Abstract
Background
Swimming is a popular and potentially health-enhancing exercise, but has received less scientific attention compared with other exercise modes.
Objective
The objective of the study was to determine the chronic (long-term) effect of pool swim training on physiological outcomes in non-elite or non-competitive swimming participants.
Design
This study was a systematic review with a meta-analysis.
Data Sources
We searched the electronic databases PubMed, EMBASE and CENTRAL from inception to March 2017.
Eligibility Criteria
The eligibility criteria included randomised controlled trials, quasi-randomised controlled trials and controlled trials of chronic (long-term) swimming interventions in non-elite or non-competitive swimming participants, with a physiological outcome measure.
Results
Our search of 6712 records revealed 29 eligible studies. Swimming had a significant and clinically meaningful effect on maximal oxygen uptake compared with the control in an analysis including multiple populations (mean difference 6.32 mL/kg/min; 95% confidence interval 4.33–8.31), and subgroup analyses of healthy children/adolescents (mean difference 7.93 mL/kg/min; 95% confidence interval 3.31–12.55) and those with asthma (mean difference 9.67 mL/kg/min; 95% confidence interval 5.84–13.51) and healthy adults (mean difference 5.87 mL/kg/min; 95% confidence interval 2.93–8.81). Swimming also resulted in significant improvements in other cardiorespiratory fitness-related outcomes such as maximal minute ventilation (mean difference 0.61 L/min; 95% confidence interval 0.17–1.05), submaximal exercise performance (standardised mean difference 0.64; 95% confidence interval 0.14–1.13) and total exercise test time (mean difference 4.27 min; 95% confidence interval 2.11–6.42). Compared with the control, swimming had significant favourable effects on body mass (mean difference − 2.90 kg, 95% confidence interval − 5.02 to − 0.78), body fat percentage in multiple populations (mean difference − 1.92%; 95% confidence interval − 3.25 to − 0.60) and healthy children/adolescents (mean difference − 1.92%; 95% confidence interval − 4.64 to − 0.80) and lean mass (mean difference 1.96 kg; 95% confidence interval 0.21–3.71), but negative effects on waist circumference in a pooled analysis of two studies involving adults with hypertension (mean difference 4.03 cm; 95% confidence interval 2.58–5.49). Regarding lung function, significant effects of swimming vs. the control were found only for peak expiratory volume in analyses including children/adolescents combined with healthy adults (mean difference 58.74 L/min; 95% confidence interval 29.70–87.78) and children/adolescents with asthma alone (mean difference 63.49 L/min; 95% confidence interval 25.01–101.97). Based on limited data, swimming had similar effects to other exercise modes, except for higher post-intervention body mass index values with swimming vs. running in healthy adults (mean difference 1.18 kg/m2; 95% confidence interval 0.54–1.81).
Conclusions
Swimming may offer robust beneficial effects on cardiorespiratory fitness and body composition across multiple populations and effects may be comparable to other exercise modes. Future randomised controlled trials are required to establish the effectiveness of swimming on physiological outcomes in healthy populations and those with non-communicable disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Swim training can significantly and meaningfully improve cardiorespiratory fitness across a number of populations, including healthy children/adolescents and those with asthma and healthy adults. |
Swim training may result in small or unclear effects on resting lung function in children/adolescents with asthma, and significant but modest improvements in body composition in healthy children/adolescents and adults, and in adults with hypertension. |
From the limited data available, swim training had similar physiological effects compared to other modes of exercise (e.g. walking, running and cycling). |
1 Introduction
Swimming is an important life-saving skill but also a type of physical activity that might provide health benefits for young and adult populations, as well as patients with non-communicable disease (NCD) [1]. Swimming is one of the most popular modes of physical activity in USA, Europe and the UK [2,3,4] while at the same time it represents an appealing form of exercise for the elderly and individuals with NCD, owing to its low-impact nature [5]. However, despite its popularity and potential for benefit, swimming has received much less attention in the scientific literature compared with running and cycling. This may be because of the difficulty in taking physiological measures during swimming, the need to acquire a certain level of skill and technique to achieve a prescribed exercise intensity, and concerns over the safety of swimming for populations with NCD [6].
The physical properties of water, including its density, pressure, thermal capacity and conductivity, represent significant challenges and elicit physiological effects in an attempt to meet these demands. Within an evidence-based framework, knowledge of the chronic (long-term) physiological responses to swimming is crucial to optimise the design and application of safe and effective exercise prescription. Furthermore, a greater understanding of swimming physiology would allow clinicians to identify the populations that may benefit most from swimming and those for which this particular mode of exercise might be contraindicated or require certain modifications or supervision to ensure safety.
Previous reviews of the physiology of swimming have focused more on competitive swimming [7,8,9,10,11,12,13,14,15,16,17,18] with less scientific attention on swimming in healthy or chronic disease populations. Most of the reviews dedicated to the latter have focused on the effects of swimming on cardiovascular physiology [5, 6, 19] or respiratory and asthma-related conditions [20,21,22,23,24,25,26]. Given the lack of relevant studies in this field, the aim of the present systematic review and meta-analysis was to investigate the long-term physiological effects of recreational swimming in both healthy populations and those at risk of or diagnosed with NCD.
2 Methods
This review was written as part of a commissioned work on the physiological effects of swimming by the Amateur Swimming Association. The review has been reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
2.1 Search Methods for Identification of Studies
Criteria for considering studies for this review can be found in Table 1. We searched the following databases to identify eligible studies: PubMed, EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL) [via the Cochrane Library, Issue 3, 2017] on the 1 March, 2017. We also screened references in relevant reviews and in published eligible studies. A search algorithm was developed for PubMed [see Table S1 of the Electronic Supplementary Material (ESM)] and was modified for CENTRAL and EMBASE. We searched all databases from their inception to the present. We included only full publications and did not exclude based on the language of publication.
