Abstract
Background
Previous systematic reviews concluded that pneumococcal vaccination in the elderly was cost effective. However, recently published economic evaluations state that it may not be cost effective when children are vaccinated with higher-valent pneumococcal conjugate vaccines. The literature suggests that the outcomes of vaccination in the elderly are strongly influenced by the vaccine effectiveness (VE) against the vaccine-type pneumococcal diseases (PD) and the impact of childhood vaccination on the vaccine-type PD incidence in the elderly, but the extent remains unclear.
Methods
We conducted a systematic literature search of cost-effectiveness studies on vaccination in the elderly in the PubMed database starting from 2006. We included studies that consider the presence of a childhood vaccination with pneumococcal conjugate vaccine (PCV) 10 and PCV13. We focus on methods and assumptions used in modeling VE and epidemiology of PD over time.
Results
Twenty-eight economic evaluations underwent full-text review and data extraction. Thirteen were selected for quality assessment. The studies with a higher quality score provide evidence that vaccinating the elderly with PCV13 is not cost effective, when an ongoing rapid decline in the incidence of PCV13-type PD is modeled. A moderate persistence of PCV13 serotypes, in particular due to PCV10 childhood vaccination, makes vaccination of the elderly with PCV13 more attractive. There is no agreement that combining PCV13 with polysaccharide vaccine PPSV23 is cost effective. PPSV23 is attractive when it is effective against non-invasive PD.
Conclusion
Methodological approaches and assumptions in modeling VE and the indirect effects of childhood vaccination have a major impact on outcomes of decision-analytic models and cost-effectiveness estimates. Considering recently observed trends in the epidemiology of pneumococcal serotypes, there is currently inconclusive evidence regarding the cost effectiveness of pneumococcal vaccination of the elderly due to lack of studies that model key serotypes such as serotype 3 separately from other groups of serotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Vaccination of susceptible groups is the most effective measure to fight diseases caused by Streptococcus pneumoniae (S. pneumoniae) on a population level. Pneumococcal conjugate vaccination of infants and young children has been established in many developed countries and indisputably has proven to be an effective preventive measure [1]. Following the 7-valent pneumococcal conjugate vaccine (PCV7), the introduction of higher valent conjugate vaccines (PCV10 and PCV13) has further reduced the burden of the pneumococcal diseases (PD) [1, 2]. The vaccination of the elderly in the presence of routine childhood vaccination with the higher valent pneumococcal conjugate vaccines (PCVs) still poses questions on general cost effectiveness, optimal age of vaccine administration, and which vaccine should be used. Conclusions of previously published systematic literature reviews [3,4,5,6] suggest that the polysaccharide vaccine PPSV23 and the conjugate vaccine PCV13 can be considered cost effective for vaccinating the elderly against pneumococcal diseases. Ogilvie et al. [3] reviewed 11 economic evaluations of vaccination of the elderly with PPSV23 and concluded that the vaccination could be cost effective compared with no program for individuals older than 65 years. These findings were supported by Nishikawa et al. [5] in a recently published review. Dirmesropian et al. [4] reviewed ten economic evaluations of usage of PCV13 in adults and the elderly and concluded that the conjugate vaccine was also cost effective [4]. However, the authors stated that the drawn estimates of the cost effectiveness should be interpreted with caution in respect to key factors that influenced the cost effectiveness, which were uncertain at that time. These included the effectiveness of PCV13 against invasive pneumococcal diseases (IPD) and non-bacteremic pneumococcal pneumonia (NBPP) in the elderly, the effectiveness of PPSV23 and the indirect effects of higher-valent PCV childhood immunization programs on the epidemiology of PD in the elderly [4].
Since the publication of the review by Dirmesropian et al. [4], new clinical evidence on PCV13 effectiveness against IPD and NBPP in the elderly has been generated in the CAPiTA trial (Community-Acquired Pneumonia Immunization Trial in Adults) [7]. CAPiTA has become a milestone in the current debates and triggered reassessments of cost effectiveness of PCV13 versus PPSV23 in view of its findings. Recently, Porchia et al. [6] conducted a review of 31 economic evaluations including the studies that informed the parameters based on the CAPiTA findings [7]. The authors concluded that both PPSV23 and PCV13 programs in the elderly were cost-effective and should be seen as a priority by decision makers [6]. Although the authors conducted a substantial review of the economic parameters, they did not address the uncertainty around vaccine effectiveness (VE) and epidemiological changes in pneumococcal diseases originated from the childhood vaccination with PCV13 and/or PCV10. By doing so, in our opinion, the authors limited their summarization of the current evidence for decision makers. In addition, since their publication a number of economic evaluations have been published that concluded that under the influence of the herd effect induced by childhood vaccination with a higher valent conjugate vaccine, vaccination of the elderly was unlikely to be cost effective [8,9,10].
In contrast to Porchia et al. [6], we investigated the cost effectiveness of vaccination strategies for the elderly in the presence of childhood pneumococcal vaccination with PCV10 and PCV13. We focus on the methods, assumptions, and data used to model the vaccine effects in order to ensure that input VE was consistent with the current knowledge and that potential epidemiological effects stemming from the childhood vaccination were not neglected or oversimplified.
2 Methods
2.1 Constituents of Vaccination Effects
The direct outcomes of a pneumococcal vaccination campaign in the elderly are determined by VE in preventing PD caused by the serotypes contained in the pneumococcal vaccine (and cross-protective serotypes [11]) as well as the disease incidence in the targeted population.
2.1.1 Vaccine Effectiveness Among the Elderly
The pneumococcal vaccine protection is expected to decline over time. PPSV23 protection has been shown to wane during and also after the first 5 years [12,13,14,15]. PCV13 is thought to provide longer protection than PPSV23 because it triggers a stronger immune response [16]. The CAPiTA results [7] show that PCV13 protection is stable over 4–5 years but its waning is still uncertain [11, 17]. Therefore, overall VE over the period of its protection can be composed of the initial VE at the time of administration and a waning pattern (see electronic supplementary material [ESM] file S1). We used the initial VE at administration and the waning of the vaccine protection reported in the studies to plot the decline of VE over time and to calculate the area under the resulting curve. This area under the curve represents the expected vaccine protection over time (EVPOT); that is, integration of the years of protection adjusted for VE at a given point in time (see ESM file S1: section 1.1). We applied a cut-off point of 20 years since vaccination when a longer period of waning was assumed. The expected vaccine protection over time is measured in efficacy-adjusted protection years (EAPY) and enables a comparative analysis of the assumptions about VE and duration of protection across the studies.
2.1.2 Incidence of Disease Due to S. pneumoniae Among the Elderly
Currently, over 90 different strains (serotypes) of S. pneumoniae have been identified, with certain strains having a higher potential to cause the disease and a higher prevalence in the susceptible groups [18]. The currently available pneumococcal vaccines contain a limited number of the serotypes; that is, PCV13 covers 13 antigens and PPSV23 includes the PCV13 serotypes, except for serotype 6A, and 11 additional antigens. Therefore, in the case of S. pneumoniae, VE could be interpreted as the proportionate reduction in occurrence of the disease caused only by the strains contained in this vaccine and included as such in the cost-effectiveness analyses.
In addition, in the countries where children are routinely vaccinated with PCV, childhood vaccination has indirect effects on pneumococcal infection caused by the vaccine strains among the elderly. The observed indirect effects include the herd effect (i.e., the indirect reduction in vaccine-type disease incidence as an effect of the childhood vaccination) and replacement disease (i.e., an indirect increase in non-vaccine-type PD incidence) [1]. The type of PCV vaccine (PCV7, PCV10, or PCV13) implemented in the infant vaccination programs plays a crucial role in the evolution of vaccine-type PD incidence among the elderly. Furthermore, the sequence of PCV infant vaccination programs (for instance PCV7 replaced by PCV13 or PCV7 followed by PCV13 and then PCV10) can also have an impact on the cost effectiveness of pneumococcal vaccination of the elderly.
Therefore, the vaccine-type PD incidence in the target population over time is determined by vaccine-serotype disease incidence before the implementation of the PCV childhood vaccination and the indirect effects on the epidemiology of S. pneumonia in this population. The key factors that determine the performance of the elderly pneumococcal vaccination, are illustrated in Fig. 1 and are described in greater detail in the ESM (file S1: section 1). Due to the complex dynamics seen in the strains of S. pneumonia, an accurate projection of vaccine-type PD incidences is challenging and subject to major assumptions. Therefore, we reviewed the assumptions and methodological choices applied in the modeling of vaccine-type IPD and NBPP incidences.
Constituents of the vaccination effects over time: a graphical representation. The outcomes of the elderly pneumococcal vaccination depend on the initial vaccine effectiveness against vaccine-type PD and its protection over time (illustrated in b) and the PD incidence caused by vaccine-type serotypes over time, which is also influenced by the indirect effects of a childhood vaccination with PCV (illustrated in a). Asterisk: vaccination rate 100% and vaccine effectiveness according to b. PD pneumococcal diseases
2.2 Review Strategy
We conducted an extensive search in the PubMed database to find studies for full-text review. The identified studies were subject to the full-text review with data extraction and selection for the following assessment of quality of economic evaluation. The search syntax, inclusion criteria for the full-text review and data extraction are described in the ESM (file S1: section 2). In accordance with the goal of this review, we defined inclusion criteria for the assessment of quality of the selected studies as follows:
-
1.
VE parameters are obtained (i) for PCV13—from or based on the CAPiTA trial [7]; (ii) for PPSV23—from a meta-analysis, a randomized clinical trial (RCT) or an observational study. These criteria are in accordance with the guidelines of the World Health Organization (WHO) for economic evaluations of vaccination programs [19].
-
2.
Childhood pneumococcal conjugate vaccination with the higher-valent vaccines is included in baseline scenarios. Post-PCV data are used to project the burden of pneumococcal diseases in the targeted population.
