Abstract
Background
Although pneumococcal conjugate vaccines (PCVs) have been available for prevention of invasive pneumococcal disease (IPD) caused by Streptococcus pneumoniae (S. pneumoniae) for over a decade, their adoption into national immunization programmes in low- and middle-income countries (LMICs) is still limited. Economic evaluations (EEs) play a crucial role in support of evidence-informed decisions.
Objective
This systematic review aims to provide a critical summary of EEs of PCVs and identify key drivers of EE findings in LMICs.
Methods
We searched Scopus, ISI Web of Science, PubMed, Embase and Cochrane Central from their inception to 30 September 2015 and limited the search to LMICs. The search was undertaken using the search strings ‘pneumococc* AND conjugat* AND (vaccin* OR immun*)’ AND ‘economic OR cost-effectiveness OR cost-benefit OR cost-utility OR cost-effectiveness OR cost-benefit OR cost-utility’ in the abstract, title or keyword fields. To be included, each study had to be a full EE of a PCV and conducted for an LMIC. Studies were extracted and reviewed by two authors. The review involved standard extraction of the study overview or the characteristics of the study, key drivers or parameters of the EE, assumptions behind the analyses and major areas of uncertainty.
Results
Out of 134 records identified, 22 articles were included. Seven studies used a Markov model for analysis, while 15 studies used a decision-tree analytic model. Eighteen studies performed a cost-utility analysis (CUA), with disability-adjusted life-years, quality-adjusted life-years or life-years gained as a measure of health outcome, while four studies focused only on cost-effectiveness analysis (CEA). Both CEA and CUA findings were provided by eight studies. Herd effects and serotype replacement were considered in 10 and 13 studies, respectively. The current evidence shows that both the 10-valent and 13-valent PCVs are probably cost effective in comparison with the 7-valent PCV or no vaccination. The most influential parameters were vaccine efficacy and coverage (in 16 of 22 studies), vaccine price (in 13 of 22 studies), disease incidence (in 11 of 22 studies), mortality from IPD and pneumonia (in 8 of 22 studies) and herd effects (in 4 of 22 studies). The findings were found to be supportive of the products owned by the manufacturers.
Conclusion
Our review demonstrated that an infant PCV programme was a cost-effective intervention in most LMICs (in 20 of 22 studies included). The results were sensitive to vaccine efficacy, price, burden of disease and sponsorship. Decision makers should consider EE findings and affordability before adoption of PCVs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pneumococcal conjugate vaccine (PCV) immunization programmes in low- and middle-income countries (LMICs) are associated with favourable cost-effective results in almost all published studies (in 20 of 22 studies). |
This systematic review highlights that vaccine efficacy, vaccine price and disease burden are the key drivers and play influential roles in the decision to implement PCV immunization programmes in LMICs. |
In addition to cost-effectiveness results, decision makers should consider feasibility, affordability and sustainability of vaccination programmes to ensure equitable access to the vaccine when deciding whether to include a PCV in the national immunization programme. |
1 Introduction
Streptococcus pneumoniae (S. pneumoniae) is a Gram-positive bacterial pathogen causing invasive pneumococcal diseases (IPDs)—including meningitis, bacteraemia, peritonitis and sepsis—and non-invasive diseases, such as acute otitis media (AOM), sinusitis and pneumonia [1–3]. Diseases caused by S. pneumoniae are serious and a major health problem leading to childhood morbidity and mortality worldwide, particularly in children under the age of 24 months [4]. The treatment of pneumococcal diseases can be difficult and inadequate to prevent sequelae and mortality [5]. Moreover, the pneumococcal strains that are resistant to antibiotics make treatment even more challenging [6].
Pneumococcal conjugate vaccines (PCVs) have become available and have been proven to be a safe and effective option for young children [5]. Given the high disease burden, the implementation of a universal childhood immunization programme and its impact on health outcomes have received much attention from policy makers and have become a high priority in many nations [6, 7]. The first PCV was the 7-valent PCV (PCV7), containing capsular polysaccharide antigens of seven serotypes (4, 6B, 9V, 14, 18C, 19F and 23F), which showed a substantial decline in vaccine-type (VT) IPD cases after 4 years following its introduction in the infant immunization schedule [8]. However, an increase in non-vaccine-type (NVT) IPD cases due to serotype replacement—notably, 7F, 19A and 22F—has also been reported [8]. Currently, the available newer PCVs containing antibodies to capsular polysaccharide antigens of S. pneumoniae include 10- and 13-valent PCVs (PCV10/Synflorix®, PCV13/Prevnar®) [9]. PCV10—containing additional antigen serotypes 1, 5 and 7F—has been claimed to confer high protection against diseases caused by non-typable Haemophilus influenzae (NTHi)—most markedly, AOM—whereas PCV13 offers seroprotection against six additional serotypes causing IPD (1, 3, 5, 6A, 7F and 19A) in addition to the seven serotypes contained in PCV7 [9, 10].
Despite the clinical benefits of PCVs in disease prevention, the introduction of vaccination programmes in low- and middle-income countries (LMICs) is limited by financial barriers. To improve access to new and underused vaccines for children living in the world’s poorest countries, the Global Alliance for Vaccines and Immunization (GAVI) was established in 2000 to bring together the public and private sectors with the shared goal of creating better access to vaccines for children in the world. Fifty-four countries are eligible to apply for GAVI support in 2016 [11]. Almost all (~84 %) of the 31 low-income countries (LICs) that are GAVI eligible have introduced the vaccine, except for Somalia, North Korea, Guinea, Comoros and Chad [12]. However, the progress of introducing PCVs into national immunization programmes in middle-income countries (MICs) has been comparatively sluggish. As of now, 134 countries have introduced PCVs into their national immunization programmes [12]. The available cost-effectiveness data for PCVs have been largely documented in high-income countries (HICs) where the vaccine has been adopted as part of immunization programmes [6]. Our previous systematic review [9], which reviewed the cost-effectiveness literature on PCV10 and PCV13 from all around the world, found that combined uncertainty related to price differences, burden of disease, vaccine effectiveness, herd immunity and serotype replacement effects determined the preference base for either PCV10 or PCV13 [9]. Of the studies that were included in that review, only eight were conducted in LMICs, where the burden of disease is usually high, and few of those countries included PCVs in their immunization programmes [7]. Thus, the generalizability of the findings was limited. Moreover, different assumptions could be made from varying time spans of economic analyses [6]. There remains a strong need for an updated systematic review of cost-effectiveness studies of PCV programmes specifically for LMICs. The current review aims to summarize the key characteristics and findings of cost-effectiveness evidence to provide relevant information for decision makers.