The results from the searches described above were merged and duplicate records of the same report were removed. The titles and abstracts were examined to remove obviously irrelevant reports. Two authors (IML and GSM) independently screened and assessed the records for eligibility. Full-text articles of potentially relevant reports were retrieved and examined for eligibility, and multiple eligible reports of the same trial were linked together. Non-English language trials were included and these trials were translated, where necessary, so that eligibility could be assessed and subsequently data extracted.
2.1.1 Data Extraction
Two authors (IML and GSM) independently extracted the following data: study design; total duration; sample size; demographic information; intervention characteristics; and physiological outcome. Multiple publications for the same trial were collated and the first or most complete report was used as the primary reference. We entered and combined the trial data using Review Manager (RevMan 5.3 Copenhagen, Denmark). We summarised the data collected from the reports in Table 2.
2.1.2 Risk of Bias Assessment of Articles
The Cochrane Collaboration’s ‘risk of bias’ tool was used to assess possible sources of bias [64]. We graded each domain as having ‘low’, ‘high’ or ‘unclear’ risk of bias and conflicts not due to assessor error were resolved by consensus (IML and GSM). If there was evidence of a large risk of bias, the findings were interpreted cautiously. The assessment of risk of bias was displayed in ‘risk of bias’ graphs. To aid interpretation, in each forest plot presented in the Results section, we included the summary of the risk of bias in each study involved.
2.2 Measures of Treatment Effect
All physiological outcome measurements were presented as continuous data across included studies. For selected outcomes, we extracted group means for change from baseline to the end of intervention and immediately post-intervention values with the corresponding standard deviations (SDs) and the number of participants assessed for each outcome per group. Where standard errors (SEs), confidence intervals (CIs) or t-values were provided instead of SDs, we used the RevMan 5.3 calculator to convert them to SDs.
For studies using the same scale to measure a continuous outcome, we calculated mean differences (MDs) and 95% CI using the change from baseline to the end of intervention values, or where not available, the immediate post-intervention values were used. Only a limited number of studies provided the change from baseline values, therefore, analyses combined change and post-intervention values. If different scales were used to measure the same continuous outcome across studies, we calculated a standardised mean difference (SMD) and 95% CI. For SMD analyses, change and post-intervention values cannot be combined, and therefore, we combined only change or post-intervention values depending on whichever was reported most. Where possible, we also interpreted the results based on the important clinically meaningful effect on outcomes [e.g. 5% loss in body mass [65] and 3.5 mL/kg/min change in maximal oxygen uptake (\( V{\text{O}}_{{ 2 {\text{max}}}} \))] [66]. We reported qualitative outcomes where data were not presented in sufficient detail or where data were available from single studies.
2.3 Unit of Analysis Issues
Where trials had more than one applicable swim training group, [34, 52] we combined outcome data from both groups as recommended in the Cochrane Handbook for Systematic Reviews of Interventions [64]. For the main analyses, we compared swim training groups with non-swimming groups for each outcome.
2.4 Assessment of Heterogeneity
A random-effects model was selected for all analyses a priori, owing to the diversity of the populations [67]. Inconsistency of results for each outcome was evaluated using the I 2 statistic, which describes the percentage of variability in the point estimates that is due to heterogeneity rather than sampling error [64]. We interpreted heterogeneity in accordance with the following recommendations of Higgins et al.: [64].
-
0–40%: might not be important;
-
30–60%: may represent moderate heterogeneity.
-
50–90%: may represent substantial heterogeneity.
-
75–100%: considerable heterogeneity.
If there was evidence of at least substantial heterogeneity, we explored its source by study population groups. There were insufficient studies available (fewer than ten) to investigate publication bias via visual inspection of planned funnel plots for signs of asymmetry.
2.5 Data Synthesis
To establish the robustness of an overall effect of swim training across populations, we combined studies that measured the same outcome in random-effects model meta-analyses where data were available from at least two studies [67]. In each analysis, we separated outcome data according to population subgroup, and presented subtotal summary statistics, in addition to the total summary statistic. Where there were insufficient studies to compare swim training with other modes of exercise (e.g. walking, running), these comparisons were presented in forest plots without meta-analyses, and reported qualitatively.
2.6 Sensitivity Analysis
Where possible, we conducted a sensitivity analysis to assess the influence of study design on the effect of swim training by removal of quasi-randomised or controlled trials from the analysis. In outcomes where at least substantial statistical heterogeneity (I 2 ≥ 50%) was found, we conducted sensitivity analyses to assess the robustness of the review results by removing the most extreme values and those studies that seemed to be estimating a different effect, and explored the contribution of each population to heterogeneity.
3 Results
3.1 Description of Included Studies
3.1.1 Results of the Search
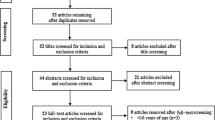
The literature search yielded 6712 potentially relevant articles. Tracking the reference lists of eligible articles and previous relevant reviews led to the inclusion of 11 additional articles. After removal of duplicates and screening of titles, abstracts, and full texts, we found 29 eligible trials, with 70 studies excluded with reasons (see Fig. 1 and Table S2 of the ESM).
3.1.2 Study Design and Population of Included Studies
The 29 eligible trials consisted of 16 randomised controlled trials (RCTs), three quasi-RCTs and ten controlled trials (CTs). Eligible studies investigated the effects of swim training on physiological outcomes in healthy children and adolescents (three CTs [27, 28, 68]), adults (six studies: three RCTs, [29, 31, 36]; three CTs, [34, 35, 37]), as well as pregnant women (one CT [38]) and individuals with obesity (two RCTs [50, 51]), asthma (nine studies: eight RCTs, [40, 42,43,44,45,46,47,48] one CT [41]), Down syndrome (one RCT [62]), cystic fibrosis (one CT [49]), hypertension (four studies: three quasi-RCT, [52, 57, 59] one CT [56]), osteoarthritis (one RCT [60]) and perforated tympanic membrane (one RCT [63]). Five trials had multiple associated publications (13 additional articles).
