Abstract
The most frequent cause of syncope in young athletes is noncardiac etiology. The mechanism of noncardiac syncope (NCS) in young athletes is neurally-mediated (reflex). NCS in athletes usually occurs either as orthostasis-induced, due to a gravity-mediated reduced venous return to the heart, or in the context of exercise. Exercise-related NCS typically occurs after the cessation of an exercise bout, while syncope occurring during exercise is highly indicative of the existence of a cardiac disorder. Postexercise NCS appears to result from hypotension due to impaired postexercise vasoconstriction, as well as from hypocapnia. The mechanisms of postexercise hypotension can be divided into obligatory (which are always present and include sympathoinhibition, histaminergic vasodilation, and downregulation of cardiovagal baroreflex) and situational (which include dehydration, hyperthermia and gravitational stress). Regarding postexercise hypocapnia, both hyperventilation during recovery from exercise and orthostasis-induced hypocapnia when recovery occurs in an upright posture can produce postexercise cerebral vasoconstriction. Athletes have been shown to exhibit differential orthostatic responses compared with nonathletes, involving augmented stroke volume and increased peripheral vasodilation in the former, with possibly lower propensity to orthostatic intolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Postexercise syncope results from not only hypotension but also hypocapnia. |
The most critical factor predisposing to postexercise hypotension is impaired postexercise vasoconstriction. |
Athletes appear to exhibit lower propensity to orthostatic intolerance and more advantageous orthostatic responses compared with nonathletes. |
1 Introduction
While a cardiac disorder can be identified during exercise in a small minority of young athletes with a history of syncope, by far the most frequent cause of syncope in this group is noncardiac syncope (NCS) [1]. A cardiac etiology in young athletes presenting with complaints of syncope should always be ruled out through a diligent diagnostic work-up, including a detailed clinical history, physical examination, electrocardiogram, echocardiogram and exercise test, before reaching the final diagnosis of NCS. NCS in athletes usually occurs either as orthostasis-induced or after the cessation of exercise. Exercise-related NCS can cause injury, especially when occurring in dangerous situations, such as in diving, motor sports and road cycling [1, 2].
The importance of the study of the mechanisms underlying NCS in athletes relies, first, on the identification of possible modifiable predisposing factors that, if changed, could decrease the incidence of NCS in athletes, and, second, on the implementation of more effective measures in the acute treatment of athletes with NCS targeting the relevant mechanisms. Similar considerations regarding the pathophysiology of NCS in competitive athletes apply in individuals engaging in routine vigorous physical exercise in the settings of recreation or occupation. Thus, this review discusses the mechanisms underlying the occurrence of NCS in athletes, as well as the pathophysiological differences in the orthostatic responses between athletes and nonathletes.
2 Methodology
A literature search based on PubMed listings to 30 December 2017 using ‘athletes AND syncope’, ‘athletes AND orthostatic intolerance’, ‘athletes AND tilt test’, ‘exercise AND syncope’, ‘exercise AND orthostatic intolerance’ and ‘exercise AND tilt test’ as the search terms identified 2330 articles. Moreover, an examination of the reference list of the articles identified by this search strategy was performed and only the articles that were judged relevant were selected for inclusion in the review.
3 Mechanisms of Noncardiac Syncope (NCS) in Athletes
NCS in young athletes is of reflex origin (i.e. neurally mediated), mostly unrelated to exercise or occurring after exercise [1]. Our study group recently demonstrated that hemodynamic responses preceding reflex syncope during the head-up tilt test (HUT) in athletes were, in decreasing order of incidence, mixed, cardioinhibitory and vasodepressor [3]. With regard to the afferent pathway of reflex syncope in athletes, the following entities are evident: vasovagal syncope mediated by orthostatic stress; situational syncope associated with specific circumstances, such as exercise and deglutition, among which the postexercise setting is the most frequent; and carotid sinus syncope [4]. A limitation of many studies evaluating athletes with a history of NCS was the overrepresentation of athletes seeking advice due to recurrent episodes of syncope that had been frequent or severe, while those with mild forms of reflex syncope were less likely to be recruited [5].
3.1 Orthostasis-Induced NCS in Athletes
Transition from a supine to upright posture is normally accompanied by a gravity-mediated reduced venous return to the heart, with reduced left ventricular (LV) filling pressures [6]. The decreased cardiac preload induces a reduction of stroke volume (SV) and cardiac output (CO), eventually leading to reduced systolic blood pressure (SBP) [6, 7]. Concomitantly, this reduction of SBP induces the baroreflex-mediated inhibition of the parasympathetic nervous system (PNS) and activation of the sympathetic nervous system (SNS) [6, 7]. Both inhibition of PNS and activation of SNS lead to upregulation of heart rate (HR), which prevents a great decrease in CO, while activation of SNS induces enhanced peripheral vasoconstriction, resulting in the upregulation of diastolic blood pressure (DBP) [6,7,8]. Thus, despite the reduction of CO, the transition from a supine to upright posture is normally accompanied by preserved mean blood pressure (MBP) due to increased peripheral vasoconstriction in the conscious athlete, while syncope appears to result from the fall of MBP below the lower limit of cerebral autoregulation [3]. Our study group recently investigated the pathophysiology of NCS in athletes and proposed a novel diagnostic strategy for HUT in athletes based on hemodynamic parameters [3]. Specifically, athletes with a history of recent NCS had decreased total peripheral resistance index (TPRI) and increased cardiac index (CI) accompanied by decreased sympathetic activation during passive HUT for 30 min compared with athletes without a history of NCS [3]. These results indicated that the most critical factor predisposing athletes to NCS during upright standing is possibly inadequate peripheral vasoconstriction due to suboptimal sympathetic activation, with the greater CI of these athletes serving to compensate for the decreased TPRI [3]. In this study, HUT yielded 34% sensitivity and 94% specificity for the identification of athletes with a history of NCS based on the occurrence of syncope during HUT, while further stratification of negative tilt test responders, characterizing their results as positive when TPRI < 2800 dyne·sec·m2/cm5 and CI > 3 L/(min·m2), resulted in 85% sensitivity and 76% specificity [3].
3.2 Exercise-Related NCS in Athletes
3.2.1 Timing of Occurrence of Exercise-Related NCS
Exercise-related NCS has been reported to occur mostly after exercise [1, 9]. With regard to NCS during exercise, the better described cases refer to older individuals with sympathetic dysfunction, where blood pressure (BP) fails to increase during exercise due to impairment of sympathetic vasoconstriction [10]. Distinguishing if the syncopal event occurred during or after exercise is crucial since it will lead to direct implications in evaluation and management. Syncopal episodes occurring during exercise without prodromal symptoms are highly indicative of cardiac etiology; however, in real practice, the distinction of whether syncope occurred during or after exercise may be quite difficult, or even impossible, for sports characterized by rapid ‘starts and stops’, such as soccer, volleyball or basketball [5]. Our study group recently reported a case of an athlete experiencing syncope during a 5-km running race, just after a sudden inversion of the route (U turn) [11]. Although the symptoms of the athlete in this report occurred during running, implying at a first glance the diagnosis of syncope occurring during exercise, a more detailed analysis of the circumstances indicated that these symptoms were, in essence, postexercise from a pathophysiological view [11]. Furthermore, there is a possibility that a modifiable noncardiac condition during exercise, such as hyperthermia, hypoglycemia or hyponatremia, may have caused discomfort, prompting the cessation of exercise, with syncope eventually occurring immediately after the sudden termination of exercise [12]. Therefore, the abovementioned considerations indicate that there is a possibility of misclassification of some postexercise episodes of syncope as falsely occurring during exercise [13].