2 Methods
This review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [13].
2.1 Search Strategy
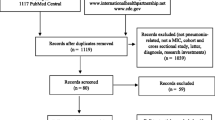
This systematic review is an extension of our previous review of economic evaluations (EEs) of PCVs in childhood [9]. Thus, the searching of the databases was limited to the time interval from 1 January 2014 to 30 September 2015, since the end date of the search for our previous review was 31 January 2014 (see Fig. 1). The literature search for the current review was undertaken in a similar fashion to that in our previous review, using the broad combined search strings ‘pneumococc* AND conjugat* AND (vaccin* OR immun*)’ AND ‘economic OR cost-effectiveness OR cost-benefit OR cost-utility OR cost-effectiveness OR cost-benefit OR cost-utility’ in the abstract, title or keyword fields. Five databases—Scopus, ISI Web of Science (SCI and SSCI), Medline (PubMed), Embase and Cochrane Central—were searched to retrieve studies of potential interest. The search was limited to LMICs using an LMIC filter (see the Appendix in the Electronic Supplementary Material).

*Articles were retrieved from Wu et al. [9]
Flow diagram of the selection process used to identify studies for inclusion in this review. CEA cost-effectiveness analysis, LMIC low- or middle-income country
2.2 Selection
All initially identified studies were considered on the basis of the title and abstract, and were included for further review if they contained a full EE [14] of a PCV in children (aged <12 years) in an LMIC. One hundred and thirty-five countries were classified as LMICs on the basis of the World Bank classification, with a per capita gross national income (GNI) less than US$12,736 [15]. Non-English-language articles, editorials, letters and review articles were excluded. A flow chart showing the selection process is illustrated in Fig. 1.
2.3 Data Extraction
The inclusion criteria were checked by two reviewers (SS and AR), and data were extracted by SS using a standardized data extraction form and confirmed by either AR, DBCW or NC. In cases of doubt, there was a consultation process to base the extraction upon consensus. As the main objective of the review was to provide LMIC decision makers with a comprehensive overview of the key issues to consider when making a decision about PCV immunization, the following data were considered: (1) study overview or characteristics of the study; (2) key drivers or parameters of the EE; (3) assumptions behind the analyses; and (4) major area of uncertainty.
3 Results
3.1 Study Selection
A total of 134 records were identified through database searching (n = 126) and other sources (n = 8). One hundred records remained after duplicates were removed. Of those remaining 100 citations, 59 records were deemed ineligible on the basis of their title and abstract. Of the 41 papers that qualified for a full-text review, 19 full-text articles were excluded because they did not meet the eligibility criteria for the review (see Fig. 1). Twenty-two studies were selected for this systematic review [16–37]. Three each were from China [25, 28, 37] and Peru [21, 29, 30]; two each were from Columbia [19, 36], Turkey [20, 24] and the Philippines [26, 32]; one each was from the Gambia [16], Brazil [18], Kenya [22], Thailand [23], Malaysia [27], Paraguay [31], Somalia [33], Georgia [34] and Egypt [35]; and one study was done in 77 MICs [17].
3.2 General Characteristics of the Included Studies
General characteristics of the EEs and vaccine prices are shown in Tables 1 and 2 and Table A-1 in the Electronic Supplementary Material. All 22 studies used economic models to evaluate the cost effectiveness of PCVs in LMICs. Eighteen studies performed a cost-utility analysis (CUA) with disability-adjusted life-years (DALYs) or quality-adjusted life-years (QALYs) as a measure of health outcome [16–18, 20–23, 25–28, 30–35, 37], while four studies focused only on cost-effectiveness analysis (CEA) [19, 24, 29, 36]. Both CEA and CUA findings were provided by eight studies [20–22, 25, 27, 34, 35, 37].
Three studies were conducted from the payer perspective, all of which were sponsored by pharmaceutical companies [25, 27, 37]. The costs for the perspective included the direct medical costs, the cost of the vaccine per dose and the direct cost associated with vaccine administration. In the study conducted in Malaysia, the authors declared their full independence in study execution [25]. Eight studies used a societal perspective [16–19, 22, 23, 28, 31], whereas the healthcare perspective was used in seven studies [18, 20, 21, 24, 32, 33, 36]. Among these, three studies were sponsored by manufacturers [18, 19, 34], two studies were sponsored by GlaxoSmithKline (GSK) with the sponsor’s involvement at all stages [18, 19] and one study funded by Pfizer declared full independence of the authors in the study conduct [34]. One study used both societal and healthcare perspectives [18]. Six studies used a government perspective [26, 29–31, 34, 35], of which one study was supported by GSK Vaccines with their full involvement from study design to data analysis [24], and one study adopted the perspectives of both the government and society [31]. The government perspective included direct medical costs related to treatment borne by the government, whereas the health sector perspective included all direct medical costs related to PCV treatment, both inpatient and outpatient. The cost inputs into the model for both perspectives were generally similar in LMIC settings.
In terms of the impacts of herd immunity and serotype replacement exploration, 10 studies modelled the positive effects of herd protection [17, 23, 25, 27, 28, 32, 34–37], and the negative impact of serotype replacement was considered in 13 studies as their base-case analysis [16, 17, 21–24, 26, 27, 30, 32, 34, 35, 37]. Of these, seven studies considered both herd effects and serotype replacement (often collectively termed ‘indirect effects’) in their evaluations [17, 23, 27, 32, 34, 35, 37].
