Abstract
The National Institute for Health and Care Excellence (NICE) invited Gilead, the company manufacturing ledipasvir-sofosbuvir (LDV/SOF), to submit evidence for the clinical effectiveness and cost effectiveness of LDV/SOF for treating chronic hepatitis C. The School of Health and Related Research (ScHARR) Technology Assessment Group was commissioned as the Evidence Review Group (ERG). This paper describes the company’s submission (CS), the ERG review and the subsequent decision of the NICE Appraisal Committee (AC). The ERG produced a critical review of the clinical effectiveness and cost-effectiveness evidence of LDV/SOF based upon the CS. The clinical effectiveness data for LDV/SOF were taken from ten trials: three phase III trials and seven phase II trials. Trials compared different durations of LDV/SOF, with and without ribavirin (RBV). There were no head-to-head trials comparing LDV/SOF with any comparator listed in the NICE scope. Data from the trials were mostly from populations with genotype 1 (GT1) disease, although some limited data were available for populations with genotypes 3 and 4. For GT1 treatment-naïve patients, sustained viral response for 12 weeks (SVR12) rates for LDV/SOF ranged from 93.1 to 99.4 % for subgroups of patients with non-cirrhotic disease, whilst SVR rates of 94.1 to 100 % were reported for subgroups of patients with compensated cirrhosis. For GT1 treatment-experienced patients, SVR12 rates ranging from 95.4 to 100 % were reported for subgroups of non-cirrhotic patients, and SVR rates ranging from 81.8 to 100 % were reported within subgroups of patients with compensated cirrhosis. Comparator data were not searched systematically as part of the submission, but were based on the company’s previous NICE submission of sofosbuvir, with additional targeted searches. The ERG’s critical appraisal of the company’s economic evaluation highlighted a number of concerns. The ERG’s base case analyses suggested that the incremental cost-effectiveness ratios (ICERs) for LDV/SOF (+RBV) are dependent on (a) treatment durations, (b) whether patients have been previously treated and (c) whether patients have liver cirrhosis or not. The AC concluded that it was appropriate to use the approach taken in the ERG’s exploratory analyses, in line with the marketing authorisation, which considered people with and without cirrhosis separately, and estimated the cost effectiveness for each recommended treatment duration of LDV/SOF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The clinical effectiveness data for ledipasvir-sofosbuvir (LDV/SOF) were taken from ten trials: three phase III trials and seven phase II trials. Trials compared different durations of LDV/SOF, with and without ribavirin (RBV). Data from the trials were mostly from populations with genotype 1 disease, although some limited data were available for populations with genotypes 3 and 4. |
The analysis suggested that the incremental cost-effectiveness ratios for LDV/SOF (+RBV) are dependent on duration of treatment, whether the patients are previously treated and cirrhosis status. In particular, the treatment duration chosen [within the European Medicine Agency (EMA)-recommended treatment durations] for the corresponding patient group has a marked impact on the cost-effectiveness results. |
1 Introduction
The National Institute for Health and Care Excellence (NICE) is an independent organisation responsible for providing national guidance on promoting good health and preventing and treating ill health in priority areas with significant impact. Health technologies must be shown to be clinically effective and to represent a cost-effective use of resources to be recommended for use within the National Health Service (NHS) in England. The NICE Single Technology Appraisal (STA) process covers new technologies, within single indications, usually soon after the UK marketing authorisation [1]. Within the STA process, the company provides a written submission, alongside a health economic model which summarises their estimates of the cost effectiveness of the technology. This submission is reviewed by an external academic organisation, the Evidence Review Group (ERG), which consults with clinical specialists to produce an ERG report. After consideration of the company’s submission (CS), the ERG report and testimony from experts and other stakeholders, the NICE Appraisal Committee (AC) formulates their preliminary guidance, on which stakeholders are invited to comment. Following this, a subsequent Appraisal Consultation Document (ACD) may be produced or a Final Appraisal Determination (FAD) issued, which is open to appeal.