3.2.2 Postexercise NCS
The incidence of syncope during postexercise HUT has been found to be increased compared with during HUT performed before exercise, with this effect appearing to attenuate with increasing time interval between the cessation of exercise and the start of HUT [8, 14]. The transition from supine to upright standing has been associated with greater increase in HR and reduction of SBP after exercise in individuals with postexercise orthostatic intolerance (OI) compared with subjects not experiencing postexercise OI. In addition, DBP has been shown to decrease in postexercise OI subjects in contrast to the normal elevation of DBP in the non-postexercise OI group [8]. Postexercise OI may be determined more by the postexercise MBP per se rather than postexercise hypotension (PEH), defined as the drop of postexercise MBP from preexercise MBP, due to the fact that the most critical factor for the occurrence of postexercise syncope appears to be the fall of postexercise MBP below the lower limit of cerebral autoregulation, and not the change from preexercise to postexercise MBP [3, 15]. More intense exercise has been shown to reduce the time to postexercise presyncope during combined HUT and application of lower body negative pressure (LBNP) 15 min after the end of exercise [16]. The reported greater PEH after more intense exercise possibly reduces cardiovascular reserve, positioning MBP closer to the lower limit of cerebral autoregulation at the beginning of postexercise orthostatic testing and thus decreasing the time to occurrence of syncope during postexercise orthostatic testing [16].
The most widely accepted underlying mechanism of postexercise reflex syncope is the sudden reduction of venous return to the heart, resulting from the abrupt cessation of muscle pump function when exercise stops suddenly, with a subsequent fall in LV preload and SV [15]. Immediately after the cessation of exercise of the lower limbs, the blood flow through the previously working muscles of the lower extremities continues to be increased, while the lack of contraction in the muscles of the lower extremities leads to inactivation of the skeletal muscle pump, and thus to inefficient venous return from the lower extremities to the right atrium [15]. Impaired postexercise vasoconstriction due to suboptimal sympathetic stimulation accompanied by increased HR was found to characterize individuals with postexercise OI [17]. This higher baroreflex-mediated HR in patients with postexercise OI translates into higher CO, tending to compensate for the decreased total peripheral resistance (TPR) [17]. Thus, postexercise syncope is possibly the result of a persistent drop in TPR that is not completely offset by the increase in CO [17, 18]. Vagal baroreflex sensitivity has been found to not differ between subjects with and without postexercise OI, indicating a normal response of HR due to the fall of MBP in the former group [17]. The reported positive correlation between postexercise sympathetic drive and preexercise serum potassium levels, along with the reduced circulating preexercise potassium in subjects with postexercise OI, indicate a potential role of potassium to protect against postexercise OI [17]. Further studies are needed to confirm this mechanism. The common denominator of the cases of postexercise OI after endurance running appears to be postexercise orthostatic hypotension with increased serum creatinine levels, rather than elevated core temperature, plasma volume reduction or dehydration [12, 15, 17, 19]. Therefore, postexercise syncope is possibly pathophysiologically related more to a reduction of effective blood flow to vital organs, rather than to total plasma volume, as indicated by deteriorated renal function, reflecting reduced renal perfusion. From this point of view, the most critical predisposing factor for postexercise syncope and accompanied orthostatic hypotension may be the redistribution of blood from the vital organs to the periphery, i.e. previously working muscles and cutaneous vasculature [12].
Syncope after exercise can be prevented despite the occurrence of PEH, due to the autoregulation of cerebral circulation, with syncope ensuing only when MBP falls below the lower limit of cerebral autoregulation [3]. Presyncope has been reported to occur at comparable levels of cerebral hypoperfusion, both before and after exercise [16]. Although cerebral autoregulation appears to be well-maintained following exercise of mild or moderate intensity, more intense exhaustive exercise may produce a postexercise cerebral autoregulatory deficit, providing less protection of cerebral perfusion from PEH [20,21,22].
In individuals with a history of reflex syncope occurring only after exercise, syncope can predictably be elicited after maximal exercise testing, but the majority of such individuals also appear to experience syncope within 30 min of passive HUT, indicating that subjects with postexercise syncope may have a general propensity to neurally-mediated syncope, whether it occurs in the setting of exercise or not [9, 23]. Furthermore, exercise-related syncope is not uncommon in subjects with a history of general vasovagal syncope [24]. The majority of individuals with a history of vasovagal syncope have been found to exhibit either impaired vasoconstriction or paradoxical vasodilation in the forearm beds of resistance vessels, as well as in the splanchnic venous beds during dynamic exercise of the lower limbs, which is in contradiction to the normal vasoconstriction of nonexercising vascular beds during exercise [24,25,26]. Among these subjects with a history of vasovagal syncope, impaired vasoconstriction during exercise was more prevalent when the episodes of vasovagal syncope were exercise-related [24]. Thus, exercise-related reflex syncope may be a presentation of individuals with a general propensity to reflex syncope, which can be attributed to impaired vasoconstriction of nonexercising vascular beds. A possible mechanism underlying the defective vasoconstriction during exercise in subjects with a history of vasovagal syncope is inadequate activation of the SNS, as indicated by the reported attenuated elevation of serum noradrenaline levels compared with individuals without a history of vasovagal syncope [18, 27].
3.3 Deglutition Syncope in Athletes
Swallowing of cold sports drinks after exercise can elicit deglutition syncope, caused by vagal afferent activation due to esophageal stimulation [28].
3.4 Carotid Sinus Syncope in Athletes
Carotid sinus syncope may occur during exercise if adequate pressure is applied to the carotid arteries. This mechanism has been implicated in the pathophysiology of some cases of syncope during rotation of the head of golf players or cyclists, and during judo choking or wrestling strangle holds [29, 30].
4 Mechanisms of Postexercise NCS
4.1 Postexercise Hypotension
PEH is usually defined as preexercise BP − postexercise BP. The pattern of changes of SBP and DBP following an acute session of exercise includes an initial elevation of SBP and MBP within the first 5 min after the cessation of exercise, mainly due to increased CO, as well as a reduction of DBP, reflecting the sudden withdrawal of the impedance provided by the intermittently contracting musculature pressing on the dilated arterioles of the exercising muscles [31, 32]. After this phase, a gradual fall of SBP and MBP, and increase in DBP, occur, all of which remain below preexercise values in the postexercise recovery period [32, 33]. PEH appears to result from a persistent drop in TPR. Endo et al. showed that approximately two-thirds of the decrease in postexercise TPR after moderate-intensity cycling for 60 min resulted from increased vascular conductance in the lower and upper limbs, while the contributions of renal and splanchnic vascular beds were much smaller [34]. Postexercise vasoconstriction in the nonexercising limbs is considered a reflex response to vasodilation in the exercising muscles after exercise of low or moderate intensity, serving to prevent a major decline in postexercise TPR, while generalized vasodilation has been reported in the nonexercising limbs after exercise of high intensity [35, 36].
Peak reductions of SBP and DBP after dynamic exercise have been reported to be up to 40 and 14 mmHg, respectively, in hypertensive subjects without antihypertensive medication, and 30 and 11 mmHg, respectively, in normotensive individuals [37,38,39]. PEH has been shown to last up to 20 h after dynamic exercise compared with a nonexercise control day [40]. PEH can be induced by exercise sessions of only a few seconds’ duration [41].