Different durations of time horizon were assumed. Three studies projected the outcomes over a year [20, 25, 37], one study each over 2 and 3 years [29, 33], six studies over 5 years [16, 24, 28, 32, 35, 36], two studies over 10 years [31, 34] and one over 25 years [18]. Seven studies used a lifetime horizon [17, 19, 21, 23, 26, 27, 30]. The time span used was not reported in one study [22]. The issue of the time horizon is critical, as it should be long enough to fully capture long-term costs and health outcomes. Since meningitis is one of the crucial IPDs that is associated with long-term sequelae—such as hearing loss or mental retardation—a lifetime horizon should be used unless the studies have provided clear justification for not using such a time horizon. Most studies used discount rates between 3 and 6 % for costs and benefits, in which rates of 3 % were applied in most studies.
A cost-effectiveness threshold of 1–3 times the per capita gross domestic product (1–3× GDP) was clearly stated, based on the cost per DALY averted (according to the recommendation from the World Health Organization (WHO) Choosing Interventions That Are Cost Effective (CHOICE) project [38]), in nine studies [16–18, 22, 30, 31, 33–35]. Five studies estimated incremental cost-effectiveness ratios (ICERs) for costs per QALY or costs per life-year gained (LYG) [39] and used 1–3× GDP as the threshold. Two studies used their own country-specific thresholds [23, 32], while two studies did not use cost-effectiveness thresholds [20, 29]. It is important to note that the remaining four studies cited the WHO-CHOICE paper as the cost-effectiveness threshold (1–3× GDP per capita), but they reported outcomes as QALYs and LYGs [25, 27, 28, 36] rather than DALYs.
The main input parameters and the results of EEs of PCVs in children in LMICs are described in Table A-2 in the Electronic Supplementary Material. Fifteen studies provided evaluations of both PCV10 and PCV13 [16, 17, 19–24, 26, 27, 29–32, 36]. Three studies included only PCV10 [18, 33, 34], of which one study done in Somalia evaluated PCV10 administered together with H. influenzae type B vaccine (Hib) versus no vaccination to aid policy decision makers regarding vaccine adoption during a humanitarian emergency [33]. Three studies provided an analysis only of PCV7 [25, 28, 37], while one study evaluated only the cost effectiveness of PCV13 versus no vaccination [35].
Although it is essential to use a relevant incremental approach to perform a direct comparison of all potential PCV candidates, most studies reported the cost effectiveness of 1–3 vaccines (PCV7, PCV10, PC13) versus no vaccination rather than evaluating the incremental CEA of using PCV10 versus PCV13 [16–19, 22–25, 28, 34, 35]. Only eight studies provided head-to-head analyses of PCV13 versus PCV10 [20, 26, 27, 29–32, 36].
All 22 studies contained analyses of the effectiveness of infant PCV immunization with no catch-up vaccination in children. However, this should not have had a substantial impact on the results, as the burden of the disease is usually highest among infants under 1 year of age. Although the four-dose schedule has been recommended on the basis of clinical trial data [40, 41], only six studies adopted this regimen in their programmes [18, 20, 24, 25, 27, 37]. The three-dose schedule, which has become acceptable practice, was employed in the majority of studies selected, of which nine studies [16, 19, 21, 23, 26, 28–32] used a 2 + 1 schedule (two primary doses at 2 and 4 months, with a booster at 13 months), whereas a 3p regimen (three primary doses at 6, 10 and 14 weeks or at 2, 4 and 6 months) was implemented in three studies [17, 34, 35]. According to a study done in Kenya, the three doses of PCV10/13 were delivered during the infant immunizations at 4, 6 and 10 weeks, and this schedule was found to be highly cost effective [22]. No study accounted for the efficacy reduction of the three-dose schedule, except for the CUA done in Thailand, in which an overall 8 % reduction in vaccine efficacy was estimated [23].
All of these analyses were based on static population models, in which Markov cohort and decision-tree analytical models were applied in seven studies [16, 19, 21, 23, 26, 27, 32] and 15 studies [17, 18, 20, 22, 24, 25, 28–31, 33–37], respectively. Of the 15 studies in which the decision-tree analytical models were utilized, four studies [30, 31, 34, 35] used integrated TRIVAC cost-effectiveness models. The TRIVAC model was developed jointly by the Pan American Health Organization (PAHO) ProVac Initiative and the London School of Hygiene & Tropical Medicine as a tool for estimating the cost effectiveness of Hib, rotavirus and PCV introduction.
As shown in Table 3, all studies conducted one-way sensitivity analyses. Threshold analyses were conducted in only two studies [18, 23]. Probabilistic sensitivity analyses (PSAs) were carried out in 11 studies [16, 19–23, 26, 28, 29, 32, 36], seven of which also provided cost-effectiveness acceptability curves (CEACs) [16, 19, 23, 28, 29, 32, 36]. Scenario analyses were conducted in 11 studies [16, 18, 19, 21, 26, 29–32, 34, 35]. Vaccination coverage varied between from 80 to 100 % on the basis of the immunization coverage of the ongoing programmes, except in one study done in Somalia, in which a 70 % vaccination uptake rate was assumed for one-dose vaccination and 50 % for two doses [33].
Estimates of vaccine efficacy against invasive and non-invasive diseases varied widely between studies (see Table A-2 in the Electronic Supplementary Material). The efficacy of vaccines against specific serotypes was estimated on the basis of adjusted US data on the serotype-specific efficacy of PCV7 against IPD and suspected pneumococcal pneumonia [40, 42, 43], using local serotype distribution in many studies [18, 19, 24, 28]. The effectiveness against AOM was mainly based on data from either the Finished Otitis Media Vaccine (Finish OM) study [44] or the meta-analysis of PCV7 trials [45] for PCV7/13 and the POET trial [46] for PCV10 [18–21, 23, 24, 26–28, 30, 31]. The data from a recent meta-analysis by Klugman et al. [47] was applied in a cost-effectiveness study in 77 MICs to estimate efficacy against IPD, as well as being used as a proxy for efficacy against pneumonia [17], while the data from a matched case–control study reported for PCV7 [48] was employed in three studies to calculate vaccine efficacy against IPD, and an equal assumption for serotypes shared between all three vaccines (PCV7, PCV10 and PCV13) was made [20, 21, 26].
