletal muscle movement with energy expenditure above sedentary conditions, such as recreational, household, and occupational activity) and exercise (planned, structured, and repetitive with the objectives of improving or maintaining physical fitness) [41]. All maternal subjects received interventions during the period of pregnancy and before labor (e.g., labor contraction or water breaking). Because confounders (e.g., dietary nutrition, alcohol drinking, tobacco, food borne infections) were often associated with maternal exercise, to discern “the main effect of exercise”, we performed subgroup analysis if confounders were used, including ‘maternal exercise-only interventions’ and ‘maternal exercise + confounders’ in both MNW and MO groups.
2.1.3 Comparison
Quantified outcomes from maternal exercise and physical activity during pregnancy were compared with control group outcomes from no or less exercise with various types, frequencies, durations, and enrolled time [e.g., treadmill (type); three times per week (frequency); 60 min per session (duration); 13 gestational weeks (enrolled time)].
2.1.4 Outcomes
Maternal exercise outcomes included child obesity indicators in early life [neonates (birth) and infants (0 < age < 2 years)] and childhood (2–15 years)], including birth weight; preterm (< 37 gestational weeks); SGA (birth weight < 10th percentile for age and sex, or < 2,500 g, or > 2 standard deviations below the mean); LGA (birth weight > 90th percentile for age and sex, or > 4000g, or > 2 standard deviations above the mean); childhood weight and obesity (body mass index ≥ 95th percentile for age and sex); the circumference of the head, chest and waist; body length; fat mass (arm, thigh, abdominal) and fat volume (visceral, subcutaneous, and abdominal).
2.1.5 Study design
All randomized controlled trials were included. Other types of studies (e.g., reviews and abstracts) were not included in the current meta-analysis.
2.2 Data Extraction and Quality Assessment
Data search was done by two authors (YT, GL) independently through the NCBI PubMed MeSH, Web of Science, ScienceDirect, and Cochrane Library database. The search was limited to randomized human studies with English language restrictions. Keywords were constructed including primary items of exercise, pregnancy, BMI, obesity and offspring with detailed search keys (Electronic Supplementary Material Table S2). Searching was from inception date to Feb 15, 2020. Library and author contacts were necessarily made for information completeness.
Two authors (YH, QY) independently screened the titles and abstracts and then checked the full texts. PICOS guidelines were used to extract the necessary information. If decisions regarding study quality were not consistent between the reviewers, the article was further sent to the Article Decision Committee (MD, MJ) for a final decision. Before rapid title screening, duplicates were removed by DistillerSR (Evidence Partners, Ottawa, Ontario, Canada). Criteria for study inclusion were data completeness and sufficient sample size (n ≥ 2). The extracted content included the first author, year, title, study design, country, population, selection criteria, participant number, age, prepregnancy BMI, prepregnancy weight, parity, height, tobacco use, alcohol use, labor hours, gestational age, physical treatment details (e.g., exercise type, frequency, intensity, and duration), preterm birth, 1 min Apgar score, cesarean section percentage, stillbirth rate, birth weight, LGA, SGA, fat mass, fat volume, muscle mass, infant weight, infant obesity, childhood weight, child obesity, head and waist circumference, and other child obesity related data. When data were not fully reported in the articles, the authors contacted librarians for data requests. In total, 42 articles were requested from librarians; full manuscripts were received for all requests. Selection, information and confounding biases were determined according to the Newcastle–Ottawa Quality Assessment for cohort studies (Electronic Supplementary Material Table S3) [42].
The quality of each study and publication bias were assessed by two individuals (GL, YT) according to the Cochrane Handbook and Newcastle–Ottawa scale method [42]. The following criteria were used for quality assessment: (a) research hypothesis related to the impacts of exercise-only interventions or exercise + other interventions in MNW and MO during pregnancy on obesity indicators in fetuses, neonates, infants, and children; (b) randomized controlled design; (c) blinded subjects assignment; (d) the similarity of the control group; (e) the similarity of the treatment group; (f) result standardization; (g) bias risks. Microsoft Excel (Excel, Microsoft Corp., Redmond, WA, USA) was used for data collection.
2.3 Statistical Analysis
All analyses were conducted using R v.3.4.3 software [43] and the metafor package [44]. Data were pooled using a random effects model. Dichotomous variable comparison was performed with the effect size (ES) of two group comparison—log odds ratio (OR) and transferred back to an OR with the ‘transf’ method [45]. The effects of prenatal exercise on continuous variables (e.g., child weight) were analyzed by the ES of the standardized mean difference (SMD) [44, 46, 47].
A random model was chosen for meta-analysis according to the DerSimonian and Laird method [48]. The Knapp and Hartung methods were used to adjust the confidence interval [49]. Q test of χ2 and I2 was used to analyze heterogeneity [49]. Values of I2 in the range of 0–50%, 50–75%, and 75–100% indicate low, medium, and high data heterogeneity, respectively [50]. Publication bias was assessed by a funnel plot with Egger’s regression [51, 52]. Sensitivity was analyzed by the trim-and-fill method, which trimmed publication bias, added missing values and checked the study stability [53]. Subgroup analysis was prioritized as follows: (a) normal-weight subjects with normal prepregnancy BMI 18.5–24.9 kg/m2; and (b) MO subjects with prepregnancy BMI ≥ 25 kg/m2. In meta-regression, logOR and SMD variables were analyzed by the random model [54]. Linear regression of the explanatory variables was conducted to examine the existence of the dose–response relationship between maternal exercise volumes and child obesity outcomes [55, 56]. The exercise volume was quantified using metabolic equivalent (MET)-min per week, which is the product of exercise intensity and the minutes of exercise per week [57]. A permutation test was used to analyze the robustness of the meta-regression model [58]. Only studies with complete data (mean, replicates, standard deviation or standard error of both control and treatment groups) were included in the meta-analysis.
3 Results
3.1 Search Results and Study Characteristics
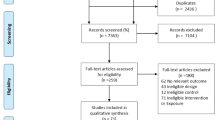
Initial searching with no language restriction yielded a total of 37,418 records (Fig. 1). After screening for English language, title, abstract, and removing duplicates, 315 eligible full texts remained. After excluding reviews and original studies lacking interventions, outcomes, and replicates (Electronic Supplementary Material Table S4), 99 studies were eligible for this meta-analysis, representing 250,028 pregnancies (births) associated with exercise interventions during pregnancy and 346,848 control pregnancies (births) not associated with exercise interventions (Electronic Supplementary Material Table S5) [35, 36, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155].
All studies included in the analysis had a randomized controlled design, and were conducted in Asia, Australia, America, Europe, Ireland, Netherlands, New Zealand or West Africa (Electronic Supplementary Material Table S5) [35, 36, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155]. Studies included varied exercise types, including leisure activity (walking, standing, and household) and training (yoga, bicycling, treadmill exercise, running, jogging, and other ACOG-recommended exercise) [156]. Exercise duration also varied, with a range from 8 weeks to full pregnancy, and exercise volume ranged from 80 to 6000 MET-min per week. Various exercise outcomes were described in different studies, with birth weight as the most commonly reported (81 studies). Because of considerable clinical variations and heterogeneity within and between studies, we used the Newcastle–Ottawa scale to assess study quality [42], a funnel plot-Egger’s test to assess publication bias [52], and a trim-and-fill analysis to assess result sensitivity [53].