Quality of economic evaluations of the studies that met both selection criteria was assessed using the “Evidence and Value: Impact on DEcisionMaking” (EVIDEM) instrument: “assessment of quality of economic evaluations” [20]. In compliance with the EVIDEM instrument, we developed a form consisting of two parts: (i) completeness and consistency of reporting of economic evaluation, and (ii) relevance and validity of economic evaluation. The instrument allowed a sequential and structured assessment of economic evaluations across 11 dimensions and provided a way of transparent reporting ensuring full traceability of the reviewers’ work. We placed a particular focus on completeness, consistency and relevance of the assumptions and methods applied in modeling VE and the indirect effects of the childhood PCV vaccination. Two evaluators (MT, SMS) selected the studies and independently completed the developed form and assigned a score (between one for low and four for high relevance/validity) for each study with a summarizing rationale for the given score. The decisions were compared, and any disagreement was resolved under arbitration by the third reviewer (AK). The studies excluded from the assessment of quality are summarized in the ESM, including the reason for their exclusion.
The assessed economic evaluations were further summarized in a comparative analysis of the assumptions and the methods applied to model the VE among the elderly, the incidence of invasive and non-invasive diseases due to S. pneumoniae, and the indirect effects of the vaccination programs with higher valent conjugate vaccines in children on the PD incidence in the elderly population. Thereafter, the cost effectiveness of the elderly vaccination programs was described using the findings of the studies that were evaluated with an EVIDEM score of three or higher for relevance and validity of economic evaluation. To facilitate the comparison of incremental cost-effectiveness ratio (ICER) estimates between the studies, the reported ratios were firstly time-adjusted to the year 2017 by applying the country-specific consumer price indices from the organisation for economic co-operation and development (OECD) [21]. Afterwards, the 2017 country-specific values were standardized to 2017 US dollars using the purchasing power parity (PPP) index [22] and exchange rates for 2017 from the OECD [23].
3 Results
3.1 Literature Search and Selection
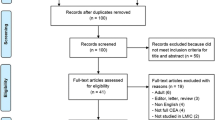
The search was conducted on 18 October 2017 and updated twice on 2 March 2018 and 28 May 2018 to identify recently published studies. Overall, it resulted in 28 studies selected for the full-text review (see Fig. 2). Of these, 13 economic evaluations were selected for the assessment of quality [8,9,10, 24,25,26,27,28,29,30,31,32,33], and the remaining 15 studies [34,35,36,37,38,39,40,41,42,43,44,45,46] were excluded from further analysis and are summarized in the ESM (file S2).
The extracted data from the selected studies are summarized in three tables. Table 1 gives the extracted data on base-case VE parameters and calculated EVPOT; Table 2 gives an overview of the applied methods and the quality assessment scores of the economic evaluations; and Table 3 describes the main characteristics of the health economic evaluations, the reported ICERs, and the conclusions given in the evaluations. The sources for VE cited in the reviewed studies as well as the extracted incidence rates with their sources are summarized in the ESM (files S3 and S4, respectively). The results of the quality assessment of economic evaluation with EVIDEM are given in the ESM (file S5).
3.2 Assumptions and Methods Used to Model the Vaccine Effects Over Time
3.2.1 Initial Vaccine Effectiveness
Although we included only the evaluations that had obtained the initial PCV13 VE from the CAPiTA trial [7], the reported values varied between the studies (see Table 1). For instance, in the age group 65–74 years, the average initial PCV13 VE against IPD ranged from 75 to 84.5%. The main reasons for this variation are methodological differences in the application of vaccine-age-interaction effects and in the estimates of effect of age on the initial VE. Eight [8,9,10, 25, 27, 30, 31, 33] of 12 studies (which evaluated PCV13) applied age-interaction effects of the initial VE. Of them, five studies [8,9,10, 25, 30] derived estimates of the vaccine-age-interaction effect from a post-hoc analysis of the CAPiTA data [50, 51] (see Table 2). Another study [33] assumed that the age-vaccine-interaction effect for PCV13 is 50% lower than the effect for PPSV23. Two studies [27, 31] applied age-group-specific variations of the initial PCV13 VE. Heo et al. [31] adjusted VE assumptions by Smith et al. [40]. Rodriguez Gonzalez-Moro et al. [27] did not describe the methods for the calculation of age-group-specific VE parameters.
We found a great variation in the applied values of the initial PPSV23 VE with two major sources of the variation of the VE values: the application of the vaccine-age-interaction effects and sources of the initial VE (see Tables 1, 2). In the age group 65–74 years, the average PPSV23 VE against IPD ranged from 55 to 82% (see Table 1). In eight studies [8, 9, 26,27,28, 30, 31, 33], PPSV23 VE against NBPP was assumed to be 0% based on the findings of several reviews and observational studies [53, 54, 59, 60] (see Table 2 for VE sources). Three studies applied VE of 39% [24], 30.8% [10], and 19.6% [32] based on other empirical studies [48, 57]. Four studies [27, 30, 31, 33] applied vaccine-age-interaction effects for PPSV23. Only Kuchenbecker et al. [33] and Dirmesropian et al. [30] reported single-age-specific initial VE for PPSV23. Dirmesropian et al. [30] applied a logistic function that was calibrated for ≥ 75 year-olds based on data from the study by Andrews et al. [15]. Kuchenbecker et al. [33] calculated the initial PPSV23 VE using a linear interpolation of VE estimates for 50-, 65- and 80-year-olds reported by Smith et al. [61]. In order to facilitate comparison of the initial VE assumptions between the studies, we defined four age groups and reported the extracted VE values in Table 1 according to these groups. For the studies that apply functions to calculate VE, Table 1 gives the unweighted average.
3.2.2 Vaccine Protection Over Time
Figure 3 gives an overview of the protection waning patterns among the reviewed evaluations. Applied waning patterns varied in their structure and, hence, showed different shapes of the curves. It resulted in differences of the calculated expected vaccine protection over time. The most common waning pattern included a period of stable VE followed by a step-wise linear decline of VE to 0% [8, 9, 25, 29, 30, 32, 33]. Three studies [27, 28, 31] applied age-group-specific waning patterns and one study [33] used single-age-specific patterns. We calculated EVPOT for the generic age groups to enable comparison between the studies. Figure 4 shows the resulting values. In this section we focus on the resulting EVPOT for immunocompetent people aged 65–74 years. For PPSV23, the calculated EVPOT against IPD ranges from 1.5 to 5.6 EAPY depending on both initial VE parameter and the waning of protection. The commonly applied period of constant protection was 1–5 years. In five studies [8, 9, 26, 30, 32], VE declined to 0% within 5–6 years of vaccination, while in the other four studies [27, 28, 31, 33] there was a (small) protective effect at least until the ninth year after vaccination. EVPOT for PCV13 is commonly larger than for PPSV23 and ranges from 3.8 to 12.6 EAPY due to a longer duration of stable protection and the slower decline of the VE afterwards. Nine [8, 9, 25,26,27, 30, 32, 33] of the 12 studies that model PCV13 protection apply a 4- to 5-year period of stable vaccine protection. Other assumptions include 9 years of stable protection [29] and a shorter period with waning starting in the second year after the initial vaccine administration [28, 31]. Blommaert et al. [26] is the only study that applied in the base-case scenario an equal duration of protection for PCV13 and PPSV23. Furthermore, the study was unique in assuming an instant drop to 0% VE after a stable period of protection for 5 years.
Representation of constructed waning patterns reported in the selected studies by the first author, vaccine and age group (when reported). For the study by Stoecker et al. [28], for the age group 50–64 years, the plot represents the unweighted average curve due to the assumption of no waning for adults aged 50–64 years, and each single age cohort from 50 to 64 years had a different length of time with stable vaccine protection. The graph for Blommaert et al. [26] illustrates the assumption of the same waning pattern for both PCV13 and PPSV23
Dot plot of the calculated expected vaccine protection over time (for details about the methods for the calculation see electronic supplementary material [ESM] file S1: section 1). EAPY efficacy-adjusted protection years, EVPOT expected vaccine protection over time, IPD invasive pneumococcal diseases, y.o. years old
In the Australian studies, Dirmesropian et al. [30] and Chen et al. [8, 9] applied 5 years of waning after the stable protection. In the studies by Rodriguez Gonzalez-Moro et al. [27], Heo et al. [31], Thorrington et al. [32], and Willem et al. [10], the waning to 0% was modeled over 10 years with different waning rates. Mangen et al. [25] and Kuchenbecker et al. [33] applied a 15-year period of waning to 0%. Van Hoek and Miller [29] and Stoecker et al. [28] assumed remaining PCV13 VE even after year 20, with Stoecker et al. [28] assuming very low waning rates compared with all other studies. Due to the assumptions of a longer period of waning, the resulting EVPOT for the studies by Mangen et al. [25], Kuchenbecker et al.[33], van Hoek and Miller [29], and Stoecker et al. [28] lies above 8.5 EAPY. In contrast, the assumption of no waning period by Blommaert et al. [26] resulted in EVPOT of 3.7 EAPY.
In contrast to the common linear decline of protection over time, Willem et al. [10] applied parametric functions of waning after the period of constant protection; these include logistic waning of PCV13 protection and an exponential decay for PPSV23. The calculation of EVPOT with the reported parameterization of these functions led to values similar to other studies: PCV13 against IPD of 7.2 EAPY and PPSV23 against IPD of 2.3 EAPY. The duration of vaccine protection against non-invasive PD was modeled analogously with the lower initial VE. The EVPOT values range from 2.1 to 7.6 EAPY.
3.2.3 Modeling Incidence Over Time (Indirect Herd and Replacement Effects)
To model the PD incidence over time, pneumococcal serotypes were typically summarized into groups according to the considered pneumococcal vaccines. Only the study by Thorrington et al. [32] included individual serotypes that are covered in PCV13 and not in PCV10 into the projection, with an assumption about the stable incidence for serotypes 3 and 6A and an indirect reduction in the incidence due to serotype 19A caused by the replacement of PCV10 with PCV13 in the childhood vaccination.
3.2.3.1 Indirect Effects of Childhood Vaccination with PCV13 on IPD Incidence Among the Elderly
Out of the 13 included studies, 11 [8,9,10, 25,26,27,28,29,30,31, 33] evaluated pneumococcal vaccination of the elderly in the presence of a PCV13 program in children, which had replaced the PCV7 program.