Increases in both intensity or duration of dynamic exercise have been found to increase the duration of PEH, as well as the peak reductions of SBP and DBP [33, 35, 42, 43]. Duration of PEH after an exercise session of a given duration was found to decrease in the following order: aerobic, mixed (aerobic and resistance) and resistance exercise [44]. Regarding PEH after resistance exercise, both the magnitude and duration of the response appear to increase with increasing volume of exercise [45,46,47,48,49]. Chronic resistance exercise training has been shown to reduce preexercise BP, as well as the magnitude of PEH [50].
The most well-established underlying mechanisms of PEH include decreased adrenergic regulation of muscle vasomotion, histamine (H) receptor-mediated vasodilation and modulation of cardiovagal baroreflex. The decreased adrenergic regulation of muscle vasomotion with resultant postexercise vasodilation is indicated by both resetting of the baroreflex control of sympathetic outflow (a downward shift in the operating point of the baroreceptor reflex) and decreased postexercise transduction of muscle sympathetic activity into vascular resistance [51,52,53]. The model for the mechanism of postexercise resetting of the arterial baroreflex was described by Chen and Bonham [54]. Notably, both resetting of the arterial baroreflex and a decrease in neurovascular transduction following exercise were reported only 60 min after large muscle-mass exercise, but not during the first 60 min following small muscle-mass exercise, possibly explaining the absence of PEH after small muscle-mass exercise [53, 55]. Additionally, early PEH within 30 min after exercise has been demonstrated to be mediated in large part by an H1 receptor-mediated vasodilation in vascular endothelial cells, while stimulation of H2 receptors in smooth muscle cells appears to mediate late PEH at 60–90 min after exercise [56, 57]. Furthermore, cardiovagal baroreflex has been found to be downregulated early after an exercise bout, possibly contributing to the occurrence of early PEH, and upregulated later after exercise, serving to attenuate the magnitude of PEH [58,59,60,61]. This time course of the postexercise responses of the cardiovagal baroreflex appears to occur more consistently with maximal- compared with moderate-intensity exercise, with the downregulation occurring mostly within the first 30 min after the end of exercise and the upregulation after 60 min [58,59,60,61].
Apart from the above-mentioned mechanisms that appear to always be present, exercise-induced dehydration has been shown to increase the magnitude of PEH and decrease postexercise baroreflex sensitivity, while adequate fluid ingestion during exercise to prevent dehydration can attenuate these dehydration-associated changes [62,63,64]. Furthermore, thermoregulatory cutaneous vasodilation has been found to contribute to PEH only in warm environments [65, 66]. In brief, the mechanisms of PEH can be divided into obligatory (which are always present and include sympathoinhibition, histaminergic vasodilation and downregulation of the cardiovagal baroreflex) and situational (which include dehydration, hyperthermia and gravitational stress) (Fig. 1). The relative contribution of each mechanism to the occurrence of PEH remains to be elucidated.
4.2 Postexercise Hypocapnia
Postexercise syncope appears to result from not only impaired postexercise hemodynamics but also hypocapnia [16]. Indeed, end-tidal partial pressure of carbon dioxide (PETCO2) has been shown to be positively correlated with cerebral blood flow (CBF) after exercise, implying that hyperventilation-induced hypocapnia after exercise may be implicated in the pathophysiology of postexercise syncope [16, 67, 68]. Notably, CBF at presyncope following exercise was reported to be related more strongly to PETCO2 than to hypotension [16]. Furthermore, postural reduction of middle cerebral artery velocity (MCAv) was shown to be exacerbated after 30 min of dynamic exercise, and its best independent predictor was the exercise-induced reduction of PETCO2 rather than the fall of MBP [69]. Moreover, OI was found to be improved during slow controlled breathing at six breaths/min compared with spontaneous breathing, indicating a relevant role of hypocapnia for the induction of OI [70]. Additionally, a decrease in PETCO2 occurs upon assuming the erect posture, attributed mainly to the increased tidal volume and the reduced SV during upright standing, while both a gravity-induced ventilation/perfusion mismatch and an increase in functional residual capacity can also contribute to hypocapnia [71]. Therefore, both hyperventilation-induced hypocapnia during recovery from exercise and orthostasis-induced hypocapnia, when recovery occurs in an upright posture, may produce postexercise cerebral vasoconstriction. In this way hypocapnia possibly diminishes cerebral oxygen delivery independently of any change in cerebral perfusion pressure (Fig. 1).
4.3 Postexercise Bradycardia
Apart from the classic vasodepressive postexercise syncope, another uncommonly reported cause of postexercise syncope is sinus bradycardia immediately followed by sinus arrest due to excessive vagal stimulation [72,73,74]. Consistently, this type of postexercise syncope was found to be effectively treated with the anticholinergic agent disopyramide [75]. These episodes have been reported to resolve spontaneously within a few seconds, followed by a gradual increase in both BP and HR, before resuscitative efforts could be initiated [76]. Importantly, postexercise asystole appears to occur in individuals with normal function of sinus node and without atrioventricular conduction abnormalities, implying impaired autonomic control of the heart after exercise rather than an intrinsic myocardial electrophysiologic abnormality [74, 76].
4.4 Bezold–Jarisch Reflex
Stimulation of the Bezold–Jarisch reflex can underlie the induction of some episodes of reflex syncope in an upright posture, although this mechanism has not been demonstrated to be operative in the postexercise setting [77]. The sudden cessation of muscular activity of the previously exercising lower limbs immediately after exercise may reduce venous return to the heart, compromising cardiac filling. At the same time, postexercise upregulation of plasma catecholamines can induce forceful ventricular contractions against a diminished ventricular volume. These ventricular contractions can stimulate LV mechanoreceptors (c-fibers) and, through the cardiac depressor reflex, known as Bezold–Jarisch reflex, may induce hypotension and bradycardia [77].
5 Mode of Exercise and Postexercise NCS
The overwhelming majority of studies investigating postexercise NCS refer to long-distance running [12]; however, postexercise NCS has also been well-reported after cycling [73], and a few case reports after marathon swimming have been published [78]. Although the typical form of postexercise NCS occurs when the subject is in an upright posture after exercise, there is a possibility of postexercise NCS in the seated position, such as in rowers [79]. Runners participating in endurance events collapse more frequently near cut-off times for medals or race closure, implying that extreme physical effort beyond the athlete’s capability may induce undue stress to the athlete’s circulatory system that could predispose to syncope [12]. SAFER (Strategies to reduce Adverse medical events For the ExerciseR) studies I, II and III found that the incidence of postural hypotension was higher in 56-km running races than in 21-km running races, in females, and in slow-pace runners [80,81,82]. Although the non-randomized nature of these case–control studies cannot exclude the presence of confounders, these data imply that postural hypotension may be more likely to occur when the duration of a race is longer since females usually run at a slower pace than males and a slower pace results in a longer duration of running for a given distance.