3.2 Study Quality, Bias, and Result Sensitivity
All studies included in this meta-analysis were of fair to good quality as indicated by ≥ 5 scores on the Newcastle–Ottawa scale tests (Electronic Supplementary Material Table S6) [35, 36, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155]. Funnel plot analysis further assessed the risk of publication bias if data were available, including exercise-only interventions in MNW, exercise + confounders in MNW, exercise-only intervention in MO, and exercise + confounders in MO (Electronic Supplementary Material Fig S1) [52]. Primary outcomes included birth weight, the OR of preterm birth, the OR of SGA, the OR of LGA, infant and child body weight, and the OR of child obesity. Egger’s test did not find significant publication bias and asymmetry (P > 0.05) in funnel plots, indicating a low publication bias and high quality of the included studies (Electronic Supplementary Material Fig S1) [35, 36, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155]. To assess result sensitivity, after excluding asymmetric outlying studies in the funnel plot using the trim-and-fill method, the results were also highly consistent with the meta-results (Electronic Supplementary Material Table S7), demonstrating the high repeatability and sensitivity of the meta-results.
3.3 Birth Weight
Eighty-one studies (n = 246,340 births) showed no overall effect (including both MNW and MO; SMD, 0.01; 95% CI − 0.10, 0.12; I2 = 98.3%; P = 0.25) of exercise in pregnancy on birth weight (Electronic Supplementary Material Fig S2) [35, 36, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75, 78, 81, 82, 84, 87,88,89,90,91,92,93,94, 96,97,98,99, 101,102,103,104, 108,109,110,111, 116,117,118, 120,121,122,123,124,125,126,127,128,129,130,131,132,133,134, 136,137,138,139,140,141,142,143, 145,146,147, 149, 151,152,153]. In subgroups, exercise-only interventions in MNW (n = 235,725 births; Fig. 2 and Electronic Supplementary Material Fig S2) tended to reduce birth weight (SMD, − 0.09; 95% CI − 0.19, 0.01; I2 = 98.7%; P = 0.08;) [35, 36, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75, 78, 81, 82, 84, 87,88,89, 91, 92, 94, 96, 97, 99, 101, 103, 104, 108,109,110, 116, 118, 120,121,122, 125, 126, 128, 131,132,133, 136,137,138, 140, 141, 143, 146, 151, 152], which was also observed in exercise-only interventions in MO (n = 4389 births; SMD, − 0.15; 95% CI − 0.33, 0.02; I2 = 81.7%; P = 0.08) [90, 93, 98, 117, 123, 124, 127, 130, 139, 153]. However, exercise + confounders in MNW tended to increase the birth weight (n = 5,461 births; SMD, 0.19; 95% CI − 0.01, 0.39; I2 = 88.3%; P = 0.07; Fig. 2 and Electronic Supplementary Material Fig S2) [102, 111, 125, 129, 142, 145, 147, 149]. No significance was observed in exercise + confounders in MO (n = 765 births; SMD, 0.06; 95% CI − 0.10, 0.21; I2 = 0.0%; P = 0.47; Fig. 2 and Electronic Supplementary Material Fig S2) [90, 111, 134, 153].
Summary of forest plot displaying efficacies of exercise-only interventions and exercise + confounders during pregnancy in mothers with maternal normal weight (MNW; prepregnancy BMI 18.5–24.9 kg/m2) or maternal overweight and obesity (MO; prepregnancy BMI ≥ 25 kg/m2) in altering birth weight (standard mean difference, SMD), odds ratio (OR) of preterm birth (< 37 gestational week), OR of small for gestational age (SGA; birth weight < 10th percentile, or < 2500 g, or > 2 standard deviation below the mean), OR of large for gestational age (LGA; birth weight > 90th percentile, or > 4000 g, or > 2 standard deviation above the mean), infant (SMD; age ≤ 2 years) and childhood weight (SMD; age 2–15 years), and OR of childhood obesity (BMI ≥ 95th percentile; age 2–15 years). Error bars indicate 95% confidence interval
3.4 Preterm Birth
Preterm birth and fetal growth restriction contributed to the reduced birth weight, which increased the risks of later life obesity and other metabolic dysfunctions [157]. Thirty-two studies (overall) reported an OR of preterm birth in pregnancies with exercise interventions (n = 81,897 births), showing a reduced overall risk of preterm birth by 18% (OR 0.82; 95% CI 0.70, 0.96; I2 = 80.7%; P = 0.01; Fig. 3) [65, 66, 76, 81, 84, 86, 88, 96, 102,103,104,105,106, 108, 111, 114, 115, 118, 121, 130, 140,141,142, 146,147,148,149, 152]. In subgroup analyses, a decrease in preterm birth risk was also observed in exercise-only interventions in both MNW (n = 76,132 births; OR 0.85; 95% CI 0.72, 1.01; I2 = 83.3%; P = 0.06; Figs. 2 and 3) and MO (n = 3,050 births; OR 0.67; 95% CI 0.49, 0.93; P = 0.01; Figs. 2 and 3) [65, 66, 76, 81, 84, 86, 88, 96, 103,104,105,106, 108, 114, 115, 118, 121, 130, 140, 141, 146,147,148, 152], showing the benefits of exercise-only interventions in reducing the risk of preterm birth. However, exercise + confounders in MNW did not affect preterm birth risk (n = 2715 births; OR 0.62; 95% CI 0.35, 1.10; I2 = 15.8%; P = 0.10; Figs. 2 and 3) [102, 111, 142, 149]. No eligible studies reported the OR of preterm birth in the exercise + confounders in MO subgroup.
Forest plot displaying effects of exercise-only interventions and exercise + confounders of pregnant women with prepregnancy normal weight (MNW; BMI 18.5–24.9 kg/m2) and overweight and obesity (MO; BMI ≥ 25 kg/m2) on odds ratio (OR) of preterm birth. Error bars indicate 95% CI. MET means metabolic equivalent
3.5 SGA
Fifty-three studies (overall) included an OR of SGA following maternal exercise intervention in pregnancy (n = 100,588 births), showing a reduced risk of SGA by 18% (OR 0.82; 95% CI 0.72, 0.93; I2 = 65.7%; P = 0.003; Fig. 4) [35, 65, 76,77,78,79,80, 87, 90, 91, 93, 97, 102, 105, 114,115,116, 119, 121,122,123, 126, 129,130,131,132, 134, 136, 138, 139, 141, 142, 145, 146, 148, 149, 153]. A notable decrease in SGA risk was also observed in exercise-only interventions in both MNW (n = 92,351 births; OR 0.83, 95% CI 0.71, 0.98; I2 = 74.5%; P = 0.02; Figs. 2 and 4) and MO (n = 3,909 births; OR 0.73, 95% CI 0.50, 1.05; I2 = 40.4%; P = 0.09; Figs. 2 and 4) [35, 65, 76,77,78,79,80, 87, 91, 93, 97, 105, 114,115,116, 119, 121,122,123, 126, 130,131,132, 136, 138, 139, 141, 146, 148, 153], showing that exercise-only interventions in pregnancy effectively reduced the risk of low birth weight. For maternal exercise with confounders, the OR of SGA was not significantly affected in either MNW (n = 3565 births; OR 0.98; 95% CI 0.76, 1.27; I2 = 0.0%; P = 0.89; Figs. 2 and 4) or MO exercise (n = 763 births; OR 0.70; 95% CI 0.36, 1.38; I2 = 0.0%; P = 0.31; Figs. 2 and 4) [90, 102, 129, 134, 142, 145, 149, 153].