We found different methods to project the epidemiological development of the serotype groups in the presence of routine childhood vaccination with PCV13. The Australian studies by Dirmesropian et al. [30] and Chen et al. [8, 9] started with an epidemiological steady state based on the national surveillance data and kept the future serotype-specific PD burden constant. Chen et al. [8] also looked into the potential changes in the PCV13-serotype epidemiology.
Other method to project indirect effects of PCV13 infant vaccination was to simulate an ongoing gradual decline of infections due to six additional (contained in PCV13 but not in PCV7) serotypes until elimination or near-elimination is reached, and include an increase of infections due to the non-PCV13 strains. In the Belgian studies, Blommaert et al. [26] and Willem et al. [10] applied an annual indirect reduction in PD incidence preventable by vaccination with PCV13 and did not report post-vaccination stabilization of the serotype distribution. Blommaert et al. [26] modeled the herd effect of the childhood PCV13 vaccination as an exponential decay with a 24% annual decrease in circulation of the PCV13-type serotypes and the vaccine-induced replacement effect was applied as a compensation of the reduction in incidence originating from the herd effect by 50% in the base case. The values were based on the observed PCV7-serotype circulation in Belgian children. Willem et al. [10] applied the indirect reduction of PD incidence in adults as an annual 16% decline of PCV13 serotypes in PCV13-serotype IPD incidence. Of this decline, 76.3% was compensated with occurrence of non-PCV13 serotypes in IPD incidence as the vaccine-induced serotype replacement. Stoecker et al. [28] and van Hoek and Miller [29] projected a decline of the six PCV13-minus-PCV7 serotypes, similar to the indirect reduction of PCV7 serotypes in IPD incidence in the elderly observed in the national epidemiological data, with a post-vaccination stabilization during the modeled time horizon comparable to the remaining PCV7 PD disease burden.
Jiang et al. [24] estimated the PD incidence from the German surveillance data and modeled the indirect effects of a PCV13 program in children on the IPD incidence among adults to follow the PCV7 effects observed in the USA. The authors applied stabilization of serotypes in 2012, 7 years after the introduction of the PCV7 vaccination and only 2 years after the replacement of PCV7 with PCV13. The evaluated vaccination of the elderly started in 2011; therefore, the cost effectiveness was evaluated under the assumptions that IPD incidence had stabilized after 1 year since the start of the program in the elderly. Therefore, the projection applied by Jiang et al. [24] can be considered to resemble the steady-state scenarios in the Australian studies [8, 30].
Thorrington et al. [32] analyzed a scenario in which PCV13 replaced PCV10 in the infant vaccination. It was assumed that the low PCV7 PD incidence remained stable. The indirect effects of higher valent PCV immunization of infants were projected based on the (historical) observed herd and replacement effects of PCV7 childhood vaccination. Under the PCV10 childhood vaccination, the incidence of IPD caused by the three additional serotypes included in PCV10 among the elderly was projected to decline over 5 years while the incidence of PCV13-minus-PCV10 serotypes remained stable. PCV13 infant vaccination was assumed to have the same effects on PCV10 serotypes. Additionally, the impact of PCV13 on vaccine serotypes 3, 19A, and 6A was analyzed based on the post-PCV13 serotype epidemiology observed in other countries. Based on this data, the authors projected a 40% decrease in the 19A serotype and no impact of PCV13 on serotypes 3 and 6A. The IPD incidences caused by PPSV23-minus-PCV13 and non-vaccine serotypes were projected to increase with the same rate as the observed serotype replacement induced by PCV7 but over a longer period in case of a replacement of PCV10 by PCV13.
Three studies [27, 31, 33] applied an all-serotype approach (described in ESM file S1: section 1.2.2) to project the indirect effects in IPD among the elderly. This approach assumes a perfect correlation between all-serotype PD incidence and serotype-specific PD incidence (i.e., a 2% reduction in IPD of pneumococcal origin results in a 2% reduction of IPD caused by PCV13 serotypes).
3.2.3.2 Indirect Effects of Childhood Vaccination with PCV10 on IPD Incidence Among the Elderly
One study [25] assessed the cost effectiveness of PCV13 vaccination in the elderly in the presence of PCV10 infant vaccination, which had replaced the PCV7 program. Two studies [10, 32] analyzed the possible impact of changing one higher-valent PCV with another; that is, replacement of PCV10 with PCV13 [32] and replacement of PCV13 with PCV10 [10].
The study by Mangen et al. [25] was the only analysis that evaluated pneumococcal vaccination of the elderly in a scenario with PCV10 infant vaccination present, which had replaced PCV7 vaccination program. The study applied equilibrium of PCV13 serotypes assuming no indirect effects of PCV10 vaccination. In addition, no projections of serotypes 3, 6A, and 19A were made.
In an additional scenario, Willem et al. [10] analyzed the impact of replacing PCV13 with PCV10 infant vaccination on the cost effectiveness of pneumococcal vaccination in the elderly. For this scenario, a relapse of PCV13 serotype incidence within 7 or 15 years was assumed and modeled by applying a logistic curve.
3.2.3.3 Indirect Effects of PCV Childhood Vaccination on Non-bacteremic Pneumococcal Pneumonia (NBPP) Incidence Among the Elderly
Nine studies [8,9,10, 25, 26, 28,29,30, 32] assumed the same serotype-specific dynamics in NBPP incidence as for IPD. Jiang et al. [24] did not describe the effects of infant vaccination on NBPP in the elderly. We also identified three studies [27, 31, 33] that used all-cause and all-serotype approaches (described in ESM file S1: section 1.2.2) to project indirect effects on NBPP incidence among the elderly. The all-cause approach assumes a perfect correlation between percentage changes in all-cause non-bacteremic pneumonia (NBP) and serotype-specific NBPP (i.e., a 2% reduction in all-cause NBP results in a 2% reduction of PCV13-NBPP). Both approaches are not valid and misrepresent possible real evolution of the serotype-specific PD incidences as described in the ESM in detail (file S1: section 1.2.2).
3.2.4 Selection of Studies for Reporting the Cost Effectiveness
The selected studies were evaluated on 11 dimensions of economic evaluation for consistency in reporting and relevance to decision making, and the scores were classified as low (≤ 2), middle (3), and high (4) (see Table 2). A short summary and the complete assessment of the quality of the economic evaluation can be found in the ESM (file S5). For the studies by Jiang et al. [24], Rodriguez Gonzalez-Moro et al. [27], Heo et al. [31], and Kuchenbecker et al. [33] we found weaknesses in the reporting and in the applied methods of modeling the VE and the indirect effects and hence considered them of low validity for decision making. These studies were excluded from the reporting of the cost-effectiveness estimates in this review.
In the Korean study by Heo et al. [31], the main limitations were seen using the all-serotype approach in the modeling of the indirect effects of the childhood vaccination, which may have led to the contrastingly low ICER estimates. Weaknesses were also found for other dimensions of the economic evaluation such as calculation of the indirect costs, time horizon, discounting, and sensitivity analysis (SA). In the Spanish study by Rodriguez Gonzalez-Moro et al. [27], vaccination with PCV13 of adults with chronic obstructive pulmonary disease (COPD) aged 50 years was evaluated, however, the φ with the population of the CAPiTA trial [7], which was used for definition of PCV13 VE. The authors applied a reduction in all-cause NBP incidence when modeling the herd effect of PCV13 childhood vaccination in NBPP. The all-cause approach was also used in modeling PCV13 VE against NBPP. The same methodological choices in modeling the vaccine effects in NBPP were seen in a recent German study by Kuchenbecker et al. [33]. The authors applied PCV13 VE of 3.9% against all-cause NBP and, in addition, used a longer duration of PCV13 and PPSV23 protection demonstrating high values of EVPOT. The herd effect for all-cause NBP was calculated based on the data from the study by Pletz et al. [64], who reported serotype distributions in NBPP. Kuchenbecker et al. [33] used the data on the serotype distributions for the calculation of the herd effect for both IPD and NBPP incidence, however, the authors did not report the methods for translation of the serotype distribution into the incidence of PD. We also excluded the German study by Jiang et al. [24] because of their assumption about the rapid stabilization of the vaccine-type serotypes in the PD incidence among the elderly in 2012 and absence of the indirect effects on NBPP incidence among the elderly.
3.2.5 Funding Source
Nine [8,9,10, 26, 28,29,30,31,32] of the 13 included studies reported industry-independent funding sources and the other four [24, 25, 27, 33] were funded by the industry. Three [24, 27, 33] of the industry-funded evaluations and one [31] of the studies with industry-independent funding were considered of low validity for the decision-making because of the flaws in modeling the indirect effects of the childhood vaccination. These studies are likely to underestimate the indirect PD reduction due to the PCV13 program in children. The assumptions about the duration of the vaccine protection seem to vary among the studies irrespectively of the funding source (see Fig. 4).
3.3 Cost Effectiveness of the Elderly Pneumococcal Vaccination in the Presence of a Routine Childhood Pneumococcal Conjugate Vaccination
In order to facilitate a comparative analysis of the findings on cost effectiveness of the adult vaccination, we included nine studies [8,9,10, 25, 26, 28,29,30, 32] that were considered relevant for decision making. We summarize the results by grouping the studies according to the vaccine used for the childhood vaccination and its effects on the PD incidence among the elderly. The results are summarized accordingly in Table 3. All reported prices and ICERs in this section are converted to 2017 US dollars (see ESM file S6 for the original and converted values of ICERs).
3.3.1 Childhood Vaccination with PCV13
3.3.1.1 Ongoing Indirect Effects
Five [8, 10, 26, 28, 29] studies projected a future decline of PCV13 preventable PD incidence among the adult population due to the herd effect of the childhood vaccination with PCV13.