3.1.3 Intervention and Comparison Details of Included Studies
There were a total of 1499 participants in the eligible studies, with 718 participating in a swim training group and 501 in a non-exercise control (23 studies), 108 in a walking group (four studies [31, 36, 50, 51]), 13 in a walking-in-water group (one study [50]), 35 in a running group (two studies [29, 36]), 65 in a cycling group (four studies [29, 36, 51, 60]), 26 in a golf group (one study [47]) and 20 in a soccer group (one study [52]). Three of the studies that compared swimming with other modes of exercise, also had a non-exercise control group [29, 36, 52]. Two of the studies compared two different swim interventions, with one comparing swimming in a chlorinated pool vs. an ozone pool [34] and the other comparing moderate- with high-intensity swimming [52].
Most of the studies were of short duration, with 22 (76%) of the studies consisting of intervention durations of 15 weeks or less (mode duration = 12 weeks in ten studies; range = 4 weeks to 2 years). Two studies had a duration of 1 year, [28, 41] whereas one study consisted of a 2-year intervention [27].
3.1.4 Outcome Details of Included Studies
Sixteen (55%) eligible studies included anthropometric or body composition measures. Seventeen (59%) studies consisted of cardiorespiratory fitness outcomes, with ten of these studies including either a direct (nine studies [28, 35, 37, 43, 44, 47, 49, 57, 68]) or an estimated (three studies [29, 31, 50]) measure of \( V{\text{O}}_{{ 2 {\text{max}}}} \). Only one study measured \( V{\text{O}}_{{ 2 {\text{max}}}} \) directly during pool (tethered) swimming [37]. Muscular strength measures were included in three studies [29, 36, 60]. Six (22%) studies included a resting cardiovascular measure (e.g. resting heart rate and blood pressure), [31, 51, 52, 57, 58, 60] whereas two studies included these measures in the swim group only [38, 56]. Lung function outcomes were reported in 12 (41%) studies [34, 40,41,42,43,44,45,46,47,48,49, 68]. Blood biomarker assessments were reported in eight (28%) studies [29, 31, 34, 52, 57, 58, 60, 68]. Table 2 summarises the characteristics and outcomes of the studies included in the analysis.
3.2 Risk of Bias of Included Studies
A summary of risk of bias across all studies is presented in Fig. 2 (see also a summary of the risk of bias for each study in Fig. S1 of the ESM). Only five (17%) studies were at a low risk of selection bias because they reported to have adequately generated their randomised sequence and concealed allocation to the intervention. All included trials included were at high risk for performance bias because, owing to the nature of swimming, it was not possible to blind the trial personnel and participants. Twenty-five (90%) studies were considered to be at a high risk for detection bias because they did not state that outcome assessors were blinded to group assignment. Nine (31%) studies with high participant withdrawal rates were judged to be at high risk of attrition bias [38, 40, 41, 46,47,48,49,50,51]. We considered 27 (93%) studies to be at an unclear risk for reporting bias because no study protocol paper or trial registration was available, and the information was insufficient to judge this item for those studies. One study [63] was at a low risk of reporting bias because the published paper included all outcomes that were reported in a prospective trial registration. Another study [60] was considered to be at high risk of reporting bias because one of the outcomes (C-reactive protein) listed in a trial registration was not included in the published paper. Three (10%) studies were at high risk for selection bias because groups were insufficiently similar at baseline [38, 49, 58].
3.3 Effects of Intervention
Summaries of findings for each pooled analysis are presented in Tables S3–5 and Figs. S2–40 of the ESM.
3.3.1 Cardiorespiratory Fitness and Muscular Strength Outcomes
A statistically significant and clinically important (≥ 3.5 mL/kg/min) effect on relative \( V{\text{O}}_{{ 2 {\text{max}}}} \) was found for swimming, compared with the control, in an analysis of children/adolescents who were healthy and those with asthma or cystic fibrosis, and adults who were healthy and those with hypertension (MD 6.32 mL/kg/min, 95% CI 4.33–8.31, I 2 = 55%, nine studies, 208 participants) (see Fig. 3). In a sensitivity analysis, the removal of the two most extreme values [29, 57] reduced the heterogeneity to 0% and maintained the overall significant effect. The effect was also robust with the inclusion of only RCTs in the analysis (MD 3.00 mL/kg/min, 95% CI 0.21–5.79, I 2 = 76%, three studies, 55 participants). A significant increase in absolute \( V{\text{O}}_{{ 2 {\text{max}}}} \) was also found for swimming compared with the control, in an analysis of healthy children/adolescents and adults (MD 0.41 L/min, 95% CI 0.27–0.55, I 2 = 22%, three studies, 74 participants).
Effect of swimming vs. control on relative maximal oxygen uptake (\( V{\text{O}}_{{ 2 {\text{max}}}} \), mL/kg/min) [combined change from baseline to end of intervention and post-intervention follow-up values analysis]. change values change from baseline to end of intervention, CI confidence interval, IV inverse variance, SD standard deviation
Separate subgroup analyses revealed significantly higher post-intervention relative \( V{\text{O}}_{{ 2 {\text{max}}}} \) values after swim training vs. the control in healthy children/adolescents (MD 7.93 mL/kg/min, 95% CI 3.31–12.55, I 2 = 0%, two studies, 44 participants) and those with asthma (MD 9.67 mL/kg/min, 95% CI 5.84–13.51, I 2 = 0%, two studies, 32 participants). Similarly, swim training had a significant effect on relative \( V{\text{O}}_{{ 2 {\text{max}}}} \) compared with the control in healthy adults (MD 5.87 mL/kg/min, 95% CI 2.93–8.81, I 2 = 76%, three studies, 77 participants). Removal of the most extreme value [29] in this analysis reduced heterogeneity to 0% and the effect was maintained, but an RCT-only analysis was not possible owing to the availability of only one RCT. An increase in absolute \( V{\text{O}}_{{ 2 {\text{max}}}} \) was also found for healthy children/adolescents after swim training compared with the control (MD 0.30 mL/kg/min, 95% CI 0.12–0.48, I 2 = 0%, two studies, 44 participants). No significant differences were found on relative \( V{\text{O}}_{{ 2 {\text{max}}}} \) for swimming compared with running in a pooled analysis of healthy adults, or single studies comparing swimming to golfing in children/adolescents with asthma, [47] and cycling in healthy adults [29]. One study found superior effects on \( V{\text{O}}_{{ 2 {\text{max}}}} \) for 6 months of walking compared with swimming [31].