Forest plot displaying effects of exercise-only interventions and exercise + confounders of pregnant women with prepregnancy normal weight (MNW; BMI 18.5–24.9 kg/m2) and overweight and obesity (MO; BMI ≥ 25 kg/m2) on odds ratio (OR) of small for gestational age (SGA). Error bars indicate 95% CI. MET means metabolic equivalent
3.6 LGA
Forty-four studies (overall) reported an OR of LGA following maternal exercise interventions during pregnancy (n = 171,893 births), showing a 28% decrease in LGA (OR 0.72; 95% CI 0.55, 0.95; I2 = 95.8%; P = 0.02; Fig. 5) [83,84,85, 87, 88, 90, 91, 93, 97, 98, 101,102,103, 105, 108, 111, 112, 115,116,117, 120,121,122, 124,125,126,127, 129,130,131,132, 134, 137, 138, 140, 142, 145, 146, 148, 149, 153]. In subgroup analyses, exercise-only interventions in MNW also reduced the risk of LGA by 17% (n = 84,310 births; OR 0.83; 95% CI 0.74, 0.95; I2 = 60.4%; P = 0.005; Figs. 2 and 5) [84, 85, 87, 88, 91, 97, 98, 101, 103, 105, 108, 112, 116, 120,121,122, 125, 126, 131, 132, 137, 138, 140, 146, 148], and exercise-only interventions in MO reduced the risk of LGA by 55% (n = 81,581 births; OR 0.45; 95% CI 0.18, 1.11; I2 = 98.3%; P = 0.08; Figs. 2 and 5) [83, 90, 93, 115, 117, 124, 127, 130, 153], showing a substantial decrease in risk of large birth weight. For exercise with confounders, the OR of LGA was not affected in either MNW (n = 5080 births; OR 0.99; 95% CI 0.78, 1.25; I2 = 0.0%; P = 0.93; Figs. 2 and 5) or MO exercise (n = 922 births; OR 1.43; 95% CI 0.92, 2.24; I2 = 0.0%; P = 0.11; Figs. 2 and 5) [90, 102, 111, 125, 129, 134, 142, 145, 149, 153].
Forest plot displaying effects of exercise-only interventions and exercise + confounders of pregnant women with prepregnancy normal weight (MNW; BMI 18.5–24.9 kg/m2) and overweight and obesity (MO; BMI ≥ 25 kg/m2) on odds ratio (OR) of large for gestational age. Error bars indicate 95% CI. MET means metabolic equivalent
3.7 Childhood Weight and Obesity Risk
Seven studies (overall) reported infant and child body weight following maternal exercise interventions during pregnancy (n = 43,040) and showed no significant change in body weight (SMD, − 0.05; 95% CI − 0.23, 0.14; I2 = 90.5%; P = 0.63) [89, 94, 100, 107, 111, 150, 154]. In subgroup analysis, three studies reported infant body weight (age < 2 years, n = 224 infants; Fig. 2 and Electronic Supplementary Material Fig S3) [89, 107, 150], and two studies reported child body weight (2–15 years; n = 42,602 children) following exercise-only interventions in MNW [94, 100]. No significant effect was observed in either infant (SMD, − 0.16; 95% CI − 0.62, 0.29; I2 = 57.6%; P = 0.48) or child body weight (SMD, 0.01; 95% CI − 0.16, 0.18; I2 = 88.9%; P = 0.88) following exercise-only interventions in MNW (Fig. 2 and Electronic Supplementary Material Fig S3). Only one study reported infant [111] or child body weight [114] from exercise + confounders in MNW, which was insufficient for subgroup analysis. Furthermore, no eligible studies reported infant and child body weight from exercise-only intervention or exercise + confounders in MO.
Two studies (overall) reported an OR of childhood obesity (2–15 years) following maternal exercise during pregnancy (n = 27,410 children; Fig. 2 and Electronic Supplementary Material Fig S4) [111, 135], and showed significant decrease in child obesity risk by 35% (OR 0.65; 95% CI 0.45, 0.94; I2 = 80%; P = 0.02; Fig. 2 and Electronic Supplementary Material Fig S4), disclosing a long-term effect of maternal exercise in reducing the risk of child obesity. Furthermore, exercise-only interventions in MNW also reduced childhood obesity risk by 53% (n = 6920 children; OR 0.47; 95% CI 0.36, 0.63; I2 = 77%; P < 0.001) [111]. Insufficient studies were available for assessing the OR of childhood obesity from exercise-only interventions or exercise + confounders in MO.
3.8 Meta-regression of Exercise Volume on Outcomes and Sensitivity Analysis
We also conducted meta-regression to investigate the dose (volume) effects of exercise on fetal growth and childhood obesity outcomes (Table 1). Birth weight was negatively and dose-dependently associated with the volume of exercise-only interventions in MNW (P = 0.01; n = 235,725 births; Table 1) [35, 36, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75, 78, 81, 82, 84, 87,88,89, 91, 92, 94, 96, 97, 99, 101, 103, 104, 108,109,110, 116, 118, 120,121,122, 125, 126, 128, 131,132,133, 136,137,138, 140, 141, 143, 146, 151, 152]. The reduced birth weight was particularly driven by exercise volume over 810 MET-min per week (Electronic Supplementary Material Fig S5). The volume of exercise + confounders in MNW was positively and linearly associated with birth weight (P = 0.01; n = 5461 births; Table 1), ranging from 180 to 945 MET-min per week (Electronic Supplementary Material Fig S5) [102, 111, 125, 129, 142, 145, 147, 149]. Except for the birth weight, no significant exercise dose effect was observed for other outcomes (Table 1). To test the meta-regression sensitivity, we conducted a permutation test [58] and the results observed were consistent with the meta-regression, showing a high sensitivity of the meta-regression results (Table 1).
4 Discussion
4.1 Summary of Evidence
The prevalence of child obesity has dramatically increased in recent decades, becoming a serious public health concern [158]. The “developmental origins of health and disease” suggest that the intrauterine environment programs fetal organ/tissue development, projecting a trajectory of metabolic diseases in the later life of offspring [159,160,161]. Previous systematic reviews have summarized that exercise during pregnancy benefits maternal health, including reduced occurrence of gestational and postpartum weight gain, gestational diabetes, hypertension, pre-eclampsia, depression, and anxiety [18, 19, 22, 162]. Exercise in women with normal pregnancies or with gestational diabetes also has no adverse effect on fetal heart rate, hyperthermia, neonatal morbidity or mortality [163, 164]. Notably, a recent meta-analysis showed that maternal exercise during pregnancy reduces the risk of macrosomia [165], which may exert preventive effects against childhood obesity [166, 167], but the programming impacts of exercise in MNW and MO during pregnancy on fetal growth and childhood obesity are largely unknown. The current meta-analysis of 99 randomized controlled trials, including exercise interventions in MNW and MO (250,028) and controls (346,848) during pregnancy, showed that maternal exercise-only interventions reduced the risks of adverse fetal growth and birth outcomes, including the OR of preterm birth, SGA, and LGA (Fig. 2). In addition, exercise interventions in MNW substantially reduced risk of childhood obesity. The beneficial outcomes were more evident in maternal exercise-only interventions relative to exercise with confounders. In meta-regression, exercise-only volume in MNW dose-dependently reduced birth weight, particularly for exercise volumes greater than 810 MET-min per week (ACOG and DHHS recommendation: 500 MET-min per week equals approximately 150 min/week moderate aerobic exercise) [32, 33], but no significant dose effect was observed in other maternal exercise-only outcomes. Overall, this analysis suggests that exercise in pregnant women improves fetal growth, effectively reducing susceptibility to childhood obesity.
SGA is a leading cause of neonatal mortality and morbidity that primarily results from preterm birth and fetal growth restriction [157]. SGA babies have a greater risk for complications from immature fetal development, including in the brain, pancreas, skeletal muscle, and adipose tissue, contributing to an increased risk of childhood obesity and type 2 diabetes [168]. Exercise during pregnancy significantly increases oxygen and nutrient consumption in maternal skeletal muscle, which potentially reduces their availability to growing fetuses during and shortly after exercise [169, 170]. In addition, exercise may stimulate the release of oxytocin and uterine contraction [171], which may increase the risks of preterm birth [36, 63, 89] and fetal growth restriction [36, 65, 66]. Although these negative impacts are not assured, they discourage participation in active physical activity during pregnancy, especially in the last trimester [34]. By synthesizing a large body of available data, our meta-analysis showed that exercise-only interventions in both MNW and MO have a tendency to reduce birth weight, but the decrease in birth weight is not associated with an increased risk of preterm birth and SGA; indeed, maternal exercise-only interventions significantly reduced the risks of preterm birth (MNW, 15% decrease; MO, 33% decrease) and SGA (MNW, 17% decrease; MO, 27% decrease), revealing that maternal exercise has no adverse effects but rather improves birth outcomes by reducing the risks of preterm birth and restricted fetal growth. Notably, the exercise volume from exercise-only interventions in MNW dose-dependently reduced birth weight, and the association was dominantly driven by exercise volumes over 810 MET-min per week. Because this dose is much greater than the 500 MET-min per week recommended by ACOG and DHHS [32, 33], moderate aerobic exercise in pregnancy is safe and has a minimal impact on fetal growth. An impairment of placental vascular branching and nutrient perfusion is mainly responsible for the fetal growth restriction observed in obese pregnant women [172]. Recent studies have showed that maternal exercise promotes placental angiogenesis, branching, and blood flow in healthy and obese pregnant mice [173], which enhances placental nutrient exchange, likely contributing to the reduced risks of fetal growth restriction in exercise interventions in both MNW and MO.