Adult vaccination with PPSV23 compared with no vaccination In the presence of ongoing effects of the childhood vaccination with PCV13, only the study by Blommaert et al. [26] reported the cost effectiveness of vaccinating the elderly with PPSV23 compared with no vaccination program. The authors investigated vaccinating different age groups of the Belgian adult population and stated that the vaccination with PPSV23 (75% coverage) might be considered cost effective with US$88,415/QALY for adults aged 65–74 years and with US$65,468/QALY for those 75–90 years old. The authors further state that the vaccine is cost effective (with willingness to pay [WTP] of US$46,062/QALY) for the 65- to 74-year-olds if there is no reduction in PPSV23-serotype IPD incidence in the population and the stable vaccine protection lasts longer than 5 years. Of note, the authors applied a conservatively low PPSV23 effectiveness against IPD of 55% taken as the lower value of the given confidence interval reported in the review by Moberley et al. [47].
Adult vaccination with PCV13 compared with no vaccination The studies by Blommaert et al. [26] and Chen et al. [8] evaluated vaccinating adults with PCV13 compared with no vaccination. Both studies showed that PCV13 was unlikely to be cost effective in the presence of the ongoing herd effects of the routine PCV13 childhood vaccination. Most of the ICER estimates reported in these studies lie above US$100,000/QALY. The results reported by Blommaert et al. [26] suggest that vaccinating the elderly ≥ 75 years can be potentially cost effective with an ICER of US$88,842/QALY compared with no vaccination. However, the authors point out that price reductions are required to keep it possibly cost effective when PCV13-preventable PD incidence decreases (24% annually) due to the herd effect. Vaccinating adults aged 50–64 years (ICER of US$287,917/QALY) or 65–74 years (ICER of US$131,104/QALY) was unlikely to be cost effective (with WTP of US$46,062/QALY) at the current prices (PCV13: US$100) unless no herd effect was present and the duration of stable PCV13 protection exceeded 6 years. In the base-case scenario, Blommaert et al. [26] assumed a constant protection for 5 years, which ceased to exist afterwards, and varied the duration of PCV13 protection in relation to the vaccine price in the sensitivity analysis. The results show the distributions of the vaccine prices, which allow keeping the ICER equal to US$46,062/QALY (original €35,000/QALY) with the given duration of protection with and without waning. The results suggest that with a longer duration of protection the vaccine price can be increased.
Chen et al. [8] analyzed the implication of the decline of PCV13 serotype circulation in the adult population in greater detail. The authors investigated a scenario of the indirect reduction in the six additional PCV13 serotypes as it has been observed for the PCV7 serotypes and compared the cost-effectiveness outcomes in this scenario with the estimates obtained under the assumption of potential stabilization seen in the national data. The authors show that compared with the conditions of the stabilization of the indirect effects of the childhood vaccination, ongoing decline of the additional six serotypes covered by PCV13 substantially worsens the cost effectiveness of vaccination for the elderly with PCV13 (US$50,359/QALY vs US$121,288/QALY). The authors emphasize that changes in the serotype distribution caused by the PCV13 childhood programs are critical to the cost effectiveness of PCV13 programs in the elderly.
Adult vaccination with PCV13 compared with vaccination with PPSV23 Stoecker et al. [28] and Willem et al. [10] compared PPSV23 with PCV13 for vaccinating the elderly. The estimated ICER for vaccinating 65-year-olds reported by Stoecker et al. [28] is slightly above US$50,000/QALY. However, Willem et al. [10] showed contrasting ICERs that lie well above US$100,000/QALY.
It is important to note that Stoecker et al. [28] showed that vaccinating at 65 years old was only cost effective (ICER of US$50,891/QALY) in a short period after the introduction of the PCV13 childhood vaccination. The authors analyzed the replacement of PPSV23 with PCV13 for the elderly vaccination in the US and evaluated cost effectiveness of this strategy for the cohort of the year 2013 with PCV13 introduced for the childhood program in 2010. The authors used a single-cohort model and looked at three scenarios of vaccinating the population of 2013 at 50, 60, and 65 years old. They showed that the ICER values rose substantially after 2013, reaching over US$400,000/QALY in 2015, and the PCV13 strategy was dominated. This increase of ICERs after 2015 suggests that a mid- or a long-term vaccination program with PCV13 is unlikely to be cost effective (> US$100,000/QALY). In addition, the authors applied a step-wise waning of PCV13 protection at a rate of 10% every 5 years, resulting in the highest EVPOT in this review.
Willem et al. [10] estimated an ICER for vaccinating 65-year-olds of US$221,970/QALY that can be considered unlikely to be cost effective (relative to a WTP of US$64,773/QALY). The authors performed extensive analyses of different vaccination strategies considering uncertainty surrounding VE, the duration of the protection, vaccine uptake, and initial PD incidence in adults. The authors applied age-specific PCV13 VE obtained from CAPiTA [7], but set it to zero for ≥ 85-year-olds. Comparably to several other studies [8, 9, 26, 30], PPSV23 VE was 56% against IPD but, in contrast, PPSV23 was also assumed to be effective against NBPP with VE of 30.8%. The authors examined different scenarios of VE against NBPP (i.e., when both vaccines are either effective or not effective against NBPP and when only PPSV23 is ineffective). The cost effectiveness of PCV13 was estimated compared with several strategies involving PPSV23: the current program with a low uptake (0.79–3.01%) and a program with an increased PPSV23 uptake (15–25%). The cost effectiveness was analyzed contrasting ICER with different thresholds of WTP (up to US$453,411/QALY). The authors pointed out that PCV13 could become cost effective for people < 75 years of age if a combination of favorable changes around PCV13 occurred, including a substantial (75%) reduction of the vaccine price, an increased duration of protection, and a lower herd effect caused by PCV13 childhood vaccination (i.e., increased disease burden caused by PCV13 serotypes). For the people aged ≥ 75 years, PCV13 remained unlikely to be cost effective in comparison with PPSV23 (ICER of US$438,072/QALY). The higher uptake of PPSV23 would be considerably more efficient relative to the current vaccination situation with ICERs of US$107,282, US$74,116, and US$67,554 per QALY for the age groups 50–64 years, 65–74 years, and 75–84 years, respectively.
Adult vaccination with a combination of PCV13 and PPSV23 (sequential vaccination) The cost effectiveness of adding PCV13 to PPSV23 in vaccinations for the elderly was analyzed in four selected studies [10, 26, 28, 29]. All of them showed that this strategy was efficacious in reducing the number of cases of IPD and NBPP and related deaths. Blommaert et al. [26], van Hoek and Miller [29] and Willem et al. [10] showed that it was above US$100,000/QALY and was not cost effective. Stoecker et al. [28] showed that the sequential vaccination could possibly be cost effective (with WTP of US$50,000–US$100,000/QALY) in a short period (around 6 years) after the introduction of the PCV13 childhood vaccination.
Stoecker et al. [28] showed an ICER of US$68,078/QALY for the addition of PCV13 to PPSV23 at age 65 years and higher ICERs for vaccinating at age 50 and 60 years with estimates of US$365,482/QALY and US$261,307/QALY, respectively. Similar to the strategy of replacing PPSV23 with PCV13, the ICER for adding PCV13 to PPSV23 was demonstrated to reach over US$250,000/QALY by 2018 for the modeled cohort ≥ 65-year-olds and to be cheaper than the replacement strategy after the first year of the modeling horizon. The dramatic increase in ICER of both strategies over time was driven by the herd effect in the single cohort.
The Belgian studies by Blommaert et al. [26] and Willem et al. [10] showed that the addition of PCV13 to a vaccination scheme with PPSV23 was generally effective but not cost effective at any age. Blommaert et al. [26] stated that this strategy might be cost effective for 65- to 74-year-olds at a WTP of US$46,062/QALY, when the stable PCV13 protection lasted 15 years. Willem et al. [10] concluded that the addition of PCV13 would bring less gain than a high uptake of PPSV23 and would become an expensive strategy with a high ICER. The ICER estimates are US$218,776, US$171,765, and US$201,380 per QALY for the age groups 50–64, 65–74, and 75–84 years, respectively.
Van Hoek and Miller [29] evaluated the cost effectiveness of adding one PCV13 dose to the vaccination with PPSV23 for the elderly aged ≥ 65 years for one cohort of 65-year-olds in England. The authors simulated the disease burden in the cohort from 2016 until death comparing the outcomes of the addition of PCV13 to the current vaccination policy with an uptake of 69% in comparison with the current program without PCV13. They assumed the longest constant duration of PCV13 VE (9 years) among the selected studies, which resulted in a relatively long EVPOT for PCV13, and PPSV23 VE was not reported. The estimated cost-effectiveness ratio for adding PCV13 to PPSV23 was US$375,622/QALY. The authors pointed out that the PCV13 price should be below zero for ICER to be below the threshold of US$29,144/QALY (original £20,000/QALY).
3.3.1.2 PCV13 Serotypes Reached a New Post-vaccination Equilibrium in PD Incidence
The Australian studies [8, 9, 30] explored the expected protective impact of PCV13 on PD incidence in the elderly ≥ 65 years and its cost effectiveness when the six additional serotypes included in PCV13 reached stabilization. The new post-vaccination equilibrium was assumed based on the Australian national surveillance data, which showed that after a rapid indirect reduction, the PCV13-type PD incidence among the elderly had stabilized [8, 30]. In these studies, the PCV13-serotype incidence was kept constant.
Adult vaccination with PPSV23 compared with no vaccination The cost-effectiveness of vaccinating the elderly with PPSV23 compared with no program was reported only by Dirmesropian et al. [30], who concluded that the strategy (60% uptake) was not cost effective with an ICER of US$210,800/QALY (vs WTP of US$42,557/QALY). The researchers applied age-specific PPSV23 VE and assumed that the initial constant protection of PPSV23 lasted for 2 years and thereafter linearly declined to zero over 3 years.
Adult vaccination with PCV13 compared with no vaccination The cost effectiveness of vaccinating the elderly with PCV13 was reported to be in the range of US$50,000–US$100,000/QALY and the program could be considered to be potentially cost effective compared with no vaccination. The studies used similar methods in calculation of age effects on VE, waning of the vaccine immunity, and most of the input parameters in the economic evaluation (price, discount rate).
Dirmesropian et al. [30] estimated an ICER of US$62,417/QALY, which is similar to the estimate obtained by Chen et al. [8] for the scenarios with the post-vaccination PCV13-serotype equilibrium (ICER of US$50,359/QALY). Nevertheless, Dirmesropian et al. [30] concluded that the strategy was not cost effective relative to the WTP level of US$42,557/QALY. The authors stated that vaccination with PCV13 could be cost effective when either the vaccine price was below US$33 (A$46) or the duration of PCV13 protection exceeds 15 years.