Local data were used in cases where they were available. For countries where the country-specific input data for cost effectiveness were scarce, data from other countries in geographical proximity was adopted. For instance, the EEs in the Gambia [16] and Kenya [22] used vaccine efficacy data from a clinical trial of 9-valent PCV (PCV9) in the Gambia [49]. Similarly, data on PCV7 coverage of AOM in Korea were also used in a study done by Che et al. [28] in China, as the coverage of AOM in China was not known. Application of region-specific data was also done in some Latin American countries where data from the Latin American Network for Surveillance of Pneumonia & Bacterial Meningitis Agents (SIREVA II) [50] for S. pneumoniae serotype distribution were applied for serotype coverage adjustment [18, 19, 21, 30, 36].
The impact of PCV immunization on IPD and S. pneumoniae were evaluated in all studies, while 17 studies investigated the effect of vaccines on AOM [18–21, 23–28, 30–32, 34–37]. The duration of the protection conferred by the vaccine remains indefinite and was usually assumed to be the same as the model projection period. The impact of vaccine efficacy waning was explored in eight studies [16, 20, 23, 26, 31, 34, 35, 37]. None of the analyses accounted for possible adverse events associated with vaccination, as PCVs are generally well tolerated.
An accurate estimate of the burden of disease is essential in cost-effectiveness evaluations. Most studies investigated the burden of disease across different age categories for IPD, all-cause pneumonia and AOM [18–21, 23–28, 30–32, 34–37], whereas two studies limited the burden of disease to pneumococcal pneumonia [29, 33]. The disease burden of AOM was explored in 17 studies [18–21, 23–28, 30–32, 34–37] (see Table A-3 in the Electronic Supplementary Material). Only four studies [20, 21, 26, 27] accounted for severe disability or hospitalizations related to AOM. Complications due to AOM can be influential for PCV10 cost effectiveness, especially in high-disease-burden settings. However, AOM is generally not a severe disease, and most cases do not require hospitalization. Therefore, this small number may not have had a significant impact on the analyses. S. pneumoniae is responsible for most severe IPD in terms of mortality, and its incidence seems to vary markedly because of diagnosis divergence between countries. Most local settings measure pneumonia incidence in terms of all-cause pneumonia, and the diagnosis can be confirmed only in limited cases. Therefore, a number of approaches were taken in order to estimate the pneumonia disease burden caused by S. pneumoniae averted by PCVs. One study classified pneumonias as ‘primary-endpoint’ and ‘non-primary-endpoint’ pneumonias according to the WHO recommendation standards [51] and used a ‘vaccine probe’ approach to assume the net difference between primary-endpoint and non-primary-endpoint pneumonia occurrences that reflected the burden avoided by PCV intervention [16], while an EE conducted in Malaysia utilized International Classification of Disease (10th Revision) codes to identify pneumococcal cases [27]. Disease states within the models were considered mutually exclusive in all but two studies [16, 25].
Although IPD can lead to various sequelae, all analyses were restricted to neurological sequelae caused by pneumococcal meningitis and/or AOM. Long-term sequelae caused by meningitis infection and/or AOM were included in the models in 17 studies [16–18, 21–28, 30–32, 34, 35, 37], of which only three studies considered complications from both AOM and meningitis [21, 26, 27]. Country-specific data were applied in only nine studies [18, 23, 24, 26–28, 32, 34, 36], whereas the majority of studies used published literature or data derived from other countries with similar geographical settings, as well as expert recommendations, to extrapolate the disease incidence (see Table A-3 in the Electronic Supplementary Material).
3.3 Study Results
3.3.1 Main Findings
Of 22 studies, 14 studies compared vaccination programmes with no vaccination. Of these, the vaccination programmes in 11 studies [16–19, 22, 24, 25, 33–35, 37] were considered cost-effective interventions, while in two studies [23, 28], they were considered unlikely to be cost-effective. Among 11 studies confirming cost effectiveness, the cost-effectiveness threshold was based on either 1× GDP [19, 22, 24, 25] or 3 × GDP [16–18, 33–35, 37]. When 1× GDP or 3× GDP were used as cost-effectiveness thresholds in all studies, the vaccination programmes were cost effective in seven studies (39 %) [19, 22, 30–32, 34, 37] and in 11 studies (61 %) [16, 19, 21, 22, 30–35, 37], respectively. One study was conducted in 77 MICs [17]. The results showed that PCV13 was cost effective for all countries, and PCV10 was cost effective for 72 countries, except for Barbados, Belarus, Montenegro, Serbia and the Seychelles [17] (see Table 1). In the other two studies that reported lack of cost effectiveness, a vaccine cost reduction was suggested to achieve cost effectiveness. For PCVs to be cost effective, the vaccine price per dose would have to be reduced from US$46.2 to US$9.8 (for PCV10) or from US$61.9 to US$15.9 (for PCV13) in Thailand [23], and from US$127 to US$47.9 (for PCV7) in China [28]. It is important to note that the cost-effectiveness thresholds in Thailand [23] and China [28] were based on 1× GDP and 3× GDP, respectively.
Head-to-head analyses of PCV13 versus PCV10 were performed in eight studies [20, 26, 27, 29–32, 36]. In seven studies, PCV13 was considered cost effective on the basis of a cost-effectiveness threshold of 1–3 × GDP, but in one study by Kieninger et al. [31], PCV13 was not cost effective in comparison with PCV10. Of seven studies with cost-effectiveness findings, three studies [20, 26, 27] showed that PCV10 dominated PCV13 and four studies showed that PCV13 dominated PCV10 [29, 30, 32, 36].