The above-mentioned considerations regarding the pathophysiology of exercise-related NCS refer mainly to studies using dynamic exercise, while the relevant data regarding resistance exercise are more limited. Acute resistance exercise performed with continued normal ventilation can potentially result in the postexercise fall of CBF associated with symptoms of OI immediately after exercise [83, 84]. Several additional mechanisms may exacerbate this state and contribute to syncope after resistance exercise. First, the Valsalva maneuver is frequently performed during resistance exercise and has been shown to reduce CBF, especially when performed in the upright position [85]. Second, the commonly used hyperventilation before resistance exercise has been found to contribute to the occurrence of postexercise NCS, especially when the athlete stands upright immediately after exercise, due to the hyperventilation-induced cerebral vasoconstriction [83]. An uncommon mechanism of NCS after resistance exercise can occur in the setting of weightlifting maneuvers. Specifically, NCS has been reported after a ‘clean and jerk’ lift, the so-called ‘blackout’, and has been attributed to the ‘mess trick’ or ‘fainting lark’ mechanism, in which syncope is provoked by first hyperventilating in a squatted position and then rising quickly in combination with a maintained Valsalva maneuver [86, 87].
6 Exercise-Related Collapse
Exercise-related syncope should be distinguished from exercise-related collapse, where the athlete falls to the ground, being unable to stand or walk unaided, without true loss of consciousness or cerebral hypoperfusion [12, 88]. Exercise-related collapse commonly occurs after the completion of endurance running events and is usually associated with hyperthermia, dehydration, hyponatremia or hypoglycemia [88]. However, there is a possibility one of these medical entities could predispose to true syncope. Specifically, exertional heat illness in athletes can be manifested by OI and true syncope, the so-called heat syncope, which occurs with orthostatic hypotension, resulting from heat-induced peripheral vasodilation [89,90,91]. Indeed, skin blood flow is under baroreceptor influence, but the ability of skin to vasoconstrict during gravitational stress was shown not to be able to completely override excessive heat-induced vasodilatation [92]. Possible mechanisms of exercise-related heat syncope include not only hypotension due to inappropriate vasodilation but also heat-induced impairment of cerebral autoregulation [93]. Heat syncope often occurs in unfit or heat-unacclimatized individuals, before blood volume expands and cardiovascular adaptations are complete, especially when wearing a uniform or insulated clothing that promotes maximal skin vasodilation [89, 94]. Impaired consciousness can be induced by hypoglycemia because the brain is normally dependent on glucose for oxidative metabolism. In addition, hypoglycemia, which is frequently observed in endurance sports, can predispose to true exercise-related syncope by reducing baroreflex sensitivity and the response of the SNS to orthostatic stress [95].
7 Comparison of Orthostatic Responses Between Athletes and Nonathletes
The issue of the impact of physical fitness on orthostatic responses has been studied in three settings. First, prospective studies investigating whether chronic exercise training can change orthostatic responses in untrained individuals; second, cross-sectional comparisons between trained and untrained individuals; and third, prospective studies investigating whether detraining can change orthostatic responses in trained individuals. The majority of relevant studies were performed with the first or second design. The first setting constitutes the most appropriate approach for the evaluation of the effects of chronic exercise training on orthostatic parameters and OI because the observed changes can be attributed only to exercise training, while, in the second setting, orthostatic responses can be influenced by various confounders.
7.1 The Impact of Chronic Exercise Training on Orthostatic Responses
With regard to the first setting, endurance exercise training for at least 3 months has been shown to attenuate OI, as demonstrated by both decreased number of patients with positive HUT and increased tolerance time in subjects with positive HUT [96,97,98,99,100]. The beneficial effect of chronic exercise training regarding the attenuation of OI was first demonstrated by Allen et al. in identical twins who fainted on the tiltboard [101]. After a 3-week exercise training program applied to one of the twins, a subsequent HUT-induced syncope could only be elicited in the untrained twin. The exercise-induced increase in tolerance time during orthostatic stress has been shown to not occur in subjects with high baseline orthostatic tolerance, and appears to be positively correlated with the magnitude of the exercise-induced increase in plasma volume, with the existence of a threshold of 150 mL, above which there is a clinically meaningful decrease in OI [97, 98]. Notably, orthostatic tolerance has been reported to increase after resistance exercise training of the lower limbs, rather than after resistance exercise training of the upper limbs [102, 103].
The decreased baroreflex sensitivity during orthostatic stress as a result of chronic exercise training has been convincingly demonstrated to be induced by exercise-induced hypervolemia, possibly reflecting less need for the recruitment of baroreflex mechanisms in the context of hypervolemia-induced attenuation of orthostatic stress [97, 98, 104]. Chronic exercise training appears to not influence the overall ability for upregulation of TPR during orthostatic stress, indicating the existence of effective vasoconstriction of the lower limbs, although the vasoconstrictor responses in both upper limbs and renal vasculature during orthostatic stress have been found to be attenuated, reflecting the downregulation of baroreflex responses during orthostatic stress [104, 105].
Additionally, the beneficial effects of chronic exercise training to reduce both symptoms of OI and HR during HUT have been convincingly demonstrated in patients with postural orthostatic tachycardia syndrome (POTS), which is characterized by an excessive increase in HR during upright standing accompanied by symptoms of OI, but without orthostatic hypotension [106, 107]. Taking into account that low SV, due to cardiac atrophy and hypovolemia, has been implicated in the pathogenesis of POTS, the reported reduction of OI by chronic exercise training in patients with POTS is possibly attributable to the increase in LV dimensions and blood volume, both of which lead to augmentation of SV [106].
The results of prospective studies evaluating the effects of chronic exercise training on OI may be confounded by the accompanied weight loss, which tends to increase OI [108,109,110]. Indeed, studies applying exercise interventions with considerable weight loss failed to demonstrate any improvement in OI, while studies without significant exercise-induced weight loss consistently found a decrease in OI [96, 97, 111].
7.2 The Relationship between Physical Fitness and Orthostatic Responses
Regarding the second setting of cross-sectional comparison of orthostatic responses between trained and untrained individuals, the inconsistency in the reported relationship between orthostatic responses and maximal oxygen consumption (VO2max) could be interpreted in light of two facts. First, VO2max is influenced more by the intensity than the volume of aerobic exercise, and, second, subjects with a higher body mass index (BMI) are characterized by both decreased VO2max and reduced OI, representing a confounding effect of BMI on the relationship between VO2max and orthostatic responses [110, 112,113,114]. The comparison of parameters of hemodynamics, HR variability (HRV) and baroreflex sensitivity between trained and untrained individuals is most prudently performed using the average values of these parameters during orthostatic stress rather than their changes from a pretest supine posture, because the latter calculations are greatly influenced by the pretest differences in supine parameters between trained and untrained subjects.
An important drawback of the previous studies investigating the differences in orthostatic responses between trained and untrained individuals was that the studied individuals were not evaluated for the presence of a history of NCS, which can profoundly influence orthostatic responses [114]. The only relevant trial that took into account the history of NCS was recently performed by our study group [114]. In this study, a full assessment of hemodynamics, HRV and baroreflex sensitivity during passive HUT was performed in four groups: athletes with a history of recent NCS; athletes without NCS; nonathletes with a history of recent NCS; and nonathletes without NCS [114]. This trial performed adjusted analysis for sex, age and BMI, which constitute the most well-established confounders of orthostatic responses. The necessity of this adjustment is further reinforced in the context of comparison between athletes and nonathletes since the usually found younger age and lower BMI of athletes compared with nonathletes are associated with diminished vasoconstrictor response of the former during orthostatic stress [110, 114]. This study found that the main underlying mechanism for the occurrence of NCS during upright standing in athletes was decreased TPRI compared with inadequate preservation of stroke index (SI) in non-athletes [114]. Moreover, this study demonstrated that HR during HUT was higher in nonathletes compared with athletes, reflecting the greater orthostatic stress of nonathletes [114]. Consistently, among the participants without NCS, the frequency of positive results of HUT was higher in nonathletes, implying a possibly reduced ability of nonathletes to cope with orthostatic stress [114]. Thus, the main mechanism explaining the decreased OI of athletes is possibly the augmented SV of athletes during orthostasis compared with nonathletes, which can be attributed to the well-known expansion of blood volume after chronic exercise training, as well as to the improved LV mechanics in athletes characterized by greater SV for any given LV filling pressure [114,115,116,117].