In another study, Chen et al. [9] demonstrated the importance of modeling the actual age of the vaccine recipients rather than assuming a certain cohort to be vaccinated all at once at a recommended age. The first approach was shown to provide more accurate estimates of the cost effectiveness and the optimal age of vaccine administration (US$59,252/QALY [recommended age scenario] vs US$46,294/QALY [actual age scenario]).
Adult vaccination with PCV13 compared with vaccination with PPSV23 Dirmesropian et al. [30] also concluded that PCV13 was cost effective versus PPSV23 with an ICER of US$25,038/QALY (vs WTP of $42,557/QALY). The authors pointed out that PCV13 was favored due to the longer duration of protection and higher VE against IPD and NBPP. The assumption that PPSV23 has no efficacy against non-invasive PD was tested in the sensitivity analyses, which resulted in larger benefits of PPSV23 and a substantial reduction in differences between ICERs.
3.3.2 Childhood Vaccination: PCV10 is Replaced with PCV13
Thorrington et al. [32] specified a hypothetical scenario of switching from PCV10 to PCV13 in the infant vaccination program and evaluated adult vaccination with PCV13 and/or PPSV23 in the Netherlands. The authors examined different scenarios of vaccinating 50% of adults over 60 years of age in combination with infant vaccination with PCV13 in comparison to the current vaccination scheme of no adult vaccination and PCV10 infant immunization. The authors projected the evolution of serotype epidemiology of IPD in Dutch adults with a routine infant vaccination with PCV10 and with PCV13, respectively. Thorrington et al. [32] showed that the strategies of vaccinating the elderly with PPSV23 with or without re-vaccination were cost effective relative to WTP of US$25,560/QALY, with vaccinating at age 70 being the most cost-effective scenario (ICER of US$7925/QALY). Vaccinating the elderly with PCV13 was shown to be more expensive, generating a higher ICER. The authors pointed out that switching to PCV13 for the infant vaccination was a rather inexpensive strategy having a beneficial health impact for the elderly as well.
3.3.3 Childhood Vaccination with PCV10
The earliest study was conducted by Mangen et al. [25] in 2015 for the Netherlands, where PCV7 had been replaced with PCV10 in the childhood pneumococcal vaccination program in 2011. The authors evaluated age- and risk-group-specific strategies of vaccinating adults aged 65–74 years with PCV13 in comparison with no vaccination from a societal perspective. Vaccination coverage varied with the health-risk group (63.9% for low-risk groups; 81.5% for medium- and high-risk groups). The resulting ICER was US$12,003/QALY for vaccinating those aged 65–74 years with a single dose of PCV13. Relative to the WTP level of US$105,900/QALY, the authors inferred that vaccination strategies with PCV13 were cost effective and vaccinating 65- to 74-year-old high-risk individuals was cost saving in the Netherlands.
3.3.4 Childhood Vaccination: PCV13 is Replaced with PCV10
Willem et al. [10] also investigated the potential impact of replacing PCV13 with PCV10 in the infant vaccination on serotype distribution. Motivation for this analysis was that PCV10 had been introduced in two regions of the country, which potentially could induce reoccurrence of three PCV13 serotypes not included in PCV10 in the elderly population. The authors analyzed a ‘quick’ (within 7 years) and a ‘slow’ (within 15 years) scenario of the potential return of PCV13 serotype incidence to the level of 2015 (current state). When PCV13 is replaced with PCV10 in the childhood vaccination, the relapse of PCV13-minus-PCV10 serotype incidence can make adult vaccination with PCV13 more beneficial.
4 Discussion
In this study, we analyzed the methods applied to model vaccine effectiveness over time as well as the indirect effects of PCV infant vaccination. In addition, we summarized the evidence of the cost effectiveness of the elderly pneumococcal vaccination in the presence of the childhood vaccination with the higher-valent PCVs. We applied very strict inclusion criteria on the assumptions and methodological choices made in the modeling of the vaccine effects. Overall, out of the 28 full-text economic evaluations that were reviewed, we selected 13 for the quality assessment with EVIDEM and included nine evaluations of the higher quality group to present the cost-effectiveness estimates.
4.1 Initial Vaccine Effectiveness
Despite our strict selection criteria regarding the input parameters for VE, we found substantial heterogeneity in the values used in the selected studies. In the case of PCV13, in order to restrain variation in the applied VE parameters we only selected studies that referred to the CAPiTA trial [7] to inform VE parameters. For PPSV23, we observed a greater variation originating from the chosen sources and applied age and health effects. Studies referencing Shapiro et al. [58] and Moberley et al. [47, 53] were seen to apply a higher PPSV23 effectiveness against IPD. In contrast to the majority of these studies, Blommaert et al. [26] applied the lowest value (55%) of the confidence interval provided by Moberley et al. [47]. This value was considerably lower but similar to VE of 56–58% used in the models referencing the review by Andrews et al. [15]. A few studies also included PPSV23 effectiveness against NBPP either in their baseline or sensitivity analyses informing the VE parameter with the data from cohort studies conducted in Spain [48, 57, 65, 66]. We found between- and (in some cases) within-study differences in the magnitude of applied age effects on the VE.
So far, the study of van Werkhoven et al. [50, 51] is the only analysis that explored vaccine-age-interaction effects of PCV13 among the elderly. The authors report statistically significant interaction effects for pneumococcal pneumonia (including invasive and non-invasive diseases). However, the applied statistical model showed a relatively poor fit for the age group ≥ 85 years, in which very few disease episodes occurred [25, 51]. Excluding this age group from the analysis, the vaccine-age-interaction was not statistically significant [25, 51]. Several observational studies [12, 15, 66,67,68] indicate vaccine-age interaction effects for PPSV23 but these have not been further investigated, although Djennad et al. [67] reported that differences in PPSV23 VE against vaccine-type IPD were significant between different age groups. Based on the current evidence about the vaccine-age-interaction effects for PCV13 and PPSV23, both age-dependent and age-independent approaches to VE can be justified and both scenarios should be evaluated in cost-effectiveness analyses. Of note, when estimating single-age or age-group-specific VE for PPSV23 based on existing observational studies, it should be considered that most observational studies stratify age groups according to the age at occurrence of the diseases and not according to the age at vaccination.
4.2 Duration of Vaccine Protection and Waning Patterns
The absence of robust empirical evidence about the duration of vaccine protection led to a great variation of methodological approaches to address this uncertainty. The majority of the studies shared the concept of composing the duration of vaccine protection from a period of stable immunity equal to VE at administration, followed by a period of waning protection. The CAPiTA trial [7] showed no waning of PCV13 effectiveness over the study period of 4–5 years and most of the studies in this review applied 4–5 years of constant protection and made assumptions regarding the subsequent waning pattern. For PPSV23, a 2- to 5-year period of stable protection was frequently used. Overall, the majority of the studies reflected in the assumptions the implication that PCV13 induced a more profound immune response than PPSV23 [7] and applied a longer-lasting protection of PCV13. A broadly used approach to modeling the waning pattern was a linear or a step-wise linear decline to 0% effectiveness over some period of time, with the annual waning rates varying across the studies. A more conservative approach, which might ease a comparative analysis of the vaccines, was to set the same duration of the protection for both vaccines, which was done in one study in this review [26]. However, the conjugate vaccine is expected to protect for longer due to the stronger vaccine-induced response [16].
In addition, upon the comparison of the reported VE values and constructed waning patterns, we calculated expected vaccine protection over time (EVPOT) to facilitate comparison of the combination of these constituents of vaccine protection between and within the studies. EVPOT was estimated by computing the area under the curve of VE over time and represents a measure of years of vaccine protection adjusted for the initial VE value, that is, it is composed of multiplicative effects of the initial VE and the applied years of waning protection. Although EVPOT allows for the comparison of modeled vaccine effects between the studies using a single value, this approach has certain limitations. Firstly, upon combining two constituents of the vaccine effects into a single value, the information about the effects and magnitude of each of them becomes hidden; therefore, we reported both constituents of EVPOT separately for each reviewed study. Secondly, when a relatively long waning period is applied, EVPOT can lead to a biased representation of the vaccine effects, which are actually simulated in the modeling. In particular, the waning patterns with a very long tail to the right may result in high EVPOT but substantial vaccine benefits resulting from the assumed long protection may not actualize in the projection, for instance, due to high mortality rates or due to the herd effect of infant vaccination. To avoid this limitation, we chose a cut-off point of 20 years since vaccination to calculate EVPOT. An alternative method of comparison of applied vaccine effects is the calculation of the half-time duration; that is, estimation of the time since vaccination at which VE is reduced to 50% of its initial value [69]. This measure, however, may introduce a bias towards the waning patterns that start with a slow waning of the initial VE followed by a rapid waning phase as compared with waning with constant rates. This, however, reflects the fact that the present effects have more value than the future effects for instance, due to mortality, quality of life decreasing with age, discounting of health outcomes and costs, and the herd effects. This preference for a slow followed by a rapid waning phase may provide a different conclusion about the outcomes of the vaccination over time as compared with the calculation of the area under the curve; that is, a vaccine may have longer half-time duration but a lower area under the curve. Furthermore, it does not include the impact of the initial VE and the combined effect of initial VE and the waning function.
4.3 Indirect Effects of Infant Vaccination
We observed different methodological approaches for predicting the serotype evolution. The first was to start from equilibrium of the childhood PCV-type serotypes in the elderly IPD incidence and assume no further herd effects, which was done in the Australian studies [8, 9, 30] and one Dutch study [25]. This approach is reasonable when the national epidemiological data sufficiently indicates that new post-PCV equilibrium has been reached or if there are no net indirect changes in the preventable PD incidence. It is important to note that Australia implemented a 3 + 0 schedule of the PCV13 program in children that is unique among high-income countries. The reported possible consequences of the schedule without a booster dose include increased PCV13 breakthrough cases and decreased herd effects in PCV13-serotype-induced IPD incidence in the older population [17]. The unique epidemiological settings of Australia make it difficult to transfer the cost-effectiveness projections reported in the Australian studies to other countries.