Gestation is coupled with an increased maternal insulin resistance, which favors glucose partitioning to growing fetuses [174]. However, aggravated insulin resistance causes excessive movements of glucose and nutrients across the placenta, leading to fetal overgrowth and resulting in LGA or macrosomia. In addition to the risks of shoulder dystocia, brachial plexus trauma, and hypoglycemia in delivery, macrosomic babies likely have excessive body fat deposition [175] and accelerated pancreatic β-cell maturation [176], contributing to childhood obesity and type 2 diabetes [7]. MO and gestational diabetes increase the risk of macrosomia [7, 11]. Exercise counteracts hyperglycemia and hyperlipidemia by increasing insulin sensitivity and glucose and lipid consumption in skeletal muscle, reducing the risk of fetal overgrowth and associated childhood obesity [166, 173]. Consistently, a recent meta-analysis showed that exercise in women with gestational diabetes can reduce the risk of babies born LGA and neonatal adiposity [177]. In this meta-analysis, exercise-only interventions in both MNW and MO reduced the risk of LGA (> 90th percentile) by 17% and 55%, respectively, clearly showing that maternal exercise during pregnancy effectively reduces the risk of fetal excessive growth regardless of prepregnancy BMI.
Childhood obesity is prevalent worldwide, and its prevention has become a public health priority [1, 2]. Though genetic variance and living environment play substantial roles in the development of childhood obesity, evidence in recent decades also shows that childhood obesity can be traced back to intrauterine life, which is affected by maternal lifestyle, including overweight and obesity, alcohol consumption and smoking [159, 160]. Our systematic review and meta-analysis showed that maternal exercise-only interventions during pregnancy reduce the risks of fetal premature birth and excessive growth, which are important indicators of later life obesity [10,11,12,13,14]. Consistently, exercise-only interventions in MNW significantly reduced the risk of childhood obesity by 53%, exerting a persistent anti-obesity effect. However, due to limited data in the current literature, we cannot evaluate the impacts of exercise interventions in MO on the OR of childhood obesity, and call for more studies in this area. Supportively, recent studies in mice show that MO exercise reduced macrosomia, and exerted anti-obesity effects in childhood and adults [166, 173]. In this meta-analysis, although maternal exercise alone showed significant benefits on fetal and child healthy growth, these effects were not observed in maternal exercise with confounders. The number of published studies involved in exercise with confounders was much lower than that for exercise-only interventions, which potentially reduced the robustness of the analyses measuring interactive effects between exercise and other confounders. Furthermore, confounders were involved in multiple lifestyle alterations, including alcohol drinking, diet, stress, and smoking [102, 111, 125, 129, 142, 145, 147, 149], which increased study variations and confounding of treatment effects. Nonetheless, developing healthy lifestyles in pregnant women, such as consuming high-quality food and limiting stress, smoking and alcohol drinking, has been demonstrated to robustly improve fetal growth and child health [161, 178, 179]; more studies are required to better understand the interactive and optimal effects of maternal exercise along with those confounders on fetal development and childhood obesity.
4.2 Strengths and Limitations
To our knowledge, there is no meta-analysis covering the effects of exercise interventions (exercise-only intervention, exercise + confounders) in MNW and MO during pregnancy on prenatal growth and childhood obesity. This systematic review and meta-analysis synthesized data from available randomized trials, which significantly increases the power of the conclusion. Publication bias risk was also assessed by funnel plots, and sensitivity was assessed by the trim-and-fill method. In this meta-analysis, sensitivity analysis was conducted along with meta-regression, showing the high repeatability of the data. In relation to dose effects, the meta-regression also revealed that high doses of maternal exercise during pregnancy were associated with reduced birth weight but were not related to the OR of preterm birth and SGA. The permutation test also showed the high sensitivity of meta-regression. A limitation of the current systematic review and meta-analysis is the existence of exercise intervention heterogeneity (varied type, intensity, duration, participating age and trimester stage), which may confound data interpretation. In addition, data on the programming impacts of exercise in MO during pregnancy on childhood weight and obesity risk are scarce, limiting the quality of assessment. Other confounding factors, such as maternal and child lifestyle behaviors (dietary nutrition, smoking, alcohol drinking, and socioeconomic status), may also increase data variations.
4.3 Future Directions
Due to limited data assessing the long-term impacts of exercise in MNW and MO on child growth and health, more follow-up studies are urgently required. In addition, metabolic, genetic, and epigenetic data of fetal and postnatal offspring affected by maternal exercise during pregnancy are also very limited in the current literature; thus, fundamental studies are needed to deepen our understanding of the beneficial impacts of maternal exercise on child health. Greater mechanistic understanding could lead to pharmaceutical intervention targets to mimic exercise, which could benefit the fetal growth and child health of pregnant women with exercise contraindications.
5 Conclusions
Overall, our findings revealed that maternal exercise-only intervention during pregnancy effectively reduced the risks of abnormal fetal growth and childhood obesity, including the risk of preterm birth (18% overall decrease), SGA (18% overall decrease), LGA (28% overall decrease), and childhood obesity (53% decrease from exercise-only interventions in MNW). Birth weight, infant, and childhood weight were not altered by exercise in either MNW or MO. Increased exercise volume in MNW was associated with reduced birth weight, particularly with exercise volume over 810 MET-min per week, but was not associated with the risk of preterm birth and SGA. Overall, maternal exercise during pregnancy, regardless of prepregnancy BMI, is a safe and beneficial nonpharmaceutical intervention for reducing the risk of adverse fetal growth and childhood obesity, providing an opportunity to prevent prevalent type 2 diabetes and cardiovascular diseases later in life.
Change history
09 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40279-021-01512-y
References
World Health Organization. Overweight and obesity. Geneva: World Health Organization; 2018.
Livingston EH. Reimagining obesity in 2018: a JAMA theme issue on obesity. JAMA. 2018;319(3):238–40.
Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa heart study. Pediatrics. 1999;103(6 Pt 1):1175–82.
Anderson PM, Butcher KF, Schanzenbach DW. Childhood disadvantage and obesity: is nurture trumping nature? National bureau of economic research; 2007. Report No.: 0898-2937.
Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73.
Wood CT, Linthavong O, Perrin EM, et al. Antecedents of obesity among children born extremely preterm. Pediatrics. 2018;142(5):e20180519.
Yu ZB, Han SP, Zhu GZ, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev. 2011;12(7):525–42.
Ou-Yang MC, Sun Y, Liebowitz M, et al. Accelerated weight gain, prematurity, and the risk of childhood obesity: a meta-analysis and systematic review. PLoS ONE. 2020;15(5):e0232238.
Strutz KL, Richardson LJ, Hussey JM. Selected preconception health indicators and birth weight disparities in a national study. Womens Health Issues. 2014;24(1):e89-97.
Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114(1):e29-36.
Heslehurst N, Vieira R, Akhter Z, et al. The association between maternal body mass index and child obesity: a systematic review and meta-analysis. PLoS Med. 2019;16(6):e1002817.
Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS ONE. 2013;8(4):e61627.
McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ. 2010;341:c3428.
Voerman E, Santos S, Patro Golab B, et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: an individual participant data meta-analysis. PLoS Med. 2019;16(2):e1002744.
Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: national health interview survey, 2012. Vital and health statistics series 10, data from the national health survey. 2014(260):1.
Martin JA, Hamilton BE, Osterman M, Driscoll A, Mathews T. Births: final data for 2018. Hyattsville: National Center for Health Statistics, CDC; 2019.
Fisher S, Kim S, Sharma A, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med. 2013;56(6):372–8.
Poyatos-León R, García-Hermoso A, Sanabria-Martínez G, Álvarez-Bueno C, Cavero-Redondo I, Martínez-Vizcaíno V. Effects of exercise-based interventions on postpartum depression: a meta-analysis of randomized controlled trials. Birth. 2017;44(3):200–8.
Ming WK, Ding W, Zhang CJP, et al. The effect of exercise during pregnancy on gestational diabetes mellitus in normal-weight women: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):440.
Wiebe HW, Boulé NG, Chari R, Davenport MH. The effect of supervised prenatal exercise on fetal growth: a meta-analysis. Obstet Gynecol. 2015;125(5):1185–94.
Davenport MH, Ruchat SM, Sobierajski F, et al. Impact of prenatal exercise on maternal harms, labour and delivery outcomes: a systematic review and meta-analysis. Br J Sports Med. 2019;53(2):99–107.
Yu Y, Xie R, Shen C, Shu L. Effect of exercise during pregnancy to prevent gestational diabetes mellitus: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2018;31(12):1632–7.
Nasiri-Amiri F, Sepidarkish M, Shirvani MA, Habibipour P, Tabari NSM. The effect of exercise on the prevention of gestational diabetes in obese and overweight pregnant women: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11(1):72.
Davenport MH, McCurdy AP, Mottola MF, et al. Impact of prenatal exercise on both prenatal and postnatal anxiety and depressive symptoms: a systematic review and meta-analysis. Br J Sports Med. 2018;52(21):1376.
Beetham KS, Giles C, Noetel M, Clifton V, Jones JC, Naughton G. The effects of vigorous intensity exercise in the third trimester of pregnancy: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):281.
Pastorino S, Bishop T, Crozier SR, et al. Associations between maternal physical activity in early and late pregnancy and offspring birth size: remote federated individual level meta-analysis from eight cohort studies. BJOG. 2019;126(4):459–70.
Bisson M, Lavoie-Guénette J, Tremblay A, Marc I. Physical activity volumes during pregnancy: a systematic review and meta-analysis of observational studies assessing the association with infant’s birth weight. AJP Rep. 2016;6(2):e170–97.
Wen J, Xun P, Chen C, et al. Non-occupational physical activity during pregnancy and the risk of preterm birth: a meta-analysis of observational and interventional studies. Sci Rep. 2017;7(1):44842.
Borodulin KM, Evenson KR, Wen F, Herring AH, Benson AM. Physical activity patterns during pregnancy. Med Sci Sports Exerc. 2008;40(11):1901–8.
Evenson KR, Wen F. Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Prev Med. 2011;53(1–2):39–43.
Santo EC, Forbes PW, Oken E, Belfort MB. Determinants of physical activity frequency and provider advice during pregnancy. BMC Pregnancy Childbirth. 2017;17(1):286.
US Department of Health and Human Services. Physical activity guidelines for Americans. Washington: HHS; 2008.
ACOG Committee Opinion No. 650. Physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol. 2015;126(6):e135–42.
Haakstad LA, Voldner N, Henriksen T, Bø K. Why do pregnant women stop exercising in the third trimester? Acta Obstet Gynecol Scand. 2009;88(11):1267–75.
Bell RJ, Palma SM, Lumley JM. The effect of vigorous exercise during pregnancy on birth-weight. Aust NZ J Obstet Gynaecol. 1995;35(1):46–51.
Clapp JF 3rd, Dickstein S. Endurance exercise and pregnancy outcome. Med Sci Sports Exerc. 1984;16(6):556–62.
Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update. 2010;16(3):255–75.
Guillemette L, Hay JL, Kehler DS, et al. Exercise in pregnancy and children’s cardiometabolic risk factors: a systematic review and meta-analysis. Sports Med Open. 2018;4(1):35.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Davies GA, Wolfe LA, Mottola MF, MacKinnon C. Joint SOGC/CSEP clinical practice guideline: exercise in pregnancy and the postpartum period. Can J Appl Physiol. 2003;28(3):330–41.
Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31.
Wells G, Shea B, O’Connell D, et al. Newcastle–Ottawa quality assessment scale cohort studies. 2014.
Team RC. R: a language and environment for statistical computing. Vienna; 2013.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48.
Rücker G, Schwarzer G, Carpenter J, Olkin I. Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat Med. 2009;28(5):721–38.
Crippa A, Orsini N. Dose-response meta-analysis of differences in means. BMC Med Res Methodol. 2016;16:91.
Ma G, Chen Y. Polyphenol supplementation benefits human health via gut microbiota: a systematic review via meta-analysis. J Funct Foods. 2020;66:103829.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693–710.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Rodney RM, Celi P, Scott W, Breinhild K, Lean IJ. Effects of dietary fat on fertility of dairy cattle: a meta-analysis and meta-regression. J Dairy Sci. 2015;98(8):5601–20.
Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600.
Schwarzer G, Carpenter J, Rücker G. Empirical evaluation suggests Copas selection model preferable to trim-and-fill method for selection bias in meta-analysis. J Clin Epidemiol. 2010;63(3):282–8.
Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407–15.
Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9.
Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73.
Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–34.
Good P. Permutation tests: a practical guide to resampling methods for testing hypotheses. Springer Science and Business Media; 2013.
Pomerance JJ, Gluck L, Lynch VA. Physical fitness in pregnancy: its effect on pregnancy outcome. Am J Obstet Gynecol. 1974;119(7):867–76.
Briend A. Maternal physical activity, birth weight and perinatal mortality. Med Hypotheses. 1980;6(11):1157–70.
Dale E, Mullinax KM, Bryan DH. Exercise during pregnancy: effects on the fetus. Can J Appl Sport Sci. 1982;7(2):98–103.
Hall DC, Kaufmann DA. Effects of aerobic and strength conditioning on pregnancy outcomes. Am J Obstet Gynecol. 1987;157(5):1199–203.
Beckmann CR, Beckmann CA. Effect of a structured antepartum exercise program on pregnancy and labor outcome in primiparas. J Reprod Med. 1990;35(7):704–9.
Clapp JF 3rd, Capeless EL. Neonatal morphometrics after endurance exercise during pregnancy. Am J Obstet Gynecol. 1990;163(6 Pt 1):1805–11.
Homer CJ, Beresford SA, James SA, Siegel E, Wilcox S. Work-related physical exertion and risk of preterm, low birthweight delivery. Paediatr Perinat Epidemiol. 1990;4(2):161–74.
Klebanoff MA, Shiono PH, Carey JC. The effect of physical activity during pregnancy on preterm delivery and birth weight. Am J Obstet Gynecol. 1990;163(5 Pt 1):1450–6.
Botkin C, Driscoll CE. Maternal aerobic exercise: newborn effects. Fam Pract Res J. 1991;11(4):387–93.
Rose NC, Haddow JE, Palomaki GE, Knight GJ. Self-rated physical activity level during the second trimester and pregnancy outcome. Obstet Gynecol. 1991;78(6):1078–80.
Hatch MC, Shu XO, McLean DE, et al. Maternal exercise during pregnancy, physical fitness, and fetal growth. Am J Epidemiol. 1993;137(10):1105–14.