This paper provides a summary of the CS [2], the ERG report [3] and the subsequent development of the NICE guidance for the use of ledipasvir-sofosbuvir (LDV/SOF) for treating chronic hepatitis C in England. Full details of all relevant appraisal documents, including the NICE scope, ERG report, CS, submissions from other consultees, the FAD and comments from consultees and commentators, can be found on the NICE website [4].
2 The Clinical Condition and Current Treatment
The CS [2] defined chronic hepatitis C as having persistent, detectable serum hepatitis C virus (HCV) ribonucleic acid (RNA) for a period greater than 6 months and stated that untreated patients with chronic hepatitis C are at progressive risk of liver fibrosis, compensated cirrhosis, decompensated cirrhosis, hepatocellular carcinoma and death, as well as extrahepatic diseases. The CS [2] also stated that chronic hepatitis C is a common cause of liver cirrhosis and a common indication for liver transplantation in Europe.
There are six major HCV RNA genotypes (GT1–6). Sentinel surveillance data in England from 2009 to 2013 show GT1 (45 %) and GT3 (45 %) predominating, with other genotypes, including GT4, comprising just 10 % of infections. LDV/SOF holds a European marketing authorisation for patients with GT1 and GT4 chronic hepatitis C, and LDV/SOF with the addition of ribavirin (RBV) is licensed for GT3 patients with cirrhosis and/or prior treatment failure [5].
At the time of submission, the CS [2] stated that current relevant treatment options include pegylated interferon (PEG-IFN), sofosbuvir (SOF), simeprevir (SMV), telaprevir (TVR), RBV and boceprevir (BOC). Other treatment options that have been licensed subsequently (e.g. daclatasvir and ombitasvir-paritaprevir-ritonavir with or without dasabuvir) were not included as comparators in the CS [2].
3 The Technology
Ledipasvir (LDV) is an HCV inhibitor targeting the HCV NS5A protein, and SOF is a pan genotypic inhibitor of the HCV NS5B RNA-dependent RNA polymerase. SOF is a nucleotide prodrug that undergoes intracellular metabolism to form the pharmacologically active uridine analogue triphosphate (GS 461203), which, when incorporated into HCV RNA by the NS5B polymerase, acts as a chain terminator. According to the CS [2], GS 461203 (the active metabolite of SOF) is neither an inhibitor of human deoxyribonucleic acid (DNA) and RNA polymerases nor an inhibitor of mitochondrial RNA polymerase.
LDV/SOF is administered in tablet form. Each tablet contains 90 mg LDV and 400 mg SOF. The recommended dose is once daily with or without food and the recommended course is either 8 weeks, 12 weeks or 24 weeks, depending on the patient’s genotype, their cirrhosis status and whether they have failed prior treatment [5]. The list price for a 28-day pack of LDV/SOF tablets is £12,993.33 [6]. The CS [2] stated that there is no requirement for response-guided therapy with LDV/SOF and no tests or investigations are required in addition to current routine hepatitis tests.
4 The Independent Evidence Review Group (ERG) Report
The ERG report [3] comprised a critical review of the evidence for the clinical effectiveness and cost effectiveness of the technology, based upon the CS [2] to NICE. As part of the process, the ERG and NICE had the opportunity to obtain clarification on specific points in the CS [2], resulting in the company providing additional evidence. The ERG used alternative parameter values and assumptions in the model to produce an ERG base case. The evidence presented in the CS [2] and the ERG’s review of that evidence is summarised here.
4.1 Clinical Evidence
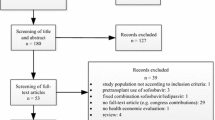
The clinical effectiveness evidence for LDV/SOF was based on ten trials. These comprised three phase III trials and seven phase II trials. Trials compared different durations of LDV/SOF, with and without RBV. There were no head-to-head trials comparing LDV/SOF with any of the comparators listed in the final NICE scope. The phase III trials, ION-1 [7], ION-2 [8] and ION-3 [9], were designed to compare different durations of LDV/SOF with or without RBV, with only historical controls for comparison.