The second method was to increase the herd effect of PCV13 serotypes step-wise and assume that the maximum reduction of the IPD incidence was reached between years 5 and 7 of the time horizon [27, 31]. A slight variation of this approach was found in two Belgian studies [10, 26], which applied an average annual decline of the PCV13-type incidence without the successive steady state. Finally, four studies predicted forward the distribution of the six additional serotypes contained in PCV13 (PCV13-minus-PCV7). The methodological choice was either to assume that the six serotypes followed a similar decline as the PCV7 serotypes followed by stabilization [24, 28, 29] or to predict a decline in the incidence using a regression equation estimated on the data for the previous years and assume stabilization after some years [33].
Another important indirect effect of the PCV childhood vaccination is the vaccine-induced serotype replacement that might counteract the beneficial impact of the herd effect in the unvaccinated population. Not all studies that modeled the herd effect included the serotype replacement. We found five studies [10, 24, 26, 28, 32] that reported the serotype replacement. The methodological approaches were either to apply an increase in non-vaccine-type PD incidence modeled as a fixed proportion of the reduction in the IPD incidence due to the herd effect or to project the effects of childhood vaccination with PCV7. It is important to note that if the pneumococcal vaccine under evaluation contains serotypes that are not included in the conjugated vaccine used for the childhood vaccination, the replacement effects potentially caused by these additional serotypes should be examined and incorporated into the model.
Extrapolation of the historical effects caused by PCV7 vaccination onto the six additional serotypes in PCV13 should be done with caution as pointed out by Chen et al. [8]. This statement was based on the findings that the circulation of the six additional serotypes declined in a shorter period than PCV7 serotypes in Australia, which may lead to a considerable shift in the steady-state predictions. However, post-PCV13-vaccination studies showed the persistence of serotype 3 in the population; that is, PCV-13 infant immunization has no or very limited indirect effect on the IPD incidence caused by serotype 3 [70]. Recently published epidemiological data also indicates that serotype 19A, after a substantial decrease due to the herd effect, may stabilize at a low level [71, 72]. Therefore, the common approach to group the serotypes by vaccine (e.g., PCV7 serotypes, PCV13 serotypes, PCV13-minus-PCV7 serotypes) may be misleading and can result in poor predictions, underestimating the remaining burden of pneumococcal diseases caused by PCV13 serotypes. The application of the serotype-specific epidemiology of invasive and non-invasive PD in the model allows a more realistic estimation of the vaccination effects. Any-serotype and all-cause approaches lead to a false presentation of the indirect effects on the selected groups of serotypes, particularly PCV13-type (see ESM file S1: section 1.2.2), favoring the outcomes of the elderly vaccination and improving its cost effectiveness. It is important to note that assumptions and input data used to inform a decision-analytic model become outdated with time and the results of an economic evaluation have to be updated when new information regarding country-specific S. pneumoniae epidemiology becomes available.
In addition, the studies by Stoecker et al. [28] and Chen et al. [9] demonstrated that model type (single cohort vs multi-cohort) substantially affected the results when time-varying serotype epidemiology is to be modeled. The authors point out that single-cohort models do not produce valid ICERs over a series of years because they do not capture variation of the serotype distribution, which in turn are differently affected by VE. The resulting ICERs over a set of calendar years in the papers by Chen et al. [9] and Stoecker et al. [28] show substantial variation between the cohorts that are vaccinated in different calendar years. Single-cohort models provide valid results only when epidemiological equilibrium has been reached before the start of the model period. Otherwise, when the dynamic indirect effects are present, application of a multi-cohort model is required to capture the ongoing changes in the PD incidence.
Furthermore, in this review we did not identify dynamic transmission models and application of PCV13 VE against the bacterial nasopharyngeal carriage. Currently, there is little empirical evidence on the protective effects of vaccine-induced reduction of the bacterial carriage in the elderly. Van Deursen et al. [73] reported that PCV13 induced a small and short-lived decline in the vaccine-type nasopharyngeal carriage in people aged ≥ 65 years. This may lead to additional herd effects with the elderly vaccination, which can be particularly beneficial in the communities with higher concentration of the elderly [74].
Overall, these considerations should be seen as relevant for the decision-making process and the studies that aim to support the decision outcomes should describe their methodological choices and assumptions and provide a transparent reporting of the disease incidence rates for all relevant serotype groups over the modeling time horizon or until a new post-vaccination equilibrium is reached.
4.4 Cost Effectiveness
The current evidence about the cost effectiveness of pneumococcal vaccination of the elderly in the presence of a higher valent PCV infant vaccination program is inconclusive. Reviewing the studies with a higher relevance and validity score, we found predominant evidence that vaccinating the elderly with PCV13 is not cost effective when an ongoing decline in the incidence of PCV13-type PD is modeled either until extinction of PCV13 serotypes or until a very low PCV13 PD incidence level is reached. However, the current epidemiologic evidence suggest that PCV13 IPD incidence initially substantially declines but then persists on a moderate level in the presence of PCV13 infant vaccination [67, 70,71,72, 75]. In the Australian studies [8, 9, 30], similar stabilization of PCV13 serotypes was applied and ICERs of PCV13 versus no vaccination were in the range of US$50,000–US$100,000/QALY. However, at the time of the evaluations, the unique 3 + 0 PCV13 infant vaccination schedule was applied. Jayasinghe et al. [17] found fast waning of PCV13 effectiveness under this schedule, which may have resulted in a higher persistent PCV13 PD incidence compared with the settings of more commonly used vaccination schedules (2 + 1 or 3 + 1). Thorrington et al. [32], who also modeled the moderate persistent incidence, reported ICERs of US$50,000–US$100,000/QALY for PCV13 versus no vaccination but the effects of the replacement of PCV10 with PCV13 in the infant vaccination was based on many assumptions. In contrast to Dirmesropian et al. [30], Thorrington et al. [32] found that PPSV23 showed better value for money than PCV13 in the vaccination of the elderly. A key difference between the studies was the application of a moderate PPSV23 VE against NBPP in the study by Thorrington et al. [32] as compared with the assumption of no protective effects by Dirmesropian et al [30].
The findings by Blommaert et al. [26], Thorrington et al. [32], and Willem et al. [10] indicate that PPSV23 is likely to be a cost-effective vaccination strategy in all reviewed epidemiologic scenarios if it is at least moderately effective against NBPP and the expected duration of protection lasts at least 3–4 years. The outcomes of the vaccination with PPSV23 were less affected by the indirect effects of the childhood vaccination due to the wide serotype coverage of the vaccine, but the childhood vaccination still played a crucial role for the vaccination benefits. In the absence of a protective effect against NBPP, evidence for the cost effectiveness of PPSV23 is not conclusive. Childhood vaccination with PCV10 makes the elderly vaccination with PCV13 more attractive due to the three additional serotypes preventable by PCV13.
Currently, there is a lack of studies that model the important PCV13 serotypes such as serotype 3 separately from other groups of serotypes when evaluating pneumococcal vaccination of the elderly in the presence of a PCV13 infant vaccination. Besides the accurate presentation of epidemiologic effects, there are still issues regarding the effectiveness of both vaccines (PCV13 and PPSV23) against serotype 3 [15, 17, 67, 76, 77], which may also require the separation of serotype 3 from other groups in the presence of PCV10 infant vaccination to investigate scenarios with the reduced vaccine effectiveness [78].
5 Conclusion
To summarize, in this review we found major differences in the methods and assumptions applied in the modeling of VE and the indirect effects of the childhood vaccination with the higher valent vaccines (PCV10 and PCV13). Results of the cost-effectiveness analyses are largely determined by the predictions of PD incidence and the estimates of pneumococcal VE over time on which they are based. Insight into the modeling of these processes can help to rationally interpret obtained cost-effectiveness estimates and to understand the variation of conclusions among the studies. It is also important to take into consideration all dimensions of economic evaluation that may drive the ICER estimates; these include characteristics of vaccination strategy (age range, dose and uptake), vaccine price, cost per PD case, utility estimates, modeling time horizon, and discount rates. Taken together, country-specific S. pneumoniae epidemiology, vaccination strategy, and country-specific economic inputs require the development of a decision-analytic model specific for this country. Any comparisons between outcomes of models from different countries should be made with caution due to the large number of parameters that determine the results.
Overall, a major pneumococcal vaccination campaign for the elderly is largely resource-consuming and with all the other social and public health challenges at hand the opportunity cost of such a vaccination campaign may be high. For this reason, the need for well-designed modeling studies that produce representative and non-biased cost-effectiveness estimates cannot be overemphasized.
References
Tin Tin Htar M, Christopoulou D, Schmitt H-J. Pneumococcal serotype evolution in Western Europe. BMC Infect Dis. 2015;15:419. https://doi.org/10.1186/s12879-015-1147-x.
Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e51–9. https://doi.org/10.1016/S2214-109X(16)30306-0.
Ogilvie I, Khoury AE, Cui Y, Dasbach E, Grabenstein JD, Goetghebeur M. Cost-effectiveness of pneumococcal polysaccharide vaccination in adults: a systematic review of conclusions and assumptions. Vaccine. 2009;27:4891–904. https://doi.org/10.1016/j.vaccine.2009.05.061.
Dirmesropian S, Wood JG, MacIntyre CR, Newall AT. A review of economic evaluations of 13-valent pneumococcal conjugate vaccine (PCV13) in adults and the elderly. Hum Vaccin Immunother. 2015;11:818–25. https://doi.org/10.1080/21645515.2015.1011954.
Nishikawa AM, Sartori AMC, Mainardi GM, Freitas AC, Itria A, Novaes HMD, de Soárez PC. Systematic review of economic evaluations of the 23-valent pneumococcal polysaccharide vaccine (PPV23) in individuals 60 years of age or older. Vaccine. 2018;36:2510–22. https://doi.org/10.1016/j.vaccine.2018.03.070.
Porchia BR, Bonanni P, Bechini A, Bonaccorsi G, Boccalini S. Evaluating the costs and benefits of pneumococcal vaccination in adults. Expert Rev Vaccines. 2017;16:93–107. https://doi.org/10.1080/14760584.2017.1242419.