Zeanah M, Schlosser SP. Adherence to ACOG guidelines on exercise during pregnancy: effect on pregnancy outcome. J Obstet Gynecol Neonatal Nurs. 1993;22(4):329–35.
Johnson AA, Knight EM, Edwards CH, et al. Selected lifestyle practices in urban African American women—relationships to pregnancy outcome, dietary intakes and anthropometric measurements. J Nutr. 1994;124(6 Suppl):963s-s972.
Henriksen TB, Hedegaard M, Secher NJ. Standing and walking at work and birthweight. Acta Obstet Gynecol Scand. 1995;74(7):509–16.
Sternfeld B, Quesenberry CP Jr, Eskenazi B, Newman LA. Exercise during pregnancy and pregnancy outcome. Med Sci Sports Exerc. 1995;27(5):634–40.
Clapp JF 3rd, Kim H, Burciu B, Lopez B. Beginning regular exercise in early pregnancy: effect on fetoplacental growth. Am J Obstet Gynecol. 2000;183(6):1484–8.
Bell R. The effects of vigorous exercise during pregnancy on birth weight. J Sci Med Sport. 2002;5(1):32–6.
Leiferman JA, Evenson KR. The effect of regular leisure physical activity on birth outcomes. Matern Child Health J. 2003;7(1):59–64.
Takito MY, Benício MH, Latorre MR. Maternal posture and its influence on birthweight. Rev Saude Publica. 2005;39(3):325–32.
Duncombe D, Skouteris H, Wertheim EH, Kelly L, Fraser V, Paxton SJ. Vigorous exercise and birth outcomes in a sample of recreational exercisers: a prospective study across pregnancy. Aust NZ J Obstet Gynaecol. 2006;46(4):288–92.
Orr ST, James SA, Garry J, Prince CB, Newton ER. Exercise and pregnancy outcome among urban, low-income, black women. Ethn Dis. 2006;16(4):933–7.
Dwarkanath P, Muthayya S, Vaz M, et al. The relationship between maternal physical activity during pregnancy and birth weight. Asia Pac J Clin Nutr. 2007;16(4):704–10.
Elden H, Ostgaard HC, Fagevik-Olsen M, Ladfors L, Hagberg H. Treatments of pelvic girdle pain in pregnant women: adverse effects of standard treatment, acupuncture and stabilising exercises on the pregnancy, mother, delivery and the fetus/neonate. BMC Complement Altern Med. 2008;8:34.
Moyer-Mileur LJ, Ball SD, Brunstetter VL, Chan GM. Maternal-administered physical activity enhances bone mineral acquisition in premature very low birth weight infants. J Perinatol. 2008;28(6):432–7.
Snapp CA, Donaldson SK. Gestational diabetes mellitus: physical exercise and health outcomes. Biol Res Nurs. 2008;10(2):145–55.
Barakat R, Lucia A, Ruiz JR. Resistance exercise training during pregnancy and newborn’s birth size: a randomised controlled trial. Int J Obes (Lond). 2009;33(9):1048–57.
Owe KM, Nystad W, Bø K. Association between regular exercise and excessive newborn birth weight. Obstet Gynecol. 2009;114(4):770–6.
Vrijkotte TG, van der Wal MF, van Eijsden M, Bonsel GJ. First-trimester working conditions and birthweight: a prospective cohort study. Am J Public Health. 2009;99(8):1409–16.
Fleten C, Stigum H, Magnus P, Nystad W. Exercise during pregnancy, maternal prepregnancy body mass index, and birth weight. Obstet Gynecol. 2010;115(2 Pt 1):331–7.
Hegaard HK, Petersson K, Hedegaard M, et al. Sports and leisure-time physical activity in pregnancy and birth weight: a population-based study. Scand J Med Sci Sports. 2010;20(1):e96-102.
Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J Clin Endocrinol Metab. 2010;95(5):2080–8.
Mottola MF, Giroux I, Gratton R, et al. Nutrition and exercise prevent excess weight gain in overweight pregnant women. Med Sci Sports Exerc. 2010;42(2):265–72.
Haakstad LA, Bø K. Exercise in pregnant women and birth weight: a randomized controlled trial. BMC Pregnancy Childbirth. 2011;11:66.
Koushkie Jahromi M, Namavar Jahromi B, Hojjati S. Relationship between daily physical activity during last month of pregnancy and pregnancy outcome. Iran Red Crescent Med J. 2011;13(1):15–20.
Nascimento SL, Surita FG, Parpinelli M, Siani S, Pinto e Silva JL. The effect of an antenatal physical exercise programme on maternal/perinatal outcomes and quality of life in overweight and obese pregnant women: a randomised clinical trial. BJOG. 2011;118(12):1455–63.
Salonen MK, Kajantie E, Osmond C, et al. Developmental origins of physical fitness: the Helsinki birth cohort study. PLoS ONE. 2011;6(7):e22302.
de Oliveria Melo AS, Silva JL, Tavares JS, Barros VO, Leite DF, Amorim MM. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: a randomized controlled trial. Obstet Gynecol. 2012;120(2 Pt 1):302–10.
Jukic AM, Evenson KR, Daniels JL, Herring AH, Wilcox AJ, Hartmann KE. A prospective study of the association between vigorous physical activity during pregnancy and length of gestation and birthweight. Matern Child Health J. 2012;16(5):1031–44.
Mudd LM, Pivarnik J, Holzman CB, Paneth N, Pfeiffer K, Chung H. Leisure-time physical activity in pregnancy and the birth weight distribution: where is the effect? J Phys Act Health. 2012;9(8):1168–77.
Oostdam N, van Poppel MN, Wouters MG, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG. 2012;119(9):1098–107.
Price BB, Amini SB, Kappeler K. Exercise in pregnancy: effect on fitness and obstetric outcomes—a randomized trial. Med Sci Sports Exerc. 2012;44(12):2263–9.
Schou Andersen C, Juhl M, Gamborg M, Sørensen TI, Nohr EA. Maternal recreational exercise during pregnancy in relation to children’s BMI at 7 years of age. Int J Pediatr. 2012;2012:920583.
Krogsgaard S, Gudmundsdottir SL, Nilsen TI. Prepregnancy physical activity in relation to offspring birth weight: a prospective population-based study in Norway-the hunt study. J Pregnancy. 2013;2013:780180.
Rauh K, Gabriel E, Kerschbaum E, et al. Safety and efficacy of a lifestyle intervention for pregnant women to prevent excessive maternal weight gain: a cluster-randomized controlled trial. BMC Pregnancy Childbirth. 2013;13:151.
Tomić V, Sporiš G, Tomić J, Milanović Z, Zigmundovac-Klaić D, Pantelić S. The effect of maternal exercise during pregnancy on abnormal fetal growth. Croat Med J. 2013;54(4):362–8.
Barakat R, Perales M, Bacchi M, Coteron J, Refoyo I. A program of exercise throughout pregnancy. Is it safe to mother and newborn? Am J Health Promot. 2014;29(1):2–8.
Currie LM, Woolcott CG, Fell DB, Armson BA, Dodds L. The association between physical activity and maternal and neonatal outcomes: a prospective cohort. Matern Child Health J. 2014;18(8):1823–30.
Ghodsi Z, Asltoghiri M. Effects of aerobic exercise training on maternal and neonatal outcome: a randomized controlled trial on pregnant women in Iran. J Pak Med Assoc. 2014;64(9):1053–6.
Kong KL, Campbell C, Wagner K, Peterson A, Lanningham-Foster L. Impact of a walking intervention during pregnancy on post-partum weight retention and infant anthropometric outcomes. J Dev Orig Health Dis. 2014;5(3):259–67.
Li Q, Cui H, Zheng D, Li N, Chang L, Liu C. Effects of walking exercise during late trimester on pregnancy outcome of low-risk primipara. Zhonghua Yi Xue Za Zhi. 2014;94(22):1722–5.