A small but statistically significant effect on post-intervention maximal minute ventilation values was found for swimming vs. controls in an analysis combining data from children/adolescents who were healthy or asthmatic, and healthy adults (SMD 0.61, 95% CI 0.17–1.05, I 2 = 0%, four studies, 88 participants), but not in a separate subgroup analysis of healthy children/adolescents. Of the remaining maximal exercise variable analyses, a significant effect of swimming compared with controls was observed only for post-intervention maximal O2 pulse in healthy children/adolescents (SMD 1.26, 95% CI 0.30–2.23, I 2 = 40%, two studies, 44 participants). A pooled analysis of seven studies revealed a significantly higher post-intervention submaximal exercise performance compared with controls (SMD 0.64, 95% CI 0.14–1.13, I 2 = 61%, 208 participants). An analysis of only RCTs revealed a significantly greater workload at lactate/ventilatory threshold intensity in children/adolescents with asthma (SMD 1.40, 95% CI 0.56–2.25, I 2 = 38%, three studies, 48 participants).
A significant improvement in exercise time during graded exercise testing was found for swimming compared with controls in a pooled analysis of two CTs involving children/adolescents with cystic fibrosis and healthy adults (MD 4.27 min, 95% CI 2.11–6.42, I 2 = 0%, 50 participants). One study reported that swimming significantly improved distance covered during Yo–Yo intermittent exercise test compared with controls in adults with hypertension [52]. Another study found a significant post-intervention between-group increase in distance covered during a 12-min swim test but no differences in 1.6-km walk test time with a swim intervention compared with a walking group in healthy women [31]. No significant differences in post-intervention peak quadriceps torque values were found between swimming and controls, walking and running groups of healthy adults, and cycling in adults with osteoarthritis [29, 36, 60].
3.3.2 Resting Cardiovascular and Vascular Function Outcomes
A pooled analysis of resting heart rate, and resting systolic, diastolic and mean arterial blood pressure was possible only for adults with hypertension. No significant effect of swimming compared with the control or to walking in analyses involving healthy women or women with obesity was found for any of these outcomes. Individual studies have found significant effects on the resting heart rate for swim training compared with cycling in adults with osteoarthritis, [60] and significant effects in favour of walking vs. swimming for systolic blood pressure and diastolic blood pressure in healthy women [31].
For vascular responses, studies involving individuals with hypertension have reported significant improvements in carotid artery compliance, flow-mediated dilation and cardiovagal baroreflex sensitivity, [57] but not in casual forearm vascular resistance, [58] after swimming interventions. Alkatan et al [60] found that endothelial function improved significantly after swimming but not post-cycling training in adults with osteoarthritis.
3.3.3 Lung Function Outcomes
In a pooled analysis of three RCTs involving healthy children/adolescents and adults, [40, 45, 46] post-intervention peak expiratory flow (PEF) was significantly greater in the swim group compared with the control group (MD 58.74 L/min, 95% CI 29.70–87.78, I 2 = 39%, 103 participants). The significant effect of swimming on PEF was also observed in a separate subgroup analysis of children/adolescents with asthma (MD 63.49 L/min, 95% CI 25.01–101.97, I 2 = 52%, two studies, 38 participants). No significant effects of swimming compared with controls were found for any other lung function measure in combined population analyses or in separate subgroup analyses of children/adolescents with asthma.
In the only study to compare the effects of swimming with other exercise modes on lung function, the swimming group had significant increases in forced expiratory volume in during the first second of forced breath percentage predicted compared with a golf group [47]. Compared with controls, studies have reported significant reductions in bronchial hyper-responsiveness in adults with asthma, [40] and in children/adolescents with asthma, improvements in exercise-induced bronchoconstriction, [42] methacholine challenge test performance, and maximal inspiratory and maximal expiratory pressure [48]. Children/adolescents with cystic fibrosis reported a significant improvement in clinical disease state after a swimming intervention vs. controls, [49] whereas an RCT found that swimming did not improve respiratory aspects of speech production in individuals with Down syndrome [62]. Another study [68] involving female high school students reported significant pre-post increases in maximal breathing capacity in a swim group but not a control group.
3.3.4 Blood Biomarker Outcomes
Only a pooled analysis of studies consisting of adults with hypertension was possible for total cholesterol, low-density lipoprotein-cholesterol, high-density lipoprotein (HDL)-cholesterol, and triglyceride levels, [52, 57, 58] and plasma glucose levels [57, 58] only. No significant differences were found between swimming and control groups for any of these blood biomarkers.
Cox and colleagues [31] found that of glucose- or insulin-related outcomes, only insulin area under the curve was significantly higher in a walking group compared with a swimming group immediately post-intervention, but not 6 months later. Conversely, the authors [31] reported significantly increased total cholesterol and low-density lipoprotein-cholesterol in the walk group compared with the swimming group 6 months after completion of the intervention, but not immediately post-intervention. With regard to other biomarkers, glycosylated haemoglobin and interleukin-6 significantly improved after both swimming and cycling in adults with osteoarthritis, [60] whereas another study found that high-intensity, but not moderate-intensity swimming improved insulin sensitivity and the expression of adhesion molecules linked with endothelial dysfunction and reduced fasting plasma insulin levels in hypertensive women [52]. The same study [52] also reported that citrate synthase, 3-hydroxyacyl-CoA dehydrogenase and complex IV in the deltoid muscle all significantly increased after both high- and moderate-intensity swimming.