Bonten MJM, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–25. https://doi.org/10.1056/NEJMoa1408544.
Chen C, Beutels P, Newall AT. Evolution over time in the cost-effectiveness of pneumococcal conjugate vaccine (PCV13) in older Australians due to herd protection from infant vaccination. Vaccine. 2018;36:2057–60. https://doi.org/10.1016/j.vaccine.2018.03.006.
Chen C, Wood JG, Beutels P, Menzies R, MacIntyre CR, Dirmesropian S, et al. The role of timeliness in the cost-effectiveness of older adult vaccination: a case study of pneumococcal conjugate vaccine in Australia. Vaccine. 2018;36:1265–71. https://doi.org/10.1016/j.vaccine.2018.01.052.
Willem L, Blommaert A, Hanquet G, Thiry N, Bilcke J, Theeten H, et al. Economic evaluation of pneumococcal vaccines for adults aged over 50 years in Belgium. Hum Vaccin Immunother. 2018. https://doi.org/10.1080/21645515.2018.1428507.
Le Polain De Waroux O, Flasche S, Prieto-Merino D, Goldblatt D, Edmunds WJ. The efficacy and duration of protection of pneumococcal conjugate vaccines against nasopharyngeal carriage: a meta-regression model. Pediatr Infect Dis J. 2015;34:858–64. https://doi.org/10.1097/inf.0000000000000717.
Suzuki M, Dhoubhadel BG, Ishifuji T, Yasunami M, Yaegashi M, Asoh N, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17:313–21. https://doi.org/10.1016/S1473-3099(17)30049-X.
Rudnick W, Liu Z, Shigayeva A, Low DE, Green K, Plevneshi A, et al. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995–2011. Vaccine. 2013;31:5863–71. https://doi.org/10.1016/j.vaccine.2013.09.049.
Gutiérrez Rodríguez MA, Ordobás Gavín MA, García-Comas L, Sanz Moreno JC, Córdoba Deorador E, Lasheras Carbajo MD, Taveira Jiménez JA, Martín Martínez F, Iniesta Fornies D, Arce Arnaez A. Effectiveness of 23-valent pneumococcal polysaccharide vaccine in adults aged 60 years and over in the Region of Madrid, Spain, 2008–2011. 2014. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES2014.19.40.20922. Accessed 24 Oct 2018.
Andrews NJ, Waight PA, George RC, Slack MP, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30:6802–8. https://doi.org/10.1016/j.vaccine.2012.09.019.
Svensson T, Kättström M, Hammarlund Y, Roth D, Andersson P-O, Svensson M, et al. Pneumococcal conjugate vaccine triggers a better immune response than pneumococcal polysaccharide vaccine in patients with chronic lymphocytic leukemia A randomized study by the Swedish CLL group. Vaccine. 2018;36:3701–7. https://doi.org/10.1016/j.vaccine.2018.05.012.
Jayasinghe S, Chiu C, Quinn H, Menzies R, Gilmour R, McIntyre P. Effectiveness of 7- and 13-valent pneumococcal conjugate vaccines in a schedule without a booster dose: a 10-year observational study. Clin Infect Dis. 2018;67:367–74. https://doi.org/10.1093/cid/ciy129.
Henriques-Normark B, Tuomanen EI. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med. 2013. https://doi.org/10.1101/cshperspect.a010215.
World Health Organization. WHO guide for standardization of economic evaluations of immunization programmes. 2008. http://www.who.int/iris/handle/10665/69981. Accessed 14 Mar 2018.
Goetghebeur MM, Wagner M, Khoury H, Levitt RJ, Erickson LJ, Rindress D. Evidence and value: impact on decisionmaking—the EVIDEM framework and potential applications. BMC Health Serv Res. 2008;8:270. https://doi.org/10.1186/1472-6963-8-270.
OECD. Inflation (CPI) (indicator): OECD; 2017. https://data.oecd.org/price/inflation-cpi.htm. Accessed 3 Sept 2018.
OECD. Purchasing power parities (PPP) (indicator): OECD; 2017. https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm. Accessed 3 Sept 2018.
OECD. Exchange rates (indicator): OECD; 2017. https://data.oecd.org/conversion/exchange-rates.htm. Accessed 3 Sept 2018.
Jiang Y, Gauthier A, Annemans L, van der Linden M, Nicolas-Spony L, Bresse X. Cost-effectiveness of vaccinating adults with the 23-valent pneumococcal polysaccharide vaccine (PPV23) in Germany. Expert Rev Pharmacoecon Outcomes Res. 2012;12:645–60. https://doi.org/10.1586/erp.12.54.
Mangen M-JJ, Rozenbaum MH, Huijts SM, van Werkhoven CH, Postma DF, Atwood M, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015;46:1407–16. https://doi.org/10.1183/13993003.00325-2015.
Blommaert A, Bilcke J, Willem L, Verhaegen J, Goossens H, Beutels P. The cost-effectiveness of pneumococcal vaccination in healthy adults over 50: an exploration of influential factors for Belgium. Vaccine. 2016;34:2106–12. https://doi.org/10.1016/j.vaccine.2016.03.003.
Rodriguez Gonzalez-Moro JM, Menendez R, Campins M, Lwoff N, Oyaguez I, Echave M, et al. Cost effectiveness of the 13-valent pneumococcal conjugate vaccination program in chronic obstructive pulmonary disease patients aged 50 + years in Spain. Clin Drug Investig. 2016;36:41–53. https://doi.org/10.1007/s40261-015-0345-z.
Stoecker C, Kim L, Gierke R, Pilishvili T. Incremental cost-effectiveness of 13-valent pneumococcal conjugate vaccine for adults age 50 years and older in the United States. J Gen Intern Med. 2016;31:901–8. https://doi.org/10.1007/s11606-016-3651-0.
van Hoek AJ, Miller E. Cost-effectiveness of vaccinating immunocompetent/= 65 year olds with the 13-valent pneumococcal conjugate vaccine in England. PLoS One. 2016;11:e0149540. https://doi.org/10.1371/journal.pone.0149540.
Dirmesropian S, Wood JG, MacIntyre CR, Beutels P, McIntyre P, Menzies R, et al. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine (PCV13) in older Australians. Vaccine. 2017;35:4307–14. https://doi.org/10.1016/j.vaccine.2017.06.085.
Heo JY, Seo YB, Choi WS, Lee J, Noh JY, Jeong HW, et al. Cost-effectiveness of pneumococcal vaccination strategies for the elderly in Korea. PLoS One. 2017;12:e0177342. https://doi.org/10.1371/journal.pone.0177342.
Thorrington D, van Rossum L, Knol M, de Melker H, Rümke H, Hak E, van Hoek AJ. Impact and cost-effectiveness of different vaccination strategies to reduce the burden of pneumococcal disease among elderly in the Netherlands. PLoS One. 2018;13:e0192640. https://doi.org/10.1371/journal.pone.0192640.
Kuchenbecker U, Chase D, Reichert A, Schiffner-Rohe J, Atwood M. Estimating the cost-effectiveness of a sequential pneumococcal vaccination program for adults in Germany. PLoS One. 2018;13:e0197905. https://doi.org/10.1371/journal.pone.0197905.
Merito M, Giorgi Rossi P, Mantovani J, Curtale F, Borgia P, Guasticchi G. Cost-effectiveness of vaccinating for invasive pneumococcal disease in the elderly in the Lazio region of Italy. Vaccine. 2007;25:458–65. https://doi.org/10.1016/j.vaccine.2006.08.005.
Rozenbaum MH, Hak E, van der Werf TS, Postma MJ. Results of a cohort model analysis of the cost-effectiveness of routine immunization with 13-valent pneumococcal conjugate vaccine of those aged or = 65 years in the Netherlands. Clin Ther. 2010;32:1517–32. https://doi.org/10.1016/j.clinthera.2010.06.016.
Akin L, Kaya M, Altinel S, Durand L. Cost of pneumococcal infections and cost-effectiveness analysis of pneumococcal vaccination at risk adults and elderly in Turkey. Hum Vaccin. 2011;7:441–50.
Neto JT, de Araujo GTB, Gagliardi A, Pinho A, Durand L, Fonseca M. Cost-effectiveness analysis of pneumococcal polysaccharide vaccination from age 60 in Sao Paulo State, Brazil. Hum Vaccin. 2011;7:1037–47. https://doi.org/10.4161/hv.7.10.15987.
Grzesiowski P, Aguiar-Ibanez R, Kobryn A, Durand L, Puig P-E. Cost-effectiveness of polysaccharide pneumococcal vaccination in people aged 65 and above in Poland. Hum Vaccin Immunother. 2012;8:1382–94. https://doi.org/10.4161/hv.21571.
Kuhlmann A, Theidel U, Pletz MW, von der Schulenburg J-MG. Potential cost-effectiveness and benefit-cost ratios of adult pneumococcal vaccination in Germany. Health Econ Rev. 2012;2:4. https://doi.org/10.1186/2191-1991-2-4.
Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307:804–12. https://doi.org/10.1001/jama.2012.169.
Weycker D, Sato R, Strutton D, Edelsberg J, Atwood M, Jackson LA. Public health and economic impact of 13-valent pneumococcal conjugate vaccine in US adults aged ≥ 50 years. Vaccine. 2012;30:5437–44. https://doi.org/10.1016/j.vaccine.2012.05.076.
Chen J, O’Brien MA, Yang HK, Grabenstein JD, Dasbach EJ. Cost-effectiveness of pneumococcal vaccines for adults in the United States. Adv Ther. 2014;31:392–409. https://doi.org/10.1007/s12325-014-0115-y.
Liguori G, Parlato A, Zamparelli AS, Belfiore P, Galle F, Di Onofrio V, et al. Adult immunization with 13-valent pneumococcal vaccine in Campania region, South Italy: an economic evaluation. Hum Vaccin Immunother. 2014;10:492–7. https://doi.org/10.4161/hv.26888.