3.3.2 Influential Parameters Reported
The influential parameters reported for each EE of pneumococcal vaccination in children in LMICs are shown in Table 4. The most frequently reported influential parameters were vaccine efficacy and coverage (in 16 of 22 studies) [16, 17, 19–21, 24, 26, 27, 29–36], vaccine price (in 13 of 22 studies) [16–19, 21, 22, 26, 28–30, 34–36], disease incidence (in 11 of 22 studies) [17, 18, 23, 25, 26, 28, 30, 31, 33–35] and mortality from IPD and pneumonia (in 8 of 22 studies) [16, 18, 19, 22, 30, 33–35]. In addition, the results of four studies [23, 25, 28, 37] were most sensitive to changes in herd effects of the vaccine on the unvaccinated population.
The review indicated that the results were sensitive to changes in vaccine efficacy and coverage. As mentioned earlier and shown in Table 4, vaccine effectiveness assumptions for both PCV10 and PCV13 were based on pivotal efficacy studies of PCV7. In countries without vaccine efficacy data, the assumptions were based on data from countries in geographical proximity. The second most influential parameter reported was vaccine price. It drove the results of cost-effectiveness analyses, especially in studies with unfavourable ICERs. For example, one study in China [28] found that the cost of the vaccine accounted for 72.2 or 73.6 % of the total cost with or without herd immunity, respectively. Another study in Thailand [23], which used the highest vaccine price in the model (see Table 2 and Table A-4 in the Electronic Supplementary Material), stated that the vaccine could become cost effective or even cost saving if the vaccine costs were reduced by 70–90 % of the current market prices. The vaccine price used in that study [23] was the highest of those in all studies in LMICs. Even the studies reporting cost effectiveness (e.g. the study by Kim et at [16]) found that the vaccine price per dose was the main driver of cost effectiveness. Of 10 studies considering herd effects in our review, the results were sensitive to changes in herd effects in only four studies [23, 25, 28, 37]. Of these, two studies [23, 28] still showed unfavourable cost-effectiveness results even when herd effects were included in the analyses.
3.4 Sponsorship
Studies with sponsorship from industry have been expected to have favourable cost-effectiveness findings [52]. In addition, sponsorship has a significant impact on the vaccine effectiveness assumption being made and the main results being shown [9]. In seven industry-sponsored studies, the findings were found to be supportive of the product manufacturer. All four studies [20, 21, 26, 27] sponsored by GSK showed that PCV10 dominated PCV13 (i.e. PCV10 saved costs and resulted in benefits in comparison with PCV13), while the studies sponsored by Pfizer showed that PCV7 was cost effective in two studies [25, 37], while PCV13 dominated PCV10 in one study [36]. Of 16 non-industry-sponsored studies, nine studies [16–19, 22, 24, 33–35] showed that providing vaccination was cost effective in comparison with no vaccination. Two studies [29, 30] showed that PCV13 was more cost effective than PCV10 and PCV7, while two studies showed that PCV7 [28], PCV10 and PCV13 [23] were not cost effective in comparison with no vaccination. In another two studies, which provided evidence from head-to-head comparisons between PCV10 and PCV13, PCV13 dominated PCV10 in one study [32] but not in the other study [31].
4 Discussion
The availability of pneumococcal vaccines (PCVs) represents a major advancement in the prevention of IPD caused by S. pneumoniae, but PCV programme implementation is still limited in LMICs, possibly because of financial barriers. To the best of our knowledge, no comprehensive review of PCV cost-effectiveness models has been published to evaluate methodological approaches in LMICs. We included 22 full PCV EEs in our systematic review. The studies primarily compared PCV7, PCV10 and PCV13 with each other or with no vaccination. Twenty studies reported that PCV programmes were cost effective or even cost saving under certain conditions. Of these, all ‘GSK studies’ reported positive results for PCV10, and all ‘Pfizer studies’ reported positive results for PCV13. However, the results were influenced by several parameters used in the models.
MICs have the same key drivers of cost-effectiveness results as HICs [9, 53]—i.e. vaccine efficacy, price, burden of disease and sponsorship. Most studies extrapolated vaccine efficacy from Western countries (e.g. the USA, UK and Canada), since no country-specific efficacy data were available. However, 16 studies applied country-specific serotype distributions [14, 16–19, 21–26, 28–30, 34, 35].
There is no denying that vaccine price has been shown to be a major barrier to implementation of PCV immunization programmes, since some LMICs are unlikely to have sufficient health budgets (unlike HICs) and are not eligible for financial aid from GAVI (unlike some other LMICs). In most GAVI-ineligible LMICs, tender prices are mostly unknown. In our review, the PCV10 price ranged from US$0.7 to US$30 per dose in all cost-effective studies. In one study with non-cost-effective results, a PCV10 price of about US$46 per dose (retrieved from GSK, Thailand) was used [23]. In addition, for the PCV13 price, most studies used a vaccine price of around US$3.5–30 per dose for analysis, but one study used a price of US$62 per dose (retrieved from Pfizer, Thailand) [23] (see Table A-4 in the Electronic Supplementary Material). Recently, another review found that the vaccine price (for human papillomavirus [HPV] vaccine) in Thailand (US$150 per dose) was also the highest of those in the included studies, while the HPV vaccine price was below US$2 per dose in some studies [54]. This is one of many other factors causing unfavourable cost-effectiveness results. Furthermore, in the studies supported by GAVI or by the Bill & Melinda Gates Foundation, the prices of PCV10 and PCV13 were always lower than US$10 per dose. Thus, vaccine price negotiations play a crucial role in determining whether PCVs are a cost-effective intervention in LMICs.