In one RCT, [29] the effects of a 30-min walking exercise on the accumulation of cartilage degeneration, assessed via serum cartilage oligomeric matrix protein, was measured after 12 weeks of swimming (non-impact), cycling (low impact) or running (high impact) training. The authors [29] reported that post-intervention 30 min of walking significantly increased serum cartilage oligomeric matrix protein levels (i.e. greater cartilage degeneration) of the swimming and cycling groups, but not of the running group.
In another study, [34] the effects of a swimming programme consisting of 20 1-h sessions in either a chlorine pool or ozone pool on levels of blood biomarkers of inflammation, injury and epithelial integrity of the lung lining surfaces (surfactant protein D and Clara cell secretory protein) were compared with a control group. Compared with baseline values, post-intervention Clara cell secretory protein levels were significantly greater in the chlorine group only, whereas levels of surfactant protein D were not significantly modified post-intervention in any group. In a study of 30 female high school students, [68] swim training resulted in significant pre-post increases in mean corpuscular volume and mean cell haemoglobin levels in the intervention group but no significant changes in the control group. No significant pre-post changes in red blood cell counts, haemoglobin, haematocrit and mean corpuscular haemoglobin were observed in either group. No significant changes were observed in any other blood biomarker in the included studies.
3.3.5 Anthropometric and Body Composition Outcomes
In our pooled analyses, significant effects on body mass or body mass index for swim training vs. controls were found only in a combined analysis of body mass data from healthy children/adolescents and those with cystic fibrosis, pregnant women, and adults who were healthy or hypertensive (MD − 2.90, 95% CI − 5.20 to − 0.78, I 2 = 90%, ten studies, 500 participants). Although no data were provided, one study [37] reported no significant changes in body mass in either a swim training or control group. However, another study [51] found significantly greater weight loss in a cycling intervention compared with swimming in women with obesity. In comparisons of exercise modes, the only significant finding was that run training reduced the body mass index to a greater extent than swimming in an analysis of two RCTs including healthy adults (MD 1.18 kg/m2, 95% CI 0.54–1.81, I 2 = 0%, 68 participants).
Swim interventions significantly reduced body fat percentage in an analysis consisting of healthy children/adolescents and adults, and adults with hypertension, compared with controls (MD − 1.92%, 95% CI − 3.25 to − 0.60, I 2 = 6%, seven studies, 216 participants) (see Fig. 4). Only one of the six studies included in this analysis was an RCT. A significant effect of swimming on post-intervention body fat percentage was observed in a subgroup analysis of healthy children/adolescents (MD − 1.92%, 95% CI − 4.64 to − 0.80, I 2 = 0%, two studies, 44 participants). No significant effects were observed in remaining subgroup analyses comparing swim training to controls, walking in healthy women/women with obesity, run training in healthy adults or in a single study of swimming vs. cycling in adults with osteoarthritis [60]. However, one study [29] found that healthy adults had a significantly greater reduction in body fat percentage with cycling compared with swimming.
Overall, there was a significant increase in lean mass for swimming vs. controls in an analysis consisting of healthy children/adolescents and adults, and adults with hypertension (MD 1.96 kg, 95% CI 0.21–3.71, I 2 = 77%, six studies, 191 participants). The removal of the most extreme value [58] reduced the heterogeneity to 0% and maintained the overall significant effect. A sensitivity analysis was not possible owing to a lack of RCTs in the analysis. No effects on lean mass were found for swim training vs. controls in subgroup analyses of healthy children/adolescents or adults with hypertension, or in single studies compared with walking either on land or in water in women with obesity [50], or cycling in adults with osteoarthritis [60].
In a pooled analysis of two studies involving 80 individuals with hypertension, post-intervention waist circumference was significantly higher in swimming vs. control interventions (MD 4.03 cm, 95% CI 2.59–5.49, I 2 = 0%), but no effects were found for hip circumference values. However, the two studies included in these analyses were quasi-RCTs, and in one study, [58] there were waist circumference imbalances at baseline. In regard to other related outcomes, the only significant findings were that arm and calf girths were significantly lower in a swimming group compared with a walking group in one study [31].
4 Discussion
4.1 Summary of Main Results
To the best of our knowledge, this is the first review synthesising the evidence about the physiological effects of swim training in non-elite and non-trained healthy and NCD populations. A statistically and clinically significant effect (≥ 3.5 mL/kg/min) on \( V{\text{O}}_{{ 2 {\text{max}}}} \) was found for swimming compared with the control in a pooled analysis of combined populations and separate analyses of children/adolescents with asthma and healthy adults. Peak expiratory flow improved significantly after swim training compared with the control in a pooled analysis of healthy adults and children/adolescents with asthma, and in a separate subgroup analysis of the latter population. Swimming was associated with significant reductions in body fat percentage and increases in lean mass in combined populations’ analyses. In adults with hypertension, there was a significant increase in waist circumference with swimming vs. the control, but no effect on any other cardiovascular, blood biomarker or anthropometric measure. Based on limited data, the effects of swimming on all outcomes analysed were similar to other exercise modes, apart from slightly higher BMI after a swimming intervention compared with running in healthy adults.
4.2 Overall Completeness and Applicability of Evidence
From a comprehensive search of the three major electronic databases (CENTRAL, EMBASE and PubMed) and reference lists of eligible studies and relevant reviews, we identified 29 studies (1499 total participants), including 16 RCTs, three quasi-RCTs, and ten CTs. However, limited studies were available for subgroup analyses and most included studies were potentially under-powered owing to small sample sizes (median, intervention n = 16 and control n = 14). Furthermore, no RCTs were available for healthy children/adolescents and those with cystic fibrosis, pregnant women and individuals with hypertension. Pooling of study data was limited by the broad range of outcomes assessed in studies, which meant many of the outcomes were examined in single studies or populations. Therefore, as a result of only a few adequately sized studies available in each population for each outcome, it is difficult to generalise the findings of the current review. In particular, blood biomarker and resting cardiovascular results are limited largely to adults with hypertension, and lung function data are predominantly based on children/adolescents with asthma. Therefore, complete evidence of the chronic physiological effects of swimming is absent for many of the populations included in the current review and those that have not yet been studied, such as individuals with arthritis, cancer, coronary heart disease, or type 1 and 2 diabetes mellitus.