Data from the trials were mostly from populations with GT1 disease, although limited data were also available for populations with GT3 and GT4. Treatment-naïve and treatment-experienced patients were represented within the trials. All ten trials reported sustained virologic response outcomes at 12 weeks post-treatment (SVR12). The phase III trials provided data on resistance, health-related quality of life (HRQoL) and adverse events (AEs). One of the phase II trials also contributed AE data.
For GT1 treatment-naïve patients, SVR12 rates for LDV/SOF ranged from 93.1 to 99.4 % for subgroups of patients with non-cirrhotic disease, whilst SVR rates of 94.1 to 100 % were reported for subgroups of patients with compensated cirrhosis. For GT1 treatment-experienced patients, SVR12 rates ranging from 95.4 to 100 % were reported for subgroups of non-cirrhotic patients and SVR rates ranging from 81.8 to 100 % were reported within subgroups of patients with compensated cirrhosis.
The most common AEs for LDV/SOF-treated patients were fatigue, headache, insomnia and nausea. Across the treatment arms of the phase III trials, 67–93 % of patients experienced at least one AE. Of these, the majority were mild to moderate in severity.
Comparator data were not searched systematically as part of the CS [2], but were instead based on the company’s previous NICE submission of SOF, with additional targeted searches.
4.1.1 The ERG’s Interpretation of Clinical Evidence
The ERG considered that all trials of LDV/SOF relevant to the NICE scope were included in the CS [2]. Despite adopting an open-label design, the three phase III LDV/SOF trials were generally considered to be at a low risk of bias. However, they were designed to compare different durations of LDV/SOF, with or without RBV, and none contained a placebo arm or a comparator without LDV/SOF. The ERG had concerns about the absence of a comparator arm and use of historical controls in the study design. Randomisation was stratified in the phase III trials, allowing a pre-specified investigation of treatment effect by subgroup. The phase II trials had small sample sizes but provided data consistent with the phase III trials.
Comparator data were not searched systematically as part of the submission. Historical controls were selected from single arms of randomised controlled trials (RCTs) or non-RCTs based on the company’s previous NICE submission of SOF, with additional targeted searches. Although reported baseline characteristics appear similar between intervention and comparator trials, the possibility that other factors differed across trials cannot be ruled out.
The approach to searching the evidence base for comparator terms and AEs was not systematic, especially given the use of targeted searches and the absence of a full systematic review. Whilst it is unlikely that there were any major omissions in the studies retrieved, there is potential for some evidence to have been missed, and the overall reporting of the searches was insufficient to allow the ERG to make a fully informed critique of this element of the appraisal.
SVR12 data were used as a measure of treatment effectiveness. Historically, sustained virologic response at 24 weeks post-treatment (SVR24) has been used to measure patient response to therapy. However, research from clinical trials has indicated a high concordance between SVR12 and SVR24 [10, 11], and SVR12 is now considered an appropriate endpoint for regulatory approval [5]. Thus, the ERG considered the use of SVR12 data to be appropriate.
4.2 Cost-Effectiveness Evidence
The CS [2] included a systematic review of published economic evaluations of treatments for hepatitis C. The company’s review was substantial, including 98 unique citations. The main body of the CS [2] summarised the economic comparisons made for the intervention and comparators defined in the NICE scope, including a list of studies in which the intervention was found to be dominant or cost-effective (acceptability criterion unspecified).
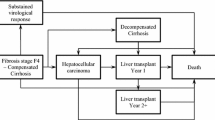
The company also submitted a de novo health economic model to evaluate the cost effectiveness of LDV/SOF ± RBV against relevant comparators for patients with GT1, GT3 and GT4. The company’s model included a total of 12 health states, including two death states, to represent the progression of liver disease and the costs and health benefits associated with curing HCV. All analyses adopted a lifetime horizon. The effectiveness of treatment was driven by SVR12 rates, which were assumed to determine whether cure is achieved, whilst the cost effectiveness of antiviral treatment was driven by the costs and benefits of the antiviral treatment and the avoidance of long-term costs and consequences associated with disease progression. Relative treatment benefits were modelled using naïve indirect comparisons between individual trial arms from multiple studies.