Przybyłowicz K, Przybyłowicz M, Grzybiak M, Janiszewska K. Effects of physical activity during pregnancy and gestational weight gain on newborn weight and length at birth in Warmińsko-Mazurskie province. Acta Sci Pol Technol Aliment. 2014;13(2):203–11.
Reid EW, McNeill JA, Alderdice FA, Tully MA, Holmes VA. Physical activity, sedentary behaviour and fetal macrosomia in uncomplicated pregnancies: a prospective cohort study. Midwifery. 2014;30(12):1202–9.
Tanvig M, Vinter CA, Jørgensen JS, et al. Anthropometrics and body composition by dual energy X-ray in children of obese women: a follow-up of a randomized controlled trial (the Lifestyle in Pregnancy and Offspring [LiPO] study). PLoS ONE. 2014;9(2):e89590.
Mudd LM, Pivarnik JM, Pfeiffer KA, Paneth N, Chung H, Holzman C. Maternal physical activity during pregnancy, child leisure-time activity, and child weight status at 3 to 9 years. J Phys Act Health. 2015;12(4):506–14.
Tanvig M, Vinter CA, Jørgensen JS, et al. Effects of lifestyle intervention in pregnancy and anthropometrics at birth on offspring metabolic profile at 2.8 years: results from the Lifestyle in Pregnancy and Offspring (LiPO) study. J Clin Endocrinol Metab. 2015;100(1):175–83.
Vamos CA, Flory S, Sun H, et al. Do physical activity patterns across the lifecourse impact birth outcomes? Matern Child Health J. 2015;19(8):1775–82.
Wang C, Zhu W, Wei Y, Feng H, Su R, Yang H. Exercise intervention during pregnancy can be used to manage weight gain and improve pregnancy outcomes in women with gestational diabetes mellitus. BMC Pregnancy Childbirth. 2015;15:255.
Daly N, Mitchell C, Farren M, Kennelly MM, Hussey J, Turner MJ. Maternal obesity and physical activity and exercise levels as pregnancy advances: an observational study. Ir J Med Sci. 2016;185(2):357–70.
Dodd JM, Deussen AR, Mohamad I, et al. The effect of antenatal lifestyle advice for women who are overweight or obese on secondary measures of neonatal body composition: the LIMIT randomised trial. BJOG. 2016;123(2):244–53.
Lindqvist M, Lindkvist M, Eurenius E, Persson M, Ivarsson A, Mogren I. Leisure time physical activity among pregnant women and its associations with maternal characteristics and pregnancy outcomes. Sex Reprod Healthc. 2016;9:14–20.
Rêgo AS, Alves MT, Batista RF, et al. Physical activity in pregnancy and adverse birth outcomes. Cad Saude Publica. 2016;32(11):e00086915.
Badon SE, Littman AJ, Chan KCG, Williams MA, Enquobahrie DA. Trajectories of maternal leisure-time physical activity and sedentary behavior during adolescence to young adulthood and offspring birthweight. Ann Epidemiol. 2017;27(11):701-7.e703.
Barakat R, Perales M, Cordero Y, Bacchi M, Mottola MF. Influence of land or water exercise in pregnancy on outcomes: a cross-sectional study. Med Sci Sports Exerc. 2017;49(7):1397–403.
da Silva SG, Hallal PC, Domingues MR, et al. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: results from the PAMELA study. Int J Behav Nutr Phys Act. 2017;14(1):175.
Daly N, Farren M, McKeating A, O’Kelly R, Stapleton M, Turner MJ. A medically supervised pregnancy exercise intervention in obese women: a randomized controlled trial. Obstet Gynecol. 2017;130(5):1001–10.
Garnæs KK, Nyrnes SA, Salvesen K, Salvesen Ø, Mørkved S, Moholdt T. Effect of supervised exercise training during pregnancy on neonatal and maternal outcomes among overweight and obese women. Secondary analyses of the ETIP trial: a randomised controlled trial. PLoS ONE. 2017;12(3):e0173937.
Hegaard HK, Rode L, Katballe MK, Langberg H, Ottesen B, Damm P. Influence of pre-pregnancy leisure time physical activity on gestational and postpartum weight gain and birth weight—a cohort study. J Obstet Gynaecol. 2017;37(6):736–41.
Norris T, McCarthy FP, Khashan AS, et al. Do changing levels of maternal exercise during pregnancy affect neonatal adiposity? Secondary analysis of the babies after SCOPE: evaluating the longitudinal impact using neurological and nutritional endpoints (BASELINE) birth cohort (Cork, Ireland). BMJ Open. 2017;7(11):e017987.
Patel N, Godfrey KM, Pasupathy D, et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. Int J Obes (Lond). 2017;41(7):1018–26.
Rodríguez-Díaz L, Ruiz-Frutos C, Vázquez-Lara JM, Ramírez-Rodrigo J, Villaverde-Gutiérrez C, Torres-Luque G. Effectiveness of a physical activity programme based on the Pilates method in pregnancy and labour. Enferm Clin. 2017;27(5):271–7.
Ronnberg AK, Hanson U, Nilsson K. Effects of an antenatal lifestyle intervention on offspring obesity—a 5-year follow-up of a randomized controlled trial. Acta Obstet Gynecol Scand. 2017;96(9):1093–9.
Wang C, Wei Y, Zhang X, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol. 2017;216(4):340–51.
Bacchi M, Mottola MF, Perales M, Refoyo I, Barakat R. Aquatic activities during pregnancy prevent excessive maternal weight gain and preserve birth weight: a randomized clinical trial. Am J Health Promot. 2018;32(3):729–35.
Badon SE, Littman AJ, Chan KCG, Williams MA, Enquobahrie DA. Associations of maternal light/moderate leisure-time walking and yoga with offspring birth size. J Phys Act Health. 2018;15(6):430–9.
Barakat R, Vargas M, Brik M, et al. Does exercise during pregnancy affect placental weight?: a randomized clinical trial. Eval Health Prof. 2018;41(3):400–14.
Chan RS, Tam WH, Ho IC, et al. Randomized trial examining effectiveness of lifestyle intervention in reducing gestational diabetes in high risk Chinese pregnant women in Hong Kong. Sci Rep. 2018;8(1):13849.
Dhana K, Haines J, Liu G, et al. Association between maternal adherence to healthy lifestyle practices and risk of obesity in offspring: results from two prospective cohort studies of mother–child pairs in the United States. BMJ. 2018;362:k2486.
Huang L, Fan L, Ding P, et al. The mediating role of placenta in the relationship between maternal exercise during pregnancy and full-term low birth weight. J Matern Fetal Neonatal Med. 2018;31(12):1561–7.
McDonald SM, Yeo S, Liu J, Wilcox S, Sui X, Pate RR. Associations between maternal physical activity and fitness during pregnancy and infant birthweight. Prev Med Rep. 2018;11:1–6.
Mizgier M, Mruczyk K, Jarząbek-Bielecka G, Jeszka J. The impact of physical activity during pregnancy on maternal weight and obstetric outcomes. Ginekol Pol. 2018;89(2):80–8.
Myrex P, Harper L, Gould S. An evaluation of birth outcomes in overweight and obese pregnant women who exercised during pregnancy. Sports (Basel). 2018;6(4):138.
Barakat R, Refoyo I, Coteron J, Franco E. Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes. A randomized controlled trial. Braz J Phys Ther. 2019;23(2):148–55.
Brik M, Fernández-Buhigas I, Martin-Arias A, Vargas-Terrones M, Barakat R, Santacruz B. Does exercise during pregnancy impact on maternal weight gain and fetal cardiac function? A randomized controlled trial. Ultrasound Obstet Gynecol. 2019;53(5):583–9.
Buckingham-Schutt LM, Ellingson LD, Vazou S, Campbell CG. The behavioral wellness in pregnancy study: a randomized controlled trial of a multi-component intervention to promote appropriate weight gain. Am J Clin Nutr. 2019;109(4):1071–9.