The majority of swimming interventions were of short duration (≥ 12 weeks), with only seven (24%) studies consisting of interventions of at least 6 months duration. Therefore, the long-term effects of swim training are not well known. Furthermore, the practicality of the interventions in the included studies varied from a more realistic one to three sessions per week (20 studies) to swim training programmes of 60 min daily [51] or six 30-min sessions a week, [42] which would be more difficult to follow and adhere to outside a study environment.
4.3 Quality of the Evidence
Most studies were judged to be at a high risk of selection bias, performance bias and detection bias. Almost a third of the studies were at a high risk of attrition bias, and all but two trials were at an unclear risk of reporting bias. In addition, there was evidence of moderate to considerable heterogeneity (I 2 = 30–100%) in many of the comparisons. However, most of this inconsistency was explained by the study population and removal of the most extreme values, in addition to study design, in the few instances an RCT-only analysis could be performed. In the 15 (52%) studies that reported adherence data, adherence to the swimming interventions was generally good (range = 76–99%). Most studies had acceptable attrition rates, although nine (31%) studies had particularly high participant withdrawal rates. Therefore, at least in the studies that reported these data, adherence and attrition rates in swimming interventions appeared to be comparable to other exercise modes.
Of particular note, given the difficulties of heart rate monitoring in water, is that equalising swimming intensity with that of other modes of exercise may always be challenging. This is supported by the fact that precise control of intensity in the different modes of exercise within the included studies was lacking. Moreover, swimming efficiency varies greatly, particularly in populations that have no previous experience in swimming or novice swimmers. As a result of this, the outcomes of a swimming programme may be greatly affected, particularly when the intensity is not precisely controlled. Therefore, there is a need for future studies to control intensity to a greater degree.
4.4 Potential Biases in the Review Process
Despite our comprehensive search, it is possible that we may have missed eligible studies. A relatively high number (n = 11) of studies were identified through searching the reference lists of eligible studies and relevant reviews, perhaps owing to inadequate cataloguing of swimming studies (older studies in particular) in these databases. Because of a lack of adequate study numbers, we could not perform publication bias analysis. Although we set no language restrictions, we included only full publications, which may contribute to publication bias. However, unpublished or studies only published in an abstract form tend be of poor methodological quality and have not undergone peer review [69, 70]. Therefore, it is unclear whether the addition of unpublished studies would have influenced the findings of this current review without adding a considerable risk of bias.
4.5 Agreements and Disagreements with Other Studies or Reviews
To the best of our knowledge, the current review is the only study to systematically review and pool data from swim training studies from all available populations. In the most recent and comprehensive systematic review and meta-analysis of the effects of swimming on lung function of children/adolescents with asthma, Beggs et al. [21] also found significant favourable effects on PEF with swimming compared with controls. However, unlike Beggs et al. [21] we found no significant effect on forced expiratory volume during the first second of forced breath predicted and average forced expiratory flow during the mid (25–75%) portion of the forced vital capacity predicted in children/adolescents with asthma. The probable reason for this discrepancy is the inclusion of data from a conference abstract [71] not included in the current review.
5 Conclusions
In a pooled analysis of combined populations and various subgroup analyses, swimming had significant favourable effects on \( V{\text{O}}_{{ 2 {\text{max}}}} \), maximal minute ventilation, submaximal exercise performance, body fat percentage and lean mass, compared with the controls. Swimming also led to significant improvements in PEF in a pooled analysis of healthy adults and children/adolescents with asthma and a subgroup analysis of the latter population only. Significant increases in waist circumference were observed in swimming interventions including adults with hypertension. Based on a meta-analysis of limited data, there were no differences between the effects of swimming compared with walking, running or cycling in any of the comparisons made, except for slightly higher post-intervention BMI values with swim vs. run training. However, the findings presented must be interpreted with caution considering the dearth of RCT evidence for the populations included in the review, risk of bias and evidence of heterogeneity across comparisons. Therefore, future well-designed and reported RCTs are required to establish the efficacy and effectiveness of swim training on physiological outcomes in various populations including children and adolescents, sedentary adults, older adults and individuals with NCD.
References
Chase NL, Sui X, Blair SN. Comparison of the health aspects of swimming with other types of physical activity and sedentary lifestyle habits. Int J Aquat Res Educ. 2008;2:151–61.
Physical Activity Council. Annual tracking sports, fitness and recreation participation in the USA. 2013. http://physicalactivitycouncil.com/. Accessed 6 Sep 2017.
Scheerder J, Vandermeerschen H, Van Tuyckom C, et al. Understanding the game sport participation in Europe: facts, reflections and recommendations. Report SPM10, Sport and Policy Management. 2011.
A.L.S. Sport England, active lives survey, year 1 report. 2016. http://www.sportengland.org/media/11498/active-lives-survey-yr-1-report.pdf. Accessed 6 Sep 2017.
Lazar JM, Khanna N, Chesler R, et al. Swimming and the heart. Int J Cardiol. 2013;168(1):19–26.
Tanaka H. Swimming exercise: impact of aquatic exercise on cardiovascular health. Sports Med. 2009;39(5):377–87.
Aspenes ST, Karlsen T. Exercise-training intervention studies in competitive swimming. Sports Med. 2012;42(6):527–43.
Barbosa TM, Bragada JA, Reis VM, et al. Energetics and biomechanics as determining factors of swimming performance: updating the state of the art. J Sci Med Sport. 2010;13(2):262–9.