HRQoL was captured within the model by assigning different health utilities to each health state. In addition, the utilities associated with on treatment health states differ for each treatment option; this was intended to reflect the disutility impacts of treatment-specific AEs. The model included costs associated with drug treatment, the management of treatment-related AEs, monitoring and health state costs (e.g. post-treatment monitoring, liver transplantation and post-transplantation follow-up).
The company’s base case analysis included separate economic comparisons for seven subgroups of patients: (i) GT1 treatment-naïve; (ii) GT4 treatment-naïve; (iii) GT1/4 treatment-experienced; (iv) GT3 treatment-naïve; (v) GT3 treatment-naïve with compensated cirrhosis; (vi) GT3 treatment-experienced IFN-ineligible; and (vii) GT3 treatment-experienced IFN-ineligible with compensated cirrhosis. The comparators considered in the company’s economic analysis differed according to the characteristics of the population and the licensed indications for each drug/combination; these include (i) PEG-IFN2a + RBV; (ii) SMV + PEG-IFN2a + RBV; (iii) TVR + PEG-IFN2a + RBV; (iv) BOC + PEG-IFN2b + RBV; (v) SOF + PEG-IFN2a + RBV; (vi) SOF + SMV; (vii) SOF + RBV; and (viii) no treatment.
In the company’s analysis of subgroups of patients with GT1 and GT4 disease, the costs and outcomes of LDV/SOF are based on a “blended” approach. This blended approach involves taking a weighted average of SVR rates (and costs) of LDV/SOF given over different treatment durations based on the company’s assumptions about the expected proportion of patients who would receive each.
The company’s model suggested that for all subgroups, LDV/SOF is expected to be the most effective treatment option (see Table 1 for the company’s cost-effectiveness results for LDV/SOF compared pairwise with each comparator). Within the GT1 treatment-naïve subgroup, the incremental cost-effectiveness ratio (ICER) for LDV/SOF versus PEG-IFN2a + RBV (the next most effective non-dominated option) was estimated to be £7985 per quality-adjusted life-year (QALY) gained. Within the GT4 treatment-naïve subgroup, the ICER for LDV/SOF versus PEG-IFN2a + RBV (the next most effective non-dominated option) was estimated to be £12,715 per QALY gained. Within the GT1/4 treatment-experienced subgroup, the ICER for LDV/SOF versus no treatment (the next most effective non-dominated option) was estimated to be £13,527 per QALY gained. Within the GT3 treatment-naïve subgroup, the ICER for LDV/SOF versus PEG-IFN2a + RBV (the next most effective non-dominated option) was estimated to be £26,491 per QALY gained. Within the GT3 treatment-naïve with compensated cirrhosis subgroup, the ICER for LDV/SOF + RBV versus SOF + PEG-IFN2a + RBV (the next most effective non-dominated option) was estimated to be £46,491 per QALY gained. Within the GT3 treatment-experienced IFN-ineligible subgroup, the ICER for LDV/SOF + RBV versus no treatment was estimated to be £28,048 per QALY gained. Within the GT3 treatment-experienced IFN-ineligible cirrhotic subgroup, the ICER for LDV/SOF + RBV versus SOF + RBV was estimated to be £6210 per QALY gained.
4.2.1 The ERG’s Interpretation of Cost-Effectiveness Evidence
The ERG’s critical appraisal of the company’s economic evaluation highlighted a number of concerns. These included (i) deviations from the NICE scope; (ii) the exclusion of relevant health effects relating to disease transmission and re-infection from the economic model; (iii) the use of naïve indirect comparisons to inform estimates of effectiveness which may be subject to bias and confounding; (iv) the use of a blended approach which takes a weighted average of efficacy and treatment duration for LDV/SOF; (v) uncertainty regarding the HRQoL benefits of LDV/SOF whilst receiving treatment; and (vi) discordance between some of the transition probabilities assumed within the company’s model and those used within previous models to inform appraisals of other antiviral therapies for the treatment of HCV.