Considering indirect effects (i.e. herd protection and serotype replacement), only 7 of 22 studies [17, 23, 27, 32, 34, 35, 37] incorporated both herd protection and serotype replacement into their models. The herd effect from the large-scale PCV7 vaccination programme showed that vaccination can decrease IPD cases by about 15 and 29 % in unvaccinated children aged <5 and >5 years, respectively [55]. Since deaths from pneumococcal disease also occur in the elderly [56], excluding the herd effect on the elderly is therefore likely to have resulted in an underestimation of the true benefit gains of the PCV7, PCV10 or PCV13 vaccines. Conversely, serotype replacement provided an opposite effect to the herd effect in terms of increased disease from non-vaccine serotypes [57]. However, the beneficial herd effects appear to outweigh negative serotype replacement [57]. Therefore, in a study that directly compared PCV7, PCV10 and PCV13 vaccines, inclusion or exclusion of indirect effects for all vaccines would not have an impact on the results, because the incremental differences between vaccines would be the same. But the herd protection effect plays an important role in ICERs when vaccines are compared with a no-vaccination strategy [58]. For example, in a study comparing six scenarios, it was found that inclusion of the herd effect but without serotype replacement showed more favourable cost-effectiveness results than scenarios considering the herd effect and serotype replacement, no herd effect and no serotype replacement, or no herd effect but serotype replacement. However, in our review, we found only four studies [23, 25, 28, 37] that were sensitive to changes in herd effects. In this review, herd immunity had a lesser effect on cost-effectiveness results than that seen in our previous review of all countries [9].
Furthermore, model structural uncertainty can potentially lead to substantial changes in cost-effectiveness results, and this is one of the key factors that should be considered. Contrary to the static models used in all of the reviewed studies, transmission dynamic models can explicitly capture herd immunity effects by allowing infection risks (sometimes referred to as the ‘force of infection’) to vary proportionally with the infectious prevalence and modelling of population immunity [59]. Application of a static model (e.g. a decision-tree model or Markov model), instead of using a dynamic model, tends to underestimate cost-effectiveness results. In our review, all studies applied static models—for example, some studies used a TRIVAC model [60], which was initially developed to help facilitate a decision-support process in countries with limited technical capacity. Because of the ability to incorporate the epidemiology of diseases and the development of herd immunity, dynamic models are generally preferred in cost-effectiveness analyses of vaccination strategies [61]. For application of static models, an exception might occur when the vaccine coverage is nearly 100 % in the whole population and thus herd immunity can be reasonably excluded. However, in reality, implementation of 100 % vaccine coverage is unlikely for vaccination strategies in LMICs. None of the studies included in this review applied a dynamic model, because it would have required more data (e.g. the force of infection and the social contact structure), which were not available in LMICs, where the disease surveillance system is not well established. Also, the results derived from a static model could be considered conservative estimates in comparison with those from a dynamic model [62]—namely, if static models indicated favourable cost effectiveness, the results from dynamic models would be cost effective.
The findings of this review were limited by our search strategies, including the inclusion criteria adopted, the databases searched and the time period of the search. Our search focused primarily on literature published in English in peer-reviewed journals. Even in the published literature, the effect sizes of the economic impact of PCV programmes may differ according to the methodological quality of the studies. Publication bias towards peer-reviewed papers may also have affected our findings. Furthermore, our review included the results of EEs from various countries with different health care systems, and data from EE models instead of real-world data were used. Thus, the results should be interpreted with caution. In addition, country-specific characteristics in terms of cost-effectiveness thresholds, budget impact analyses, equity, and health insurance schemes should be taken into account. Despite these limitations, however, we believe we have identified and synthesized the relevant articles in a thorough manner with respect to PCV immunization for decision makers in LMICs.
5 Conclusion
PCV immunization in LMICs is generally cost effective from both healthcare and societal perspectives in the current situation. The results are sensitive to vaccine efficacy, price, the burden of disease and sponsorship. Policy makers in LMICs should consider not only the cost-effectiveness evidence but also other important factors—including the feasibility, affordability and sustainability of vaccination programmes—to ensure equitable access to vaccines when deciding whether to include PCVs in national immunization schedules.
References
O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi:10.1016/s0140-6736(09)61204-6.
Fedson DS, Scott JA. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine. 1999;17(Suppl 1):S11–8.
World Health Organization. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly Epidemiol Rec. 2007;82:93–104.
Beutels P, Thiry N, Van Damme P. Convincing or confusing? Economic evaluations of childhood pneumococcal conjugate vaccination—a review (2002–2006). Vaccine. 2007;25(17208339):1355–67.
Johns Hopkins Bloomberg School of Public Health. Pneumococcal disease: prevention & treatment. 2010. http://www.jhsph.edu/research/centers-and-institutes/ivac/resources/solutions-pneumococcal-disease-prevention-treatment.html. Accessed 20 Jan 2016.
Beutels P, Thiry N, Van Damme P. Convincing or confusing? Economic evaluations of childhood pneumococcal conjugate vaccination—a review (2002–2006). Vaccine. 2007;25(8):1355–67. doi:10.1016/j.vaccine.2006.10.034.
World Health Organization Department of Immunization, Vaccines and Biologicals. Measuring impact of Streptococcus pneumoniae and Haemophilus influenzae type B conjugate vaccination. Geneva: World Health Organization; 2012.
Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11(10):760–8. doi:10.1016/S1473-3099(11)70090-1.
Wu DB, Chaiyakunapruk N, Chong HY, Beutels P. Choosing between 7-, 10- and 13-valent pneumococcal conjugate vaccines in childhood: a review of economic evaluations (2006–2014). Vaccine. 2015;33(14):1633–58. doi:10.1016/j.vaccine.2015.01.081.
Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005;5(2):83–93. doi:10.1016/s1473-3099(05)01280-6.
Global Alliance for Vaccines and Immunization (GAVI). Countries eligible for support. 2016. http://www.gavi.org/support/apply/countries-eligible-for-support/. Accessed 1 Apr 2016.
International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health. Vaccine Information and Epidemiology Window (VIEW-hub) global vaccine introduction report. 2016. http://www.jhsph.edu/research/centers-and-institutes/ivac/view-hub/. Accessed 1 Apr 20166.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
Drummond M, O’Brien B, Stoddary G, Torrance G. Methods for the economic evaluation of health care programmes. 2nd ed. Oxford: Oxford University Press; 1997.
World Bank. Country and lending groups. 2015. http://data.worldbank.org/about/country-and-lending-groups. Accessed 10 Sep 2015.
Kim S-Y, Lee G, Goldie S. Economic evaluation of pneumococcal conjugate vaccination in the Gambia. BMC Infect Dis. 2010;10:260.