Costa MJ, Bragada JA, Mejias JE, et al. Tracking the performance, energetics and biomechanics of international versus national level swimmers during a competitive season. Eur J Appl Physiol. 2012;112(3):811–20.
Cooper LW, Powell AP, Rasch J. Master’s swimming: an example of successful aging in competitive sport. Curr Sports Med Rep. 2007;6(6):392–6.
Ferreira MI, Barbosa TM, Costa MJ, et al. Energetics, biomechanics, and performance in masters’ swimmers: a systematic review. J Strength Cond Res. 2016;30(7):2069–81.
Holmer I. Physiology of swimming man. Exerc Sport Sci Rev. 1979;7:87–123.
Lavoie JM, Montpetit RR. Applied physiology of swimming. Sports Med. 1986;3(3):165–89.
Marino M. Profiling swimmers. Clin Sports Med. 1984;3(1):211–29.
Pendergast DR, Moon RE, Krasney JJ, et al. Human physiology in an aquatic environment. Compr Physiol. 2015;5(4):1705–50.
Toubekis AG, Tokmakidis SP. Metabolic responses at various intensities relative to critical swimming velocity. J Strength Cond Res. 2013;27(6):1731–41.
Troup JP. The physiology and biomechanics of competitive swimming. Clin Sports Med. 1999;18(2):267–85.
Videler JJ, Nolet BA. Costs of swimming measured at optimum speed: scale effects, differences between swimming styles, taxonomic groups and submerged and surface swimming. Comp Biochem Physiol A Comp Physiol. 1990;97(2):91–9.
Koenig J, Jarczok MN, Wasner M, et al. Heart rate variability and swimming. Sports Med. 2014;44(10):1377–91.
Bar-Or O, Inbar O. Swimming and asthma. Benefits and deleterious effects. Sports Med. 1992;14(6):397–405.
Beggs S, Foong YC, Le HC, et al. Swimming training for asthma in children and adolescents aged 18 years and under. Cochrane Database Syst Rev. 2013;(4):CD009607.
Bougault V, Boulet LP. Airway dysfunction in swimmers. Br J Sports Med. 2012;46(6):402–6.
Bougault V, Turmel J, Levesque B, et al. The respiratory health of swimmers. Sports Med. 2009;39(4):295–312.
Fisk MZ, Steigerwald MD, Smoliga JM, et al. Asthma in swimmers: a review of the current literature. Phys Sportsmed. 2010;38(4):28–34.
Geiger KR, Henschke N. Swimming for children and adolescents with asthma. Br J Sports Med. 2015;49(12):835–6.
Rosimini C. Benefits of swim training for children and adolescents with asthma. J Am Acad Nurse Pract. 2003;15(6):247–52.
Bielec G, Peczak-Graczyk A, Waade B. Do swimming exercises induce anthropometric changes in adolescents? Issues Compr Pediatr Nurs. 2013;36(1–2):37–47.
Obert P, Courteix D, Lecoq AM, Guenon P. Effect of long-term intense swimming training on the upper body peak oxygen uptake of prepubertal girls. Eur J Appl Physiol Occup Physiol. 1996;73(1–2):136–43.
Çelik Ö, Salci Y, Ak E, et al. Serum cartilage oligomeric matrix protein accumulation decreases significantly after 12 weeks of running but not swimming and cycling training: a randomised controlled trial. Knee. 2013;20(1):19–25.
Özdemir RA, Çelik Ö, Aşçı FH. Exercise interventions and their effects on physical self-perceptions of male university students. Int J Psychol. 2010;45(3):174–81.
Cox KL, Burke V, Beilin LJ, et al. Blood pressure rise with swimming versus walking in older women: the Sedentary Women Exercise Adherence Trial 2 (SWEAT 2). J Hypertens. 2006;24(2):307–14.
Cox KL, Burke V, Beilin LJ, et al. Short and long-term adherence to swimming and walking programs in older women: the Sedentary Women Exercise Adherence Trial (SWEAT 2). Prev Med. 2008;46(6):511–7.
Cox KL, Burke V, Beilin LJ, et al. A comparison of the effects of swimming and walking on body weight, fat distribution, lipids, glucose, and insulin in older women: the Sedentary Women Exercise Adherence Trial 2. Metabolism. 2010;59(11):1562–73.
Fernández-Luna Á, Gallardo L, Plaza-Carmona M, et al. Respiratory function and changes in lung epithelium biomarkers after a short-training intervention in chlorinated vs. ozone indoor pools. PLoS One. 2013;8(7):e68447.
Lieber DC, Lieber RL, Adams WC. Effects of run-training and swim-training at similar absolute intensities on treadmill VO2max. Med Sci Sports Exerc. 1989;21(6):655–61.
Lu L, Wang Y. Effects of exercises on knee cartilage volume in young healthy adults: a randomized controlled trial. Chin Med J. 2014;127(12):2316–21.
Magel JR, Foglia GF, McArdle WD, et al. Specificity of swim training on maximum oxygen uptake. J Appl Physiol. 1975;38(1):151–5.
Lynch AM, Goodman C, Choy PL, et al. Maternal physiological responses to swimming training during the second trimester of pregnancy. Res Sports Med. 2007;15(1):33–45.
Lynch AM, McDonald S, Magann EF, et al. Effectiveness and safety of a structured swimming program in previously sedentary women during pregnancy. J Matern Fetal Neonatal Med. 2003;14(3):163–9.
Arandelović M, Stanković I, Nikolić M. Swimming and persons with mild persistent asthma. Sci World J. 2007;7:1182–8.
Huang SW, Veiga R, Sila U, et al. The effect of swimming in asthmatic children–participants in a swimming program in the city of Baltimore. J Asthma. 1989;26(2):117–21.
Matsumoto I, Araki H, Tsuda K, et al. Effects of swimming training on aerobic capacity and exercise induced bronchoconstriction in children with bronchial asthma. Thorax. 1999;54(3):196–201.