The company’s blended approach used a weighted average of SVR rates and treatment durations for different options given over different treatment durations based on the expected proportion of patients who would receive each regimen. Consequently, the mean treatment duration, SVR rates, costs, treatment-specific HRQoL decrement avoided and, ultimately, the cost effectiveness of LDV/SOF were dependent upon the proportion of patients in each part of the “blend”. The ERG considered that the blended analyses presented by the company are of limited value for decision-making as these may result in the simultaneous recommendation of some options which are known to be efficient and other options which are known to be inefficient. The ERG performed “unblended” analyses using the company’s model based on European Medicine Agency (EMA)-recommended treatment durations for LDV/SOF ± RBV [5]; this analysis formed the ERG’s preferred base case.
The ERG undertook the following additional analyses to address issues identified within the critique of the company’s health economic analysis:
-
1.
The development of an ERG-preferred base case using unblended EMA-recommended treatment durations [5] for LDV/SOF ± RBV.
-
2.
The consideration of alternative EMA-recommended treatment durations for LDV/SOF [5].
-
3.
The use of alternative transition probabilities based on the previous SOF STA model [12].
-
4.
The use of on-treatment utility increment derived by Wright et al. [13].
-
5.
The use of shorter time horizons (5 and 10 years) to dampen the company’s assumptions that patients cannot be re-infected after achieving an SVR.
The results presented in Table 2 were produced after the submission of the ERG report [3] as additional analyses were undertaken by the ERG (at the request of the NICE AC). The AC requested that the comparators that were not recommended by NICE or not included in current clinical practice in England be excluded from the incremental analysis.
The ERG-preferred base case analysis suggested the following results. Within the GT1/4 treatment-naïve subgroup, in the non-cirrhotic population, the ICER for 12 weeks of LDV/SOF versus SMV + PEG-IFN2a + RBV (the next most effective non-dominated option) is estimated to be £22,676 per QALY gained; within the cirrhotic population, the ICER for 24 weeks of LDV/SOF versus SOF + PEG-IFN2a + RBV (the next most effective non-dominated option) is estimated to be £45,323 per QALY gained. Within the GT1/4 treatment-experienced subgroup, in the non-cirrhotic population, the ICER for 12 weeks of LDV/SOF versus no treatment (the next most effective non-dominated option) is estimated to be £16,566 per QALY gained; within the cirrhotic population, the ICER for 24 weeks of LDV/SOF versus SOF + PEG-IFN2a + RBV (the next most effective non-dominated option) is £32,458 per QALY gained. Within the GT3 treatment-naïve subgroup, in the non-cirrhotic population, the ICER for 24 weeks of LDV/SOF + RBV versus PEG-IFN2a + RBV (the next most effective non-dominated option) is estimated to be £88,853 per QALY gained; within the cirrhotic population, the ICER for 24 weeks of LDV/SOF + RBV versus SOF + PEG-IFN2a + RBV (the next most effective non-dominated option) is estimated to be £46,149 per QALY gained. Within the GT3 treatment-experienced subgroup, in the non-cirrhotic population, the ICER for 24 weeks of LDV/SOF + RBV versus no treatment (the next most effective non-dominated option) is estimated to be £33,576 per QALY gained; within the cirrhotic population, the ICER for LDV/SOF versus no treatment (the next most effective non-dominated option) is estimated to be £18,238 per QALY gained.