Nakamura MM, Tasslimi A, Lieu TA, Levine O, Knoll MD, Russell LB, et al. Cost effectiveness of child pneumococcal conjugate vaccination in middle-income countries. Int Health. 2011;3(4):270–81.
Sartori AM, de Soarez PC, Novaes HM. Cost-effectiveness of introducing the 10-valent pneumococcal conjugate vaccine into the universal immunisation of infants in Brazil. J Epidemiol Community Health. 2012;66(3):210–7. doi:10.1136/jech.2010.111880.
Castañeda-Orjuela C, Alvis-Guzmán N, Velandia-González M, De la Hoz-Restrepo F. Cost-effectiveness of pneumococcal conjugate vaccines of 7, 10, and 13 valences in Colombian children. Vaccine. 2012;30(11):1936–43.
Bakir M, Türel O, Topachevskyi O. Cost-effectiveness of new pneumococcal conjugate vaccines in Turkey: a decision analytical model. BMC Health Serv Res. 2012;12(1):386.
Gomez JA, Tirado JC, Rojas AAN, Alba MMC, Topachevskyi O. Cost-effectiveness and cost utility analysis of three pneumococcal conjugate vaccines in children of Peru. BMC Public Health. 2013;13:1025.
Ayieko P, Griffiths UK, Ndiritu M, Moisi J, Mugoya IK, Kamau T, et al. Assessment of health benefits and cost-effectiveness of 10-valent and 13-valent pneumococcal conjugate vaccination in Kenyan children. PLoS One. 2013;8(6):e67324.
Kulpeng W, Leelahavarong P, Rattanavipapong W, Sornsrivichai V, Baggett HC, Meeyai A, et al. Cost-utility analysis of 10- and 13-valent pneumococcal conjugate vaccines: protection at what price in the Thai context? Vaccine. 2013;31:2839–47.
Türel Ö, Kisa A, McIntosh EDG, Bakir M. Potential cost-effectiveness of pneumococcal conjugate vaccine (PCV) in Turkey. Value Health. 2013;16(5):755–9.
Hu S, Shi Q, Song S, Du L, He J, Chen C-I, et al. Estimating the cost-effectiveness of the 7-valent pneumococcal conjugate vaccine in Shanghai, China. Value Health Reg Issues. 2014;3:197–204. doi:10.1016/j.vhri.2014.04.007.
Zhang X-H, Nievera MC, Carlos J, Lucero M, Bibera G, Atienza MI, et al. Cost-effectiveness analysis of pneumococcal vaccination with the pneumococcal polysaccharide NTHi protein D conjugate vaccine in the Philippines. Value Health Reg Issues. 2014;3:156–66. doi:10.1016/j.vhri.2014.04.004.
Aljunid S, Maimaiti N, Ahmed Z, Muhammad Nur A, Md Isa Z, Azmi S, et al. Economic impact of pneumococcal protein-D conjugate vaccine (PHiD-CV) on the Malaysian national immunization programme. Value Health Reg Issues. 2014;3:146–55. doi:10.1016/j.vhri.2014.04.008.
Che D, Zhou H, He J, Wu B. Modeling the impact of the 7-valent pneumococcal conjugate vaccine in Chinese infants: an economic analysis of a compulsory vaccination. BMC Health Serv Res. 2014;14:56. doi:10.1186/1472-6963-14-56.
Mezones-Holguín E, Bolanos-Diaz R, Fiestas V, Sanabria C, Gutierrez-Aguado A, Fiestas F, et al. Cost-effectiveness analysis of pneumococcal conjugate vaccines in preventing pneumonia in Peruvian children. J Infect Dev Ctries. 2014;8(12):1552–62. doi:10.3855/jidc.5855.
Mezones-Holguín E, Canelo-Aybar C, Clark AD, Janusz CB, Jauregui B, Escobedo-Palza S, et al. Cost-effectiveness analysis of 10- and 13-valent pneumococcal conjugate vaccines in Peru. Vaccine. 2015;33(Suppl 1):A154–66. doi:10.1016/j.vaccine.2014.12.039.
Kieninger MP, Caballero EG, Sosa AA, Amarilla CT, Jauregui B, Janusz CB, et al. Cost-effectiveness analysis of pneumococcal conjugate vaccine introduction in Paraguay. Vaccine. 2015;33(Suppl 1):A143–53. doi:10.1016/j.vaccine.2014.12.078.
Haasis MA, Ceria JA, Kulpeng W, Teerawattananon Y, Alejandria M. Do pneumococcal conjugate vaccines represent good value for money in a lower-middle income country? A cost-utility analysis in the Philippines. PLoS One. 2015;10(7):e0131156. doi:10.1371/journal.pone.0131156.
Gargano LM, Hajjeh R, Cookson ST. Pneumonia prevention during a humanitarian emergency: cost-effectiveness of Haemophilus influenzae type B conjugate vaccine and pneumococcal conjugate vaccine in Somalia. Prehosp Disaster Med. 2015;30(4):402–11. doi:10.1017/s1049023x15004781.
Komakhidze T, Hoestlandt C, Dolakidze T, Shakhnazarova M, Chlikadze R, Kopaleishvili N, et al. Cost-effectiveness of pneumococcal conjugate vaccination in Georgia. Vaccine. 2015;33(Suppl 1):A219–26. doi:10.1016/j.vaccine.2014.12.070.
Sibak M, Moussa I, El-Tantawy N, Badr S, Chaudhri I, Allam E, et al. Cost-effectiveness analysis of the introduction of the pneumococcal conjugate vaccine (PCV-13) in the Egyptian national immunization program, 2013. Vaccine. 2015;33(Suppl 1):A182–91. doi:10.1016/j.vaccine.2014.12.044.
Ordóñez JE, Orozco JJ. Cost-effectiveness analysis of the available pneumococcal conjugated vaccines for children under five years in Colombia. Cost Eff Resour Alloc. 2015;13:6. doi:10.1186/s12962-015-0032-1.