Additionally, the slope MCAv/LBNP during graded LBNP was smaller in physically active elderly individuals compared with sedentary elderly subjects, implying the possible existence of improved cerebral autoregulation during orthostatic stress in trained individuals [118]. Although increased lower limb venous compliance has been shown in trained compared with untrained subjects, it may not contribute to the differential OI between trained and untrained individuals since no difference has been found in venous emptying and venous return from the lower limbs between trained and untrained individuals [119, 120]. There are a few studies reporting no relationship between the level of physical fitness and the hypotensive response to HUT after an exercise session [69, 121]; however, the issue of the impact of training status on postexercise orthostatic responses needs further investigation.
The above-mentioned differential orthostatic responses between trained and untrained individuals have been demonstrated in comparisons of individuals with different levels of aerobic fitness [120, 122, 123]. The comparison of orthostatic responses between subjects with different levels of strength power needs further investigation. Ifuku and Shiraishi showed that the baroreflex increase in TPR was greater during HUT than during head-up suspension in track and field athletes, but not in swimmers [124]. Furthermore, these changes during HUT were greater in track and field athletes compared with swimmers [124]. Taking into account that antigravity muscles of the lower limbs are activated only during HUT and not during head-up suspension, these findings suggest that the action of the antigravity muscles to prevent venous pooling in the lower limbs during upright standing is upregulated in track and field athletes compared with swimmers, serving to preserve more efficiently venous return to the heart during orthostasis in the former compared with the latter. This difference can be plausibly attributed to the fact that track and field athletes train under terrestrial gravity, while exercise training for swimmers is performed in a hypogravity environment. In this respect, chronic exercise training on land may induce more advantageous cardiovascular responses during upright standing compared with exercise training in water.
7.3 The Impact of Detraining on Orthostatic Responses
With regard to the effects of detraining on orthostatic parameters, detraining of marathon skaters for 1 month increased HR during HUT, implying an increase in orthostatic stress [125].
8 Evaluation of Athletes with Exercise-Related Syncope
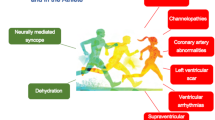
The most important clues in the evaluation of an athlete presenting with exercise-related syncope can be obtained from the clinical history (Fig. 2) [4]. In this respect, meticulous history-taking should be performed by the clinician and specific details regarding the setting in which the episode of syncope occurred can inform the clinician whether the etiology was cardiac or not. Specifically, clues from the clinical history that raise the possibility the episode of syncope was due to a cardiac disorder are the occurrence of syncope during exercise and the onset of palpitations or chest pain shortly before syncope. Other clues that suggest a cardiac etiology are rapid onset of the syncope without prodromes, and short duration of syncope lasting for a few seconds, with spontaneous complete recovery from the episode [4]. On the other hand, a noncardiac etiology is strongly indicated when syncope occurred immediately after the termination of exercise, especially when syncope was preceded by prodromes of abdominal discomfort and nausea. A long history of OI may be linked with a propensity for the occurrence of exercise-related NCS. Furthermore, the clinician should confirm that the athlete has no first-degree relatives with an inherited cardiac disorder or with a history of sudden cardiac death, unexplained drowning or unexplained motor accident at a young age. Importantly, the diagnosis of exercise-related NCS is a diagnosis of exclusion of any cardiac disorder, and thus can be reached only after an extensive diagnostic work-up, including clinical examination, electrocardiogram, echocardiography, exercise stress testing and ambulatory 24-h Holter monitoring. Cardiac magnetic imaging or genetic testing can be further considered if there is a high level of suspicion for a cardiac disorder. Finally, the demonstration of a considerable drop in BP accompanied by symptoms of OI after the termination of exercise stress testing, as well as a positive tilt testing, suggest that a previous episode of postexercise syncope was possibly of noncardiac etiology.
9 Conclusions
NCS in young athletes is of reflex origin and is usually either orthostasis-induced or occurs after the cessation of an exercise session. Postexercise syncope appears to result from not only hypotension but also hypocapnia. The most critical factor predisposing to PEH may be impaired postexercise vasoconstriction. Moreover, athletes appear to exhibit lower propensity to OI and more advantageous orthostatic responses compared with nonathletes. Taking into account that chronic exercise training has been shown to improve OI, this type of intervention may also prove valuable in the treatment of OI in sedentary individuals.
References
Colivicchi F, Ammirati F, Santini M. Epidemiology and prognostic implications of syncope in young competing athletes. Eur Heart J. 2004;25:1749–53.
Calkins H, Seifert M, Morady F. Clinical presentation and long-term follow-up of athletes with exercise-induced vasodepressor syncope. Am Heart J. 1995;129:1159–64.
Christou GA, Kouidi EJ, Anifanti MA, Sotiriou PG, Deligiannis AP. A novel strategy for evaluating tilt test in athletes with syncope. Eur J Prev Cardiol. 2016;23:1003–10.
Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631–71.
Thompson PD. Tilt table testing in the evaluation and management of athletes with recurrent exercise-induced syncope. Med Sci Sports Exerc. 1993;25:883.
Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34:375–86.
Nardo CJ, Chambless LE, Light KC, Rosamond WD, Sharrett AR, Tell GS, et al. Descriptive epidemiology of blood pressure response to change in body position. The ARIC Study. Hypertension. 1999;33:1123–9.
Bjurstedt H, Rosenhamer G, Balldin U, Katkov V. Orthostatic reactions during recovery from exhaustive exercise of short duration. Acta Physiol Scand. 1983;119:25–31.
Sneddon JF, Scalia G, Ward DE, McKenna WJ, Camm AJ, Frenneaux MP. Exercise induced vasodepressor syncope. Br Heart J. 1994;71:554–7.
Smith GD, Watson LP, Pavitt DV, Mathias CJ. Abnormal cardiovascular and catecholamine responses to supine exercise in human subjects with sympathetic dysfunction. J Physiol. 1995;484:255–65.
Christou GA, Christou KA, Christou EA, Kiortsis DN. Can noncardiac syncope occur during exercise? Cardiology. 2017;138:159–63.
Holtzhausen LM, Noakes TD, Kroning B, de Klerk M, Roberts M, Emsley R. Clinical and biochemical characteristics of collapsed ultra-marathon runners. Med Sci Sports Exerc. 1994;26:1095–101.
Edenfield KM, Stern AN, Dillon MC, Burkart TA, Clugston JR. A case of vasovagal syncope in a collegiate swimmer during competition. Curr Sports Med Rep. 2015;14:86–90.
Murrell C, Cotter JD, George K, Shave R, Wilson L, Thomas K, et al. Influence of age on syncope following prolonged exercise: differential responses but similar orthostatic intolerance. J Physiol. 2009;587:5959–69.