Ordonez JE, Orozco JJ. Cost-effectiveness analysis of pneumococcal conjugate vaccine 13-valent in older adults in Colombia. BMC Infect Dis. 2014;14:172. https://doi.org/10.1186/1471-2334-14-172.
de Soarez PC, Sartori AMC, Freitas AC, Nishikawa AM, Novaes HMD. Cost-effectiveness analysis of universal vaccination of adults aged 60 years with 23-valent pneumococcal polysaccharide vaccine versus current practice in Brazil. PLoS One. 2015;10:e0130217. https://doi.org/10.1371/journal.pone.0130217.
Zhao D, Gai Tobe R, Cui M, He J, Wu B. Cost-effectiveness of a 23-valent pneumococcal polysaccharide vaccine immunization programme for the elderly in Shanghai, China. Vaccine. 2016;34:6158–65. https://doi.org/10.1016/j.vaccine.2016.11.003.
Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2008. https://doi.org/10.1002/14651858.cd000422.pub2.
Vila-Córcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodríguez T, Llor C. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis. 2006;43:860–8. https://doi.org/10.1086/507340.
Rodriguez-Barradas MC, Goulet J, Brown S, Goetz MB, Rimland D, Simberkoff MS, et al. Impact of pneumococcal vaccination on the incidence of pneumonia by HIV infection status among patients enrolled in the Veterans Aging Cohort 5-Site Study. Clin Infect Dis. 2008;46:1093–100. https://doi.org/10.1086/529201.
van Werkhoven CH, Huijts SM, Bolkenbaas M, Grobbee DE, Bonten MJM. The impact of age on the efficacy of 13-valent pneumococcal conjugate vaccine in elderly. Clin Infect Dis. 2015;61:1835–8. https://doi.org/10.1093/cid/civ686.
van Werkhoven CH, Huijts SM, Bolkenbaas M. 13-valent pneumococcal conjugate vaccine efficacy is declining with old age: results from an exploratory analysis of the CAPiTAtrial. In: ID-Week: Philadelphia < 2014. Abstract #1099.
Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–8. https://doi.org/10.1056/NEJMoa035060.
Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013. https://doi.org/10.1002/14651858.cd000422.pub3.
Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180:48–58. https://doi.org/10.1503/cmaj.080734.
French N, Nakiyingi J, Carpenter LM, Lugada E, Watera C, Moi K, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355:2106–11.
Falkenhorst G, Remschmidt C, Harder T, Hummers-Pradier E, Wichmann O, Bogdan C. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine (ppv23) against pneumococcal disease in the elderly: systematic review and meta-analysis. PLoS One. 2017;12:e0169368. https://doi.org/10.1371/journal.pone.0169368.
Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, Gomez-Bertomeu F, Figuerola-Massana E, Raga-Luria X, Hospital-Guardiola I. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged ≥ 60 years: 3 years of follow-up in the CAPAMIS study. Clin Infect Dis. 2014;58:909–17. https://doi.org/10.1093/cid/ciu002.
Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–60. https://doi.org/10.1056/NEJM199111213252101.
Kraicer-Melamed H, O’Donnell S, Quach C. The effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: a systematic review and meta-analysis. Vaccine. 2016;34:1540–50. https://doi.org/10.1016/j.vaccine.2016.02.024.
Ortqvist A, Hedlund J, Burman LA, Elbel E, Höfer M, Leinonen M, et al. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Swedish Pneumococcal Vaccination Study Group. Lancet. 1998;351:399–403.
Smith KJ, Zimmerman RK, Lin CJ, Nowalk MP, Ko F-S, McEllistrem MC, Roberts MS. Alternative strategies for adult pneumococcal polysaccharide vaccination: a cost-effectiveness analysis. Vaccine. 2008;26:1420–31. https://doi.org/10.1016/j.vaccine.2008.01.007.
Fry AM, Zell ER, Schuchat A, Butler JC, Whitney CG. Comparing potential benefits of new pneumococcal vaccines with the current polysaccharide vaccine in the elderly. Vaccine. 2002;21:303–11.
Knol MJ, Wagenvoort GHJ, Sanders EAM, Elberse K, Vlaminckx BJ, de Melker HE, van der Ende A. Invasive pneumococcal disease 3 years after introduction of 10-valent pneumococcal conjugate vaccine, the Netherlands. Emerg Infect Dis. 2015;21:2040–4. https://doi.org/10.3201/eid2111.140780.
Pletz MW, Ewig S, Rohde G, Schuette H, Rupp J, Welte T, et al. Impact of pneumococcal vaccination in children on serotype distribution in adult community-acquired pneumonia using the serotype-specific multiplex urinary antigen detection assay. Vaccine. 2016;34:2342–8. https://doi.org/10.1016/j.vaccine.2016.03.052.
Vila-Corcoles A, Salsench E, Rodriguez-Blanco T, Ochoa-Gondar O, de Diego C, Valdivieso A, et al. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in middle-aged and older adults: a matched case-control study. Vaccine. 2009;27:1504–10. https://doi.org/10.1016/j.vaccine.2009.01.013.
Gutiérrez Rodríguez MA, Ordobás Gavín MA, García-Comas L, Sanz Moreno JC, Córdoba Deorador E, Lasheras Carbajo MD, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine in adults aged 60 years and over in the Region of Madrid, Spain, 2008–2011. Eurosurveillance. 2014. https://doi.org/10.2807/1560-7917.es2014.19.40.20922.
Djennad A, Ramsay ME, Pebody R, Fry NK, Sheppard C, Ladhani SN, Andrews NJ. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine. 2018;6:42–50. https://doi.org/10.1016/j.eclinm.2018.12.007.
Wright LB, Hughes GJ, Chapman KE, Gorton R, Wilson D. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in people aged 65 years and over in the North East of England, April 2006–July 2012. Trials Vaccinol. 2013;2:45–8. https://doi.org/10.1016/j.trivac.2013.09.004.
Bilcke J, Verelst F, Beutels P. Sponsorship bias in base-case values and uncertainty bounds of health economic evaluations? A systematic review of herpes zoster vaccination. Med Decis Making. 2018;38:730–45. https://doi.org/10.1177/0272989X18776636.
Slotved H-C, Dalby T, Harboe ZB, Valentiner-Branth P, Casadevante VFD, Espenhain L, et al. The incidence of invasive pneumococcal serotype 3 disease in the Danish population is not reduced by PCV-13 vaccination. Heliyon. 2016;2:e00198. https://doi.org/10.1016/j.heliyon.2016.e00198.
Southern J, Andrews N, Sandu P, Sheppard CL, Waight PA, Fry NK, et al. Pneumococcal carriage in children and their household contacts six years after introduction of the 13-valent pneumococcal conjugate vaccine in England. PLoS One. 2018;13:e0195799. https://doi.org/10.1371/journal.pone.0195799.
Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18:441–51. https://doi.org/10.1016/S1473-3099(18)30052-5.
van Deursen AMM, van Houten MA, Webber C, Patton M, Scott D, Patterson S, et al. The impact of the 13-valent pneumococcal conjugate vaccine on pneumococcal carriage in the community acquired pneumonia immunization trial in adults (CAPiTA) study. Clin Infect Dis. 2018;67:42–9. https://doi.org/10.1093/cid/ciy009.
Cafiero-Fonseca ET, Stawasz A, Johnson ST, Sato R, Bloom DE. The full benefits of adult pneumococcal vaccination: a systematic review. PLoS One. 2017;12:e0186903. https://doi.org/10.1371/journal.pone.0186903.
Pilishvili T, Gierke R, Farley M, Schaffner W, Thomas A, Reingold A, et al. Direct and Indirect impact of 13-valent pneumococcal conjugate vaccine (PCV13) on invasive pneumococcal disease (IPD) among children and adults in the U.S. Open Forum Infect Dis. 2017;4:S66–7. https://doi.org/10.1093/ofid/ofx162.158.
Vila-Corcoles A, Ochoa-Gondar O. Pneumococcal conjugate vaccination: correlates of protection. Lancet Infect Dis. 2014;14:784–6. https://doi.org/10.1016/S1473-3099(14)70849-7.
Domínguez Á, Ciruela P, Hernández S, García-García JJ, Soldevila N, Izquierdo C, et al. Effectiveness of the 13-valent pneumococcal conjugate vaccine in preventing invasive pneumococcal disease in children aged 7–59 months. A matched case-control study. PLoS One. 2017;12:e0183191. https://doi.org/10.1371/journal.pone.0183191.
Falkenhorst G, Remschmidt C, Harder T, Wichmann O, Glodny S, Hummers-Pradier E, et al. Background paper to the updated pneumococcal vaccination recommendation for older adults in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2016;59:1623–57. https://doi.org/10.1007/s00103-016-2466-9.
Acknowledgements
Alexander Kuhlmann and Marina Treskova developed the study design the search strategy and Marina Treskova conducted the literature search. Marina Treskova and Stefan M. Scholz screened, included, and excluded the studies and extracted the data for the following analyses and conducted quality assessment with the quality scoring via EVIDEM. Alexander Kuhlmann was consulted in any case of missing consensus. Alexander Kuhlmann conducted calculations of the expected vaccine protection over time. Marina Treskova summarized the results and drafted the paper. Alexander Kuhlmann and Stefan M. Scholz participated in drafting, proof-reading, and editing the manuscript. All three authors contributed to the preparation of the figures, tables and supplemental files.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data availability statement
The data used in the present review can be found in the studies cited in the main and supplemental text. All other data generated during the review are available from the corresponding author upon request.
Funding
This study was funded by the Center for Health Economics Research Hannover (CHERH) of Gottfried Wilhelm Leibniz Universität Hannover, Hannover, Germany.
Conflict of interest
Marina Treskova, Stefan M. Scholz, and Alexander Kuhlmann declare that they have no conflict of interest.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Treskova, M., Scholz, S.M. & Kuhlmann, A. Cost Effectiveness of Elderly Pneumococcal Vaccination in Presence of Higher-Valent Pneumococcal Conjugate Childhood Vaccination: Systematic Literature Review with Focus on Methods and Assumptions. PharmacoEconomics 37, 1093–1127 (2019). https://doi.org/10.1007/s40273-019-00805-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-019-00805-5