Clark E, Isler C, Strickland D, et al. Influence of aerobic exercise on maternal lipid levels and offspring morphometrics. Int J Obes (Lond). 2019;43(3):594–602.
Dodd JM, Deussen AR, Louise J. A randomised trial to optimise gestational weight gain and improve maternal and infant health outcomes through antenatal dietary, lifestyle and exercise advice: the OPTIMISE randomised trial. Nutrients. 2019;11(12):2911.
Dodd JM, Louise J, Deussen AR, et al. Effect of metformin in addition to dietary and lifestyle advice for pregnant women who are overweight or obese: the GRoW randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(1):15–24.
Hoffmann J, Günther J, Geyer K, et al. Associations between prenatal physical activity and neonatal and obstetric outcomes—a secondary analysis of the cluster-randomized geliS trial. J Clin Med. 2019;8(10):1735.
Huang L, Fan L, Ding P, et al. Maternal exercise during pregnancy reduces the risk of preterm birth through the mediating role of placenta. J Matern Fetal Neonatal Med. 2019;32(1):109–16.
Jochumsen S, Henriksen TB, Lindhard MS, Hegaard HK, Rode L. Physical activity during pregnancy and intelligence in sons; a cohort study. Scand J Med Sci Sports. 2019;29(12):1988–95.
Kunath J, Günther J, Rauh K, et al. Effects of a lifestyle intervention during pregnancy to prevent excessive gestational weight gain in routine care—the cluster-randomised GeliS trial. BMC Med. 2019;17(1):5.
McMillan AG, May LE, Gaines GG, Isler C, Kuehn D. Effects of aerobic exercise during pregnancy on 1-month infant neuromotor skills. Med Sci Sports Exerc. 2019;51(8):1671–6.
Rodríguez-Blanque R, Sanchez-Garcia JC, Sanchez-Lopez AM, Expósito-Ruiz M, Aguilar-Cordero MJ. Randomized clinical trial of an aquatic physical exercise program during pregnancy. J Obstet Gynecol Neonatal Nurs. 2019;48(3):321–31.
Sundgot-Borgen J, Sundgot-Borgen C, Myklebust G, Sølvberg N, Torstveit MK. Elite athletes get pregnant, have healthy babies and return to sport early postpartum. BMJ Open Sport Exerc Med. 2019;5(1):e000652.
van Poppel MNM, Simmons D, Devlieger R, et al. A reduction in sedentary behaviour in obese women during pregnancy reduces neonatal adiposity: the DALI randomised controlled trial. Diabetologia. 2019;62(6):915–25.
Huang RC, Silva D, Beilin L, et al. Feasibility of conducting an early pregnancy diet and lifestyle e-health intervention: the pregnancy lifestyle activity nutrition (PLAN) project. J Dev Orig Health Dis. 2020;11(1):58–70.
Rodríguez-Blanque R, Aguilar-Cordero MJ, Marín-Jiménez AE, Núñez-Negrillo AM, Sánchez-López AM, Sánchez-García JC. Influence of a water-based exercise program in the rate of spontaneous birth: a randomized clinical trial. Int J Environ Res Public Health. 2020;17(3):795.
ACOG Committee Obsteteric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171–3.
Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: a consensus statement of the international societies of pediatric endocrinology and the growth hormone research society. J Clin Endocrinol Metab. 2007;92(3):804–10.
Janssen I, Katzmarzyk PT, Boyce WF, et al. Comparison of overweight and obesity prevalence in school-aged youth from 34 countries and their relationships with physical activity and dietary patterns. Obes Rev. 2005;6(2):123–32.
Gillman MW, Barker D, Bier D, et al. Meeting report on the 3rd international congress on developmental origins of health and disease (DOHaD). Pediatr Res. 2007;61(5 Pt 1):625–9.
Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353(17):1848–50.
Chen YT, Hu Y, Yang QY, et al. Excessive glucocorticoids during pregnancy impair fetal brown fat development and predispose offspring to metabolic dysfunctions. Diabetes. 2020;69(8):1662–74.
Davenport MH, Ruchat SM, Poitras VJ, et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br J Sports Med. 2018;52(21):1367–75.
Davenport MH, Yoo C, Mottola MF, et al. Effects of prenatal exercise on incidence of congenital anomalies and hyperthermia: a systematic review and meta-analysis. Br J Sports Med. 2019;53(2):116–23.
Brown J, Ceysens G, Boulvain M. Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes. Cochrane Database Syst Rev. 2017;6(6):Cd012202.
Davenport MH, Meah VL, Ruchat SM, et al. Impact of prenatal exercise on neonatal and childhood outcomes: a systematic review and meta-analysis. Br J Sports Med. 2018;52(21):1386–96.
Son JS, Zhao L, Chen Y, et al. Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci Adv. 2020;6(16):eaaz0359.
Moyer C, Reoyo OR, May L. The influence of prenatal exercise on offspring health: a review. Clin Med Insights Womens Health. 2016;9:37–42.
Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300(24):2886–97.
McMurray RG, Hackney AC, Guion WK, Katz VL. Metabolic and hormonal responses to low-impact aerobic dance during pregnancy. Med Sci Sports Exerc. 1996;28(1):41–6.
Bonen A, Campagna P, Gilchrist L, Young DC, Beresford P. Substrate and endocrine responses during exercise at selected stages of pregnancy. J Appl Physiol (1985). 1992;73(1):134–42.
TambyRaja RL. Current concepts in the management of preterm labour. Singap Med J. 1989;30(6):578–83.
Hayes EK, Lechowicz A, Petrik JJ, et al. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: role of altered development of the placental vasculature. PLoS ONE. 2012;7:e33370.
Son JS, Liu X, Tian Q, et al. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J Physiol. 2019;597(13):3333–47.
Sivan E, Homko CJ, Chen X, Reece EA, Boden G. Effect of insulin on fat metabolism during and after normal pregnancy. Diabetes. 1999;48(4):834–8.
McFarland MB, Trylovich CG, Langer O. Anthropometric differences in macrosomic infants of diabetic and nondiabetic mothers. J Matern Fetal Med. 1998;7(6):292–5.
Ford SP, Zhang L, Zhu M, et al. Maternal obesity accelerates fetal pancreatic β-cell but not α-cell development in sheep: prenatal consequences. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R835–43.
Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5(5):Cd011970.
Chen Y-T, Hu Y, Yang Q-Y, et al. Embryonic exposure to hyper glucocorticoids suppresses brown fat development and thermogenesis via REDD1. Sci Bull (Beijing). 2020;66:478–89.
Borge TC, Aase H, Brantsæter AL, Biele G. The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: a systematic review and meta-analysis. BMJ Open. 2017;7(9):e016777.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
US National Institute of Health R01-HD067449 and R21-AG049976.
Conflict of interest
Yanting Chen, Guiling Ma, Qiyuan Yang, Yun Hu, Jeanene Deavila, Meijun Zhu, and Min Du declare that they have no conflicts of interest relevant to the content of this review.
Data availability statement
All data are available in the submitted manuscript or as electronic supplementary material.
Code availability
Not applicable.
Author contributions
All authors contributed to the design, data interpretation, and revising of the article. YC, GM, YH, QY, and JD screened the studies and extracted and analyzed data. YC, GM, YH, QY, JD, MZ, and MD contributed to the literature search, data extraction, and analysis. YT and GM led the quantitative analysis. MZ and MD were expert advisers. All authors read and approved the final manuscript.
Additional information
The original article has been updated: Due to Figures 3, 4 and 5 update.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Ma, G., Hu, Y. et al. Effects of Maternal Exercise During Pregnancy on Perinatal Growth and Childhood Obesity Outcomes: A Meta-analysis and Meta-regression. Sports Med 51, 2329–2347 (2021). https://doi.org/10.1007/s40279-021-01499-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-021-01499-6