Holtzhausen LM, Noakes TD. The prevalence and significance of post-exercise (postural) hypotension in ultramarathon runners. Med Sci Sports Exerc. 1995;27:1595–601.
Mündel T, Perry BG, Ainslie PN, Thomas KN, Sikken EL, Cotter JD, et al. Postexercise orthostatic intolerance: influence of exercise intensity. Exp Physiol. 2015;100:915–25.
Gratze G, Mayer H, Skrabal F. Sympathetic reserve, serum potassium, and orthostatic intolerance after endurance exercise and implications for neurocardiogenic syncope. Eur Heart J. 2008;29:1531–41.
Smith GD, Watson LP, Mathias CJ. Cardiovascular and catecholamine changes induced by supine exercise and upright posture in vasovagal syncope. Comparisons with normal subjects and subjects with sympathetic denervation. Eur Heart J. 1996;17:1882–990.
Murrell CJ, Cotter JD, George K, Shave R, Wilson L, Thomas K, et al. Syncope is unrelated to supine and postural hypotension following prolonged exercise. Eur J Appl Physiol. 2011;111:469–76.
Ogoh S, Fisher JP, Purkayastha S, Dawson EA, Fadel PJ, White MJ, et al. Regulation of middle cerebral artery blood velocity during recovery from dynamic exercise in humans. J Appl Physiol. 1985;2007(102):713–21.
Ogoh S, Dalsgaard MK, Yoshiga CC, Dawson EA, Keller DM, Raven PB, et al. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Heart Circ Physiol. 2005;288:H1461–7.
Willie CK, Ainslie PN, Taylor CE, Eves ND, Tzeng YC. Maintained cerebrovascular function during post-exercise hypotension. Eur J Appl Physiol. 2013;113:1597–604.
Sakaguchi S, Shultz JJ, Remole SC, Adler SW, Lurie KG, Benditt DG. Syncope associated with exercise, a manifestation of neurally mediated syncope. Am J Cardiol. 1995;75:476–81.
Thomson HL, Lele SS, Atherton JJ, Wright KN, Stafford W, Frenneaux MP. Abnormal forearm vascular responses during dynamic leg exercise in patients with vasovagal syncope. Circulation. 1995;92:2204–9.
Thomson HL, Atherton JJ, Khafagi FA, Frenneaux MP. Failure of reflex venoconstriction during exercise in patients with vasovagal syncope. Circulation. 1996;93:953–9.
Nobrega AC, O’Leary D, Silva BM, Marongiu E, Piepoli MF, Crisafulli A. Neural regulation of cardiovascular response to exercise: role of central command and peripheral afferents. Biomed Res Int. 2014;2014:478965.
Goldstein DS, Eisenhofer G, Kopin IJ. J Sources and significance of plasma levels of catechols and their metabolites in humans. Pharmacol Exp Ther. 2003;305:800–11.
Olshansky B. A pepsi challenge. N Engl J Med. 1999;340:2006.
Flugelman M, Halon DA, Goldblatt H. Golf syncope. Lancet. 1987;2:47.
Krediet CT, Wilde AA, Wieling W, Halliwill JR. Exercise related syncope, when it’s not the heart. Clin Auton Res. 2004;14:25–36.
Brown SP, Clemons JM, He Q, Liu S. Effects of resistance exercise and cycling on recovery blood pressure. J Sports Sci. 1994;12:463–8.
Coats AJ, Conway J, Isea JE, Pannarale G, Sleight P, Somers VK. Systemic and forearm vascular resistance changes after upright bicycle exercise in man. J Physiol. 1989;413:289–98.
Jones H, George K, Edwards B, Atkinson G. Is the magnitude of acute post-exercise hypotension mediated by exercise intensity or total work done? Eur J Appl Physiol. 2007;102:33–40.
Endo MY, Shimada K, Miura A, Fukuba Y. Peripheral and central vascular conductance influence on post-exercise hypotension. J Physiol Anthropol. 2012;31:32.
Bennett T, Wilcox RG, Macdonald IA. Post-exercise reduction of blood pressure in hypertensive men is not due to acute impairment of baroreflex function. Clin Sci (Lond). 1984;67:97–103.
Isea JE, Piepoli M, Adamopoulos S, Pannarale G, Sleight P, Coats AJ. Time course of haemodynamic changes after maximal exercise. Eur J Clin Invest. 1994;24:824–9.
Wilcox RG, Bennett T, Brown AM, Macdonald IA. Is exercise good for high blood pressure? Br Med J (Clin Res Ed). 1982;285:767–9.
Wallace JP, Bogle PG, King BA, Krasnoff JB, Jastremski CA. The magnitude and duration of ambulatory blood pressure reduction following acute exercise. J Hum Hypertens. 1999;13:361–6.
Cléroux J, Kouamé N, Nadeau A, Coulombe D, Lacourcière Y. After effects of exercise on regional and systemic hemodynamics in hypertension. Hypertension. 1992;19:183–91.
Carvalho RS, Pires CM, Junqueira GC, Freitas D, Marchi-Alves LM. Hypotensive response magnitude and duration in hypertensives: continuous and interval exercise. Arq Bras Cardiol. 2015;104:234–41.
Wieling, Harms MP, ten Harkel AD, van Lieshout JJ, Sprangers RL. Circulatory response evoked by a 3 s bout of dynamic leg exercise in humans. J Physiol. 1996;494:601–11.
Eicher JD, Maresh CM, Tsongalis GJ, Thompson PD, Pescatello LS. The additive blood pressure lowering effects of exercise intensity on post-exercise hypotension. Am Heart J. 2010;160:513–20.
Hagberg JM, Montain SJ, Martin WH 3rd. Blood pressure and hemodynamic responses after exercise in older hypertensives. J Appl Physiol. 1985;1987(63):270–6.
Keese F, Farinatti P, Pescatello L, Monteiro W. A comparison of the immediate effects of resistance, aerobic, and concurrent exercise on postexercise hypotension. J Strength Cond Res. 2011;25:1429–36.
Brito Ade F, de Oliveira CV, Brasileiro-Santos Mdo S, Santos Ada C. Resistance exercise with different volumes: blood pressure response and forearm blood flow in the hypertensive elderly. Clin Interv Aging. 2014;9:2151–8.
Figueiredo T, Rhea MR, Peterson M, Miranda H, Bentes CM, dos Reis VM, et al. Influence of number of sets on blood pressure and heart rate variability after a strength training session. J Strength Cond Res. 2015;29:1556–63.
Cavalcante PA, Rica RL, Evangelista AL, Serra AJ, Figueira A Jr, Pontes FL Jr, et al. Effects of exercise intensity on postexercise hypotension after resistance training session in overweight hypertensive patients. Clin Interv Aging. 2015;10:1487–95.
de Freitas Brito A, Brasileiro-Santos Mdo S, Coutinho de Oliveira CV, Sarmento da Nóbrega TK, de Moraes Lúcia, Forjaz C, da Cruz Santos A. High-intensity resistance exercise promotes postexercise hypotension greater than moderate intensity and affects cardiac autonomic responses in women who are hypertensive. J Strength Cond Res. 2015;29:3486–93.
Figueiredo T, Willardson JM, Miranda H, Bentes CM, Reis VM, Simão R. Influence of load intensity on postexercise hypotension and heart rate variability after a strength training session. J Strength Cond Res. 2015;29:2941–8.