Caldwell R, Roberts CS, An Z, Chen CI, Wang B. The health and economic impact of vaccination with 7-valent pneumococcal vaccine (PCV7) during an annual influenza epidemic and influenza pandemic in China. BMC Infect Dis. 2015;15:284. doi:10.1186/s12879-015-1021-x.
Edejer TT, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans D, et al. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva: World Health Organization; 2003.
World Health Organization Commission on Macroeconomics and Health. Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health. Geneva: World Health Organization; 2001.
Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19(3):187–95.
O’Brien KL, Moulton LH, Reid R, Weatherholtz R, Oski J, Brown L, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet. 2003;362(9381):355–61. doi:10.1016/s0140-6736(03)14022-6.
Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21(9):810–5. doi:10.1097/01.inf.0000027926.99356.4c.
Black S, Shinefield H, Baxter R, Austrian R, Bracken L, Hansen J, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23(6):485–9.
Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344(6):403–9. doi:10.1056/nejm200102083440602.
Pavia M, Bianco A, Nobile CG, Marinelli P, Angelillo IF. Efficacy of pneumococcal vaccination in children younger than 24 months: a meta-analysis. Pediatrics. 2009;123(6):e1103–10. doi:10.1542/peds.2008-3422.
Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367(9512):740–8. doi:10.1016/s0140-6736(06)68304-9.
Klugman KP, Cutts F, Adegbola RA, Black S, Madhi SA, O’Brien KL, et al. Meta-analysis of the efficacy of conjugate vaccines against invasive pneumococcal disease. In: Siber G, Klugman K, Mäkelä P, editors. Pneumococcal vaccines: the impact of conjugate vaccine. Washington DC: ASM; 2008. p. 317–26.
Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case–control study. Lancet. 2006;368(9546):1495–502. doi:10.1016/s0140-6736(06)69637-2.
Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in the Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365(9465):1139–46. doi:10.1016/s0140-6736(05)71876-6.
Castaneda E, Agudelo CI, Regueira M, Corso A, Brandileone MC, Brandao AP, et al. Laboratory-based surveillance of Streptococcus pneumoniae invasive disease in children in 10 Latin American countries: a SIREVA II project, 2000–2005. Pediatr Infect Dis J. 2009;28(9):e265–70. doi:10.1097/INF.0b013e3181a74b22.
Cherian T, Mulholland EK, Carlin JB, Ostensen H, Amin R, de Campo M, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83(5):353–9. doi:10.1590/S0042-96862005000500011.
Polyzos NP, Valachis A, Mauri D, Ioannidis JPA. Industry involvement and baseline assumptions of cost-effectiveness analyses: diagnostic accuracy of the Papanicolaou test. CMAJ. 2011;183(6):E337–43. doi:10.1503/cmaj.101506.
Vučina V, Filipović S, Kožnjak N, Stamenić V, Clark A, Mounaud B, et al. Cost-effectiveness of pneumococcal conjugate vaccination in Croatia. Vaccine. 2015;33(Suppl 1):A209–18. doi:10.1016/j.vaccine.2014.12.043.
Fesenfeld M, Hutubessy R, Jit M. Cost-effectiveness of human papillomavirus vaccination in low and middle income countries: a systematic review. Vaccine. 2013;31(37):3786–804. doi:10.1016/j.vaccine.2013.06.060.
Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–46. doi:10.1056/NEJMoa022823.
Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285(13):1729–35.
Melegaro A, Choi YH, George R, Edmunds WJ, Miller E, Gay NJ. Dynamic models of pneumococcal carriage and the impact of the heptavalent pneumococcal conjugate vaccine on invasive pneumococcal disease. BMC Infect Dis. 2010;10:90. doi:10.1186/1471-2334-10-90.
Rozenbaum MH, Hoek AJ, Hak E, Postma MJ. Huge impact of assumptions on indirect effects on the cost-effectiveness of routine infant vaccination with 7-valent conjugate vaccine (Prevnar). Vaccine. 2010;28(12):2367–9. doi:10.1016/j.vaccine.2010.01.005.
Brisson M, Edmunds WJ. Economic evaluation of vaccination programs: the impact of herd-immunity. Med Decis Making. 2003;23(12583457):76–82.
Clark A, Jauregui B, Griffiths U, Janusz CB, Bolanos-Sierra B, Hajjeh R, et al. TRIVAC decision-support model for evaluating the cost-effectiveness of Haemophilus influenzae type B, pneumococcal and rotavirus vaccination. Vaccine. 2013;31(Suppl 3):C19–29. doi:10.1016/j.vaccine.2013.05.045.
World Health Organization Department of Immunization. Vaccines and biologicals. WHO guide for standardization of economic evaluations of immunization programmes. Geneva: World Health Organization; 2008.
Pitman R, Fisman D, Zaric GS, Postma M, Kretzschmar M, Edmunds J, et al. Dynamic transmission modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—5. Value Health. 2012;15(6):828–34. doi:10.1016/j.jval.2012.06.011.
Cost of Living Index. http://www.numbeo.com/cost-of-living/rankings.jsp. Accessed 30 Sep 2015.
XE Live Exchange Rates. http://www.xe.com. Accessed 30 Sep 2015.
Acknowledgments
The authors thank Miss Paranya Raktanyakan for data extraction assistance with Table A-4, and Miss Mutita Piromyapron for calculation of cost conversions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors declares any conflict of interest.
Author contributions
SS and NC are the overall guarantors for the study. DBCW and NC guided the design of this review. SS developed the search methodology and created the data abstraction sheets. SS conducted the searches and abstracted the data, removed the duplicates, and screened and selected the studies, which were confirmed by AR. SS and AR drafted the article and created the tables and figures with support from NC. All authors have read and approved the manuscript for publication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saokaew, S., Rayanakorn, A., Wu, D.BC. et al. Cost Effectiveness of Pneumococcal Vaccination in Children in Low- and Middle-Income Countries: A Systematic Review. PharmacoEconomics 34, 1211–1225 (2016). https://doi.org/10.1007/s40273-016-0439-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-016-0439-3