The use of alternative EMA-recommended treatment durations had a substantial impact upon the cost effectiveness of LDV/SOF (see Table 3). Assuming a duration of treatment of 8 weeks for LDV/SOF in the GT1/4 treatment-naïve non-cirrhotic subgroup, the ICER for LDV/SOF versus PEG-IFN2a + RBV (the next most effective non-dominated option) is reduced to £8894 per QALY gained. Assuming a duration of treatment of 12 weeks for LDV/SOF within the GT1/4 treatment-naïve cirrhotic population, the ICER for LDV/SOF versus no treatment (the next most effective non-dominated option) is reduced to £4518 per QALY gained. In the treatment-experienced GT1/4 non-cirrhotic subgroup, using a duration of treatment of 24 weeks for LDV/SOF, the ICER for LDV/SOF versus SMV + PEG-IFN2a + RBV is estimated to be £77,495 per QALY gained.
The ERG’s additional analyses surrounding the company’s transition probabilities and the HRQoL increment associated with achieving SVR also produced different ICERs; however, the overall conclusions of the economic analysis remain unaffected.
The ERG’s analyses which use shorter time horizons resulted in an increase in the ICERs for LDV/SOF (all of which are higher than £75,000 per QALY gained) compared with those estimated in the ERG-preferred base case analyses. This is unsurprising since the benefits are curtailed to a short time horizon yet the costs of treatment are incurred upfront.
5 Conclusions of the ERG report
The ERG base case analyses using an unblended analysis suggested that the ICERs for LDV/SOF (+RBV) are dependent on (a) duration of treatment, (b) whether the patients are previously treated and (c) cirrhosis status. In particular, the treatment duration chosen (within the EMA-recommended treatment durations [5]) for the corresponding patient group has a marked impact on the cost-effectiveness results. In general, the economic profile of LDV/SOF (+RBV) appears considerably more favourable for shorter treatment durations because of their lower cost.
6 Key Methodological Issues Identified by the ERG
The ERG had several concerns regarding the data and assumptions incorporated with the company’s cost-effectiveness analyses and conducted exploratory analyses to quantify the impact of making alternative assumptions and using alternative parameter inputs. Issues which appeared to have the most impact on the ICER were the use of a blended approach and the choice of treatment duration.
7 NICE Guidance
7.1 Key Issues Considered by the Appraisal Committee
The AC reviewed the available evidence on clinical effectiveness and cost effectiveness of LDV/SOF, having considered evidence on the nature of HCV and the value placed on the benefits of LDV/SOF by people with the condition, those who represent them, and clinical experts. It also took into account the effective use of NHS resources.
The AC noted that the clinical effectiveness evidence was associated with considerable uncertainty, namely (i) the clinical study designs (open-label, non-randomised evidence, with no head-to-head studies), (ii) the selection of SVR rates for comparators from single studies, and (iii) the use of an naïve indirect comparison to estimate relative treatment effects.
The AC discussed the treatment durations and clinical effectiveness for LDV/SOF in GT1, GT3 and GT4 patients with and without cirrhosis. The AC was concerned that the company had selected SVR rates from single studies without justification, particularly because this breaks the randomisation and also because no uncertainty associated with them was included in the company’s estimates of cost effectiveness. The AC concluded that the company’s evidence on the relative effectiveness of LDV/SOF (with or without RBV) in people with GT1, GT3 or GT4 HCV was not robust, and that these aspects should be taken into account in decision-making.
The AC noted that the company’s economic model structure grouped mild and moderate chronic hepatitis C into a single health state, and therefore the company’s model distinguished only between people with and without cirrhosis. The clinical experts acknowledged that the model structure was consistent with how people are diagnosed in clinical practice.
The AC noted that the company’s base case analysis presented ICERs for a combined group of people with and without cirrhosis using a weighted-average approach (i.e. a blended approach). The AC was aware that the presence of cirrhosis affects the recommended regimen for LDV/SOF and a person’s likelihood of an SVR with comparator treatments, and therefore the cost effectiveness of treatment with LDV/SOF. The AC concluded that it was appropriate to use the approach taken in the ERG’s exploratory analyses, in line with the marketing authorisation, which considered people with and without cirrhosis separately, and estimated the cost effectiveness for each recommended treatment duration of LDV/SOF.