Varray AL, Mercier JG, Terral CM, et al. Individualized aerobic and high intensity training for asthmatic children in an exercise readaptation program: is training always helpful for better adaptation to exercise? Chest. 1991;99(3):579–86.
Varray AL, Mercier JG, Prefaut CG. Individualized training reduces excessive exercise hyperventilation in asthmatics. Int J Rehabil Res. 1995;18(4):297–312.
Wang JS, Hung WP. The effects of a swimming intervention for children with asthma. Respirology. 2009;14(6):838–42.
Weisgerber MC, Guill M, Weisgerber JM, et al. Benefits of swimming in asthma: effect of a session of swimming lessons on symptoms and PFTs with review of the literature. J Asthma. 2003;40(5):453–64.
Weisgerber M, Webber K, Meurer J, et al. Moderate and vigorous exercise programs in children with asthma: safety, parental satisfaction, and asthma outcomes. Pediatr Pulmonol. 2008;43(12):1175–82.
Wicher IB, Ribeiro MA, Marmo DB, et al. Effects of swimming on spirometric parameters and bronchial hyperresponsiveness in children and adolescents with moderate persistent atopic asthma. J Pediatr. 2010;86(5):384–90.
Edlund LD, French RW, Herbst JJ, et al. Effects of a swimming program on children with cystic fibrosis. Am J Dis Child. 1986;140(1):80–3.
Gappmaier E, Lake W, Nelson AG, et al. Aerobic exercise in water versus walking on land: effects on indices of fat reduction and weight loss of obese women. J Sports Med Phys Fitness. 2006;46(4):564–9.
Gwinup G. Weight loss without dietary restriction: efficacy of different forms of aerobic exercise. Am J Sports Med. 1987;15(3):275–9.
Mohr M, Nordsborg NB, Lindenskov A, et al. High-intensity intermittent swimming improves cardiovascular health status for women with mild hypertension. Biomed Res Int. 2014;2014:728289.
Mohr M, Helge EW, Petersen LF, et al. Effects of soccer vs swim training on bone formation in sedentary middle-aged women. Eur J Appl Physiol. 2015;115(12):2671–9.
Connolly LJ, Nordsborg NB, Nyberg M, et al. Low-volume high-intensity swim training is superior to high-volume low-intensity training in relation to insulin sensitivity and glucose control in inactive middle-aged women. Eur J Appl Physiol. 2016;116(10):1889–97.
Nordsborg NB, Connolly L, Weihe P, et al. Oxidative capacity and glycogen content increase more in arm than leg muscle in sedentary women after intense training. J Appl Physiol (1985). 2015;119(2):116–23.
Silva J, Geraldes A, Natali A, et al. Acute effects of swimming on the arterial pressure of hypertensive adults. Maced J Med Sci. 2009;2(4):330–4.
Nualnim N, Parkhurst K, Dhindsa M, et al. Effects of swimming training on blood pressure and vascular function in adults > 50 years of age. Am J Cardiol. 2012;109(7):1005–10.
Tanaka H, Bassett DR, Howley ET. Effects of swim training on body weight, carbohydrate metabolism, lipid and lipoprotein profile. Clin Physiol. 1997;17(4):347–59.
Tanaka H, Bassett DR, Howley ET, et al. Swimming training lowers the resting blood pressure in individuals with hypertension. J Hypertens. 1997;15(6):651–7.
Alkatan M, Baker JR, Machin DR, et al. Improved function and reduced pain after swimming and cycling training in patients with osteoarthritis. J Rheumatol. 2016;43(3):666–72.
Alkatan M, Machin DR, Baker JR, et al. Effects of swimming and cycling exercise intervention on vascular function in patients with osteoarthritis. Am J Cardiol. 2016;117(1):141–5.
Casey AF, Emes C. The effects of swim training on respiratory aspects of speech production in adolescents with Down syndrome. Adapt Phys Activ Q. 2011;28(4):326–41.
Stephen ATN, Leach AJ, Morris PS. Impact of swimming on chronic suppurative otitis media in Aboriginal children: a randomised controlled trial. Med J Aust. 2013;199(1):51–5.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Stevens J, Truesdale KP, McClain JE, et al. The definition of weight maintenance. Int J Obes. 2006;30(3):391–9.
Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Stransky AW, Mickelson RJ, van Fleet C, et al. Effects of a swimming training regimen on hematological, cardiorespiratory and body composition changes in young females. J Sports Med Phys Fitness. 1979;19(4):347–54.
McAuley L, Pham B, Tugwell P, et al. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet. 2000;356(9237):1228–31.
Schmucker CM, Blumle A, Schell LK, et al. Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research. PLoS One. 2017;12(4):e0176210.
Altintas D, Cevit O, Ergen N, et al. The effect of swimming training on aerobic capacity and pulmonary functions in children with asthma. Allergy Clin Immunol Int. 2003;1:17.
Acknowledgements
This article arose from work initially commissioned by Prof. Ian Cumming on behalf of the Swimming and Health Commission for Swim England. The authors thank Ann B. Gates, Prof. Ian Cumming and Seven Stones for their help in the original commissioned paper.
Author information
Authors and Affiliations
Contributions
Ian M. Lahart and George S. Metsios contributed to this work by drafting a narrative report after conducting a systematic review of the acute and chronic physiological effects of swimming. The current article includes the systematic review of the chronic physiological effects of swimming together with a meta-analysis of primary and secondary outcomes.
Corresponding author
Ethics declarations
Funding
This work was partly sponsored by the independent Swimming and Health Commission, Swim England, UK.
Conflict of interest
Ian M. Lahart and George S. Metsios have no conflicts of interest directly relevant to the content of this review.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lahart, I.M., Metsios, G.S. Chronic Physiological Effects of Swim Training Interventions in Non-Elite Swimmers: A Systematic Review and Meta-Analysis. Sports Med 48, 337–359 (2018). https://doi.org/10.1007/s40279-017-0805-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-017-0805-0