Moraes MR, Bacurau RF, Simões HG, Campbell CS, Pudo MA, Wasinski F, et al. Effect of 12 weeks of resistance exercise on post-exercise hypotension in stage 1 hypertensive individuals. J Hum Hypertens. 2012;26:533–9.
Brito LC, Queiroz AC, Forjaz CL. Influence of population and exercise protocol characteristics on hemodynamic determinants of post-aerobic exercise hypotension. Braz J Med Biol Res. 2014;47:626–36.
Floras JS, Sinkey CA, Aylward PE, Seals DR, Thoren PN, Mark AL. Postexercise hypotension and sympathoinhibition in borderline hypertensive men. Hypertension. 1989;14:28–35.
Halliwill JR, Taylor JA, Eckberg DL. Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol. 1996;495:279–88.
Chen CY, Bonham AC. Postexercise hypotension: central mechanisms. Exerc Sport Sci Rev. 2010;38:122–7.
Buck TM, Romero SA, Ely MR, Sieck DC, Abdala PM, Halliwill JR. Neurovascular control following small muscle-mass exercise in humans. Physiol Rep. 2015;3:e12289.
Lockwood JM, Wilkins BW, Halliwill JR. H1 receptor-mediated vasodilatation contributes to postexercise hypotension. J Physiol. 2005;563:633–42.
McCord JL, Beasley JM, Halliwill JR. H2-receptor-mediated vasodilation contributes to postexercise hypotension. J Appl Physiol. 1985;2006(100):67–75.
Somers VK, Conway J, LeWinter M, Sleight P. The role of baroreflex sensitivity in post-exercise hypotension. J Hypertens Suppl. 1985;3:S129–30.
Piepoli M, Coats AJ, Adamopoulos S, Bernardi L, Feng YH, Conway J, et al. Persistent peripheral vasodilation and sympathetic activity in hypotension after maximal exercise. J Appl Physiol. 1985;1993(75):1807–14.
Convertino VA, Adams WC. Enhanced vagal baroreflex response during 24 h after acute exercise. Am J Physiol. 1991;260:R570–5.
Willie CK, Ainslie PN, Taylor CE, Jones H, Sin PY, Tzeng YC. Neuromechanical features of the cardiac baroreflex after exercise. Hypertension. 2011;57:927–33.
Charkoudian N, Halliwill JR, Morgan BJ, Eisenach JH, Joyner MJ. Influences of hydration on post-exercise cardiovascular control in humans. J Physiol. 2003;552:635–44.
Davis JE, Fortney SM. Effect of fluid ingestion on orthostatic responses following acute exercise. Int J Sports Med. 1997;18:174–8.
Endo MY, Kajimoto C, Yamada M, Miura A, Hayashi N, Koga S, et al. Acute effect of oral water intake during exercise on post-exercise hypotension. Eur J Clin Nutr. 2012;66:1208–13.
Franklin PJ, Green DJ, Cable NT. The influence of thermoregulatory mechanisms on post-exercise hypotension in humans. J Physiol. 1993;470:231–41.
Wilkins BW, Minson CT, Halliwill JR. Regional hemodynamics during postexercise hypotension. II. Cutaneous circulation. J Appl Physiol. 1985;2004(97):2071–6.
Lucas SJ, Cotter JD, Murrell C, Wilson L, Anson JG, Gaze D, et al. Mechanisms of orthostatic intolerance following very prolonged exercise. J Appl Physiol. 1985;2008(105):213–25.
Ide K, Eliasziw M, Poulin MJ. Relationship between middle cerebral artery blood velocity and end-tidal PCO2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol. 1985;2003(95):129–37.
Murrell CJ, Cotter JD, George K, Shave R, Wilson L, Thomas K, et al. Cardiorespiratory and cerebrovascular responses to head-up tilt II: influence of age, training status and acute exercise. Exp Gerontol. 2011;46:1–8.
Lucas SJ, Lewis NC, Sikken EL, Thomas KN, Ainslie PN. Slow breathing as a means to improve orthostatic tolerance: a randomized sham-controlled trial. J Appl Physiol. 1985;2013(115):202–11.
Gisolf J, Wilders R, Immink RV, van Lieshout JJ, Karemaker JM. Tidal volume, cardiac output and functional residual capacity determine end-tidal CO2 transient during standing up in humans. J Physiol. 2004;554:579–90.
Bőhm A, Kiss RG, Bachmann B, Duray GZ. Exercise-induced vasovagal syncope. Heart Rhythm. 2014;11:1089–90.
Crisafulli A, Melis F, Orrù V, Lener R, Lai C, Concu A. Hemodynamic during a 3 postexertional asystolia in a healthy athlete: a case study. Med Sci Sports Exerc. 2000;32:4–9.
Hirata T, Yano K, Okui T, Mitsuoka T, Hashiba K. Asystole with syncope following strenuous exercise in a man without organic heart disease. J Electrocardiol. 1987;20:280–3.
Abe H, Iwami Y, Nakashima Y, Kohshi K, Kuroiwa A. Exercise-induced neurally mediated syncope. Jpn Heart J. 1997;38:535–9.
Pedersen WR, Janosik DL, Goldenberg IF, Stevens LL, Redd RM. Post-exercise asystolic arrest in a young man without organic heart disease: utility of head-up tilt testing in guiding therapy. Am Heart J. 1989;18:410–3.
Campagna JA, Carter C. Clinical relevance of the Bezold-Jarisch reflex. Anesthesiology. 2003;98:1250–60.
Finlay JB, Hartman AF, Weir RC. Post-swim orthostatic intolerance in a marathon swimmer. Med Sci Sports Exerc. 1995;27:1231–7.
Whyte G, Stephens N, Budgett R, Sharma S, Shave RE, McKenna WJ. Exercise induced neurally mediated syncope in an elite rower: a treatment dilemma. Br J Sports Med. 2004;38:84–5.
Schwabe K, Schwellnus M, Derman W, Swanevelder S, Jordaan E. Medical complications and deaths in 21 and 56 km road race runners: a 4-year prospective study in 65 865 runners – SAFER study I. Br J Sports Med. 2014;48:912–8.
Schwabe K, Schwellnus MP, Derman W, Swanevelder S, Jordaan E. Less experience and running pace are potential risk factors for medical complications during a 56 km road running race: a prospective study in 26 354 race starters—SAFER study II. Br J Sports Med. 2014;48:905–11.
Schwabe K, Schwellnus MP, Derman W, Swanevelder S, Jordaan E. Older females are at higher risk for medical complications during 21 km road race running: a prospective study in 39 511 race starters—SAFER study III. Br J Sports Med. 2014;48:891–7.
Romero SA, Cooke WH. Hyperventilation before resistance exercise: cerebral hemodynamics and orthostasis. Med Sci Sports Exerc. 2007;39:1302–7.
Pott F, Van Lieshout JJ, Ide K, Madsen P, Secher NH. Middle cerebral artery blood velocity during intense static exercise is dominated by a Valsalva maneuver. J Appl Physiol. 1985;2003(94):1335–44.
Pott F, van Lieshout JJ, Ide K, Madsen P, Secher NH. Middle cerebral artery blood velocity during a valsalva maneuver in the standing position. J Appl Physiol. 1985;2000(88):1545–50.
Compton D, Hill PM, Sinclair JD. Weight-lifters’ blackout. Lancet. 1973;2:1234–7.
Wieling W, van Lieshout JJ. The fainting lark. Clin Auton Res. 2002;12:207.
Asplund CA, O’Connor FG, Noakes TD. Exercise-associated collapse: an evidence-based review and primer for clinicians. Br J Sports Med. 2011;45:1157–62.
Casa DJ, DeMartini JK, Bergeron MF, Csillan D, Eichner ER, Lopez RM, et al. National athletic trainers’ association position statement: exertional heat illnesses. J Athl Train. 2015;50:986–1000.
Schlader ZJ, Wilson TE, Crandall CG. Mechanisms of orthostatic intolerance during heat stress. Auton Neurosci. 2016;196:37–46.
Howe AS, Boden BP. Heat-related illness in athletes. Am J Sports Med. 2007;35:1384–95.
Johnson JM, Niederberger M, Rowell LB, Eisman MM, Brengelmann GL. Competition between cutaneous vasodilator and vasoconstrictor reflexes in man. J Appl Physiol. 1973;35:798–803.
Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1443–8.
Jimenez C, Fortrat JO, Delapierre B, Melin B. Moderate exercise effects on orthostatic intolerance while wearing protective clothing. Aviat Space Environ Med. 2012;83:570–6.
Adler GK, Bonyhay I, Failing H, Waring E, Dotson S, Freeman R. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes. 2009;58:360–6.
Madden KM, Lockhart CK, Potter TF, Cuff DJ, Meneilly GS. Short-term aerobic exercise reduces nitroglycerin-induced orthostatic intolerance in older adults with type 2 diabetes. J Cardiovasc Pharmacol. 2011;57:666–71.
Mtinangi BL, Hainsworth R. Effects of moderate exercise training on plasma volume, baroreceptor sensitivity and orthostatic tolerance in healthy subjects. Exp Physiol. 1999;84:121–30.
Mtinangi BL, Hainsworth R. Increased orthostatic tolerance following moderate exercise training in patients with unexplained syncope. Heart. 1998;80:596–600.
Takahagi VC, Costa DC, Crescêncio JC, Gallo Junior L. Physical training as non-pharmacological treatment of neurocardiogenic syncope. Arq Bras Cardiol. 2014;102:288–94.
Winker R, Barth A, Bidmon D, Ponocny I, Weber M, Mayr O, et al. Endurance exercise training in orthostatic intolerance: a randomized, controlled trial. Hypertension. 2005;45:391–8.
Allen SC, Taylor CL, Hall VE. A study of orthostatic insufficiency by the tiltboard method. Am J Physiol. 1945;143:11–20.
Brilla LR, Stephens AB, Knutzen KM, Caine D. Effect of strength training on orthostatic hypotension in older adults. J Cardiopulm Rehabil. 1998;18:295–300.
Howden R, Lightfoot JT, Brown SJ, Swaine IL. The effects of isometric exercise training on resting blood pressure and orthostatic tolerance in humans. Exp Physiol. 2002;87:507–15.
Mack GW, Convertino VA, Nadel ER. Effect of exercise training on cardiopulmonary baroreflex control of forearm vascular resistance in humans. Med Sci Sports Exerc. 1993;25:722–6.
Conboy EE, Fogelman AE, Sauder CL, Ray CA. Endurance training reduces renal vasoconstriction to orthostatic stress. Am J Physiol Renal Physiol. 2010;298:F279–84.
Fu Q, Vangundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010;55:2858–68.
George SA, Bivens TB, Howden EJ, Saleem Y, Galbreath MM, Hendrickson D, et al. The international POTS registry: evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm. 2016;13:943–50.
Rubinshtein R, Ciubotaru M, Elad H, Bitterman H. Severe orthostatic hypotension following weight reduction surgery. Arch Intern Med. 2001;161:2145–7.
DeHaven J, Sherwin R, Hendler R, Felig P. Nitrogen and sodium balance and sympathetic-nervous-system activity in obese subjects treated with a low-calorie protein or mixed diet. N Engl J Med. 1980;302:477–82.
Christou GA, Kiortsis DN. The effects of body weight status on orthostatic intolerance and predisposition to noncardiac syncope. Obes Rev. 2017;18:370–9.
Stevens GH, Foresman BH, Shi X, Stern SA, Raven PB. Reduction in LBNP tolerance following prolonged endurance exercise training. Med Sci Sports Exerc. 1992;24:1235–44.
Convertino VA, Sather TM, Goldwater DJ, Alford WR. Aerobic fitness does not contribute to prediction of orthostatic intolerance. Med Sci Sports Exerc. 1986;18:551–6.
Frey MA, Mathes KL, Hoffler GW. Aerobic fitness in women and responses to lower body negative pressure. Aviat Space Environ Med. 1987;58:1149–52.
Christou GA, Kouidi EJ, Anifanti MA, Sotiriou PG, Koutlianos NA, Deligiannis AP. Pathophysiological mechanisms of noncardiac syncope in athletes. Int J Cardiol. 2016;224:20–6.
Convertino VA. Blood volume: its adaptation to endurance training. Med Sci Sports Exerc. 1991;23:1338–48.
Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, et al. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–805.
Galderisi M, Cardim N, D’Andrea A, Bruder O, Cosyns B, Davin L, et al. The multi-modality cardiac imaging approach to the Athlete’s heart: an expert consensus of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:353.
Formes K, Zhang P, Tierney N, Schaller F, Shi X. Chronic physical activity mitigates cerebral hypoperfusion during central hypovolemia in elderly humans. Am J Physiol Heart Circ Physiol. 2010;298:H1029–37.
Hernandez JP, Franke WD. Age- and fitness-related differences in limb venous compliance do not affect tolerance to maximal lower body negative pressure in men and women. J Appl Physiol. 1985;2004(97):925–9.
Louisy F, Jouanin JC, Guezennec CY. Filling and emptying characteristics of lower limb venous network in athletes. Study by postural plethysmography. Int J Sports Med. 1997;18:26–9.
Sugawara J, Komine H, Miyazawa T, Imai T, Ogoh S. Influence of regular exercise training on post-exercise hemodynamic regulation to orthostatic challenge. Front Physiol. 2014;5:229.
Convertino VA, Mathes KL, Lasley ML, Tomaselli CM, Frey MA, Hoffler GW. Hemodynamic and hormonal responses to lower body negative pressure in men with varying profiles of strength and aerobic power. Eur J Appl Physiol Occup Physiol. 1993;67:492–8.
Smith ML, Graitzer HM, Hudson DL, Raven PB. Baroreflex function in endurance- and static exercise-trained men. J Appl Physiol. 1985;1988(64):585–91.
Ifuku H, Shiraishi Y. Assessment of cardiovascular regulation during head-up tilt and suspension in swimmers. Med Sci Sports Exerc. 2004;36:155–9.
Frederiks J, Swenne CA, Bruschke AV, van der Velde ET, Maan AC, TenVoorde BJ, et al. Correlated neurocardiologic and fitness changes in athletes interrupting training. Med Sci Sports Exerc. 2000;32:571–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflicts of interest
Georgios Christou, Konstantinos Christou and Dimitrios Kiortsis declare that they have no conflicts of interest relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Christou, G.A., Christou, K.A. & Kiortsis, D.N. Pathophysiology of Noncardiac Syncope in Athletes. Sports Med 48, 1561–1573 (2018). https://doi.org/10.1007/s40279-018-0911-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-018-0911-7