The AC published preliminary recommendations for consultation and discussed the consultation comments at subsequent AC meetings. The AC also discussed the additional evidence provided by the company in response to consultation, which included cost-effectiveness results for the 12-week, treatment-experienced GT1 or GT4 with cirrhosis group deemed at low risk of clinical disease progression. This resulted in a change in the recommendations for this patient group after the first ACD. Two ACDs and a FAD were produced for this STA.
7.2 Final Guidance
The final NICE guidance published in November 2015 stated that LDV/SOF is recommended as an option for treating chronic hepatitis C in adults, as specified in Table 4.
8 Conclusions
This paper describes the STA on LDV/SOF for treating chronic hepatitis C. The “blended comparisons” in the CS combined some options which were efficient and other options which were inefficient. The ERG performed unblended analyses using the company’s model based on EMA-recommended treatment durations for LDV/SOF (+RBV). Cost effectiveness of LDV/SOF (+RBV) depended on the duration of treatment, whether the patients had been previously treated and their cirrhosis status. LDV/SOF was recommended by NICE as a possible treatment option for subgroups of GT1 and GT4 patients.
References
National Institute for Health and Clinical Excellence (NICE) Guide to the methods of technology appraisal. London, NICE 2013 Available at: http://www.nice.org.uk/. 2008.
Gilead Sciences Inc. Manufacturer/sponsor submission of evidence for Single Technology Appraisal (STA) of ledipasvir-sofosbuvir for treating chronic hepatitis C [ID742]—Company submission. 2014.
Thokala P, Simpson E, Tappenden P, Stevens JW, Dickinson K, Ryder S et al. Ledipasvir-sofosbuvir for treating chronic hepatitis C: a Single Technology Appraisal, School of Health and Related Research (ScHARR), 2015. 2015.
National Institute for Health and Care Excellence, Single Technology Appraisal (STA): ledipasvir-sofosbuvir for treating chronic hepatitis C [ID742]. 2015.
European Medicine Agency. European Public Assessment Report—Harvoni. London: EMA; 2014.
National Institute for Health and Care Excellence, Final appraisal determination ledipasvir-sofosbuvir for treating chronic hepatitis C [ID742]. 2015.
Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–98.
Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–93.
Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–88.
Martinot-Peignoux M, Stern C, Maylin S, Ripault MP, Boyer N, Leclere L, et al. Twelve weeks posttreatment follow-up is as relevant as 24 weeks to determine the sustained virologic response in patients with hepatitis C virus receiving pegylated interferon and ribavirin. Hepatology. 2010;51(4):1122–6.
Chen J, Florian J, Carter W, Fleischer RD, Hammerstrom TS, Jadhav PR, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology. 2013;144(7):1450–5.
National Instiute for Health and Care Excellence. Hepatitis C (chronic)—sofosbuvir: consultation document. ID654. London: NICE; 2014.
Wright M, Grieve R, Roberts J, Main J, Thomas HC. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess. 2006; 10(21):1–113, iii.
Contributions of Authors
Emma Simpson critiqued the clinical effectiveness evidence data reported by the company. Kath Dickinson critiqued the searches undertaken by the company. John Stevens critiqued statistical analyses undertaken by the company. Praveen Thokala and Paul Tappenden critiqued the mathematical model provided and the cost‐effectiveness evidence submitted by the company. Praveen Thokala undertook the additional analyses by the ERG. Dr Steve Ryder and Dr Phillip Harrison provided clinical advice to the ERG throughout the project. All authors were involved in drafting and commenting on the final version to be published. This summary has not been externally peer reviewed by PharmacoEconomics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (Project Number 14/59/01 STA). See the HTA programme website for further project information (http://www.hta.ac.uk). This summary of the ERG report was compiled after NICE issued the FAD. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of NICE or the Department of Health.
Conflict of interest
Dr. Steve Ryder has attended paid advisory boards for Gilead and other companies with products in this therapeutic area (Roche, MSD, Abbvie, Janssen and Novartis). None of the other authors have any conflicts to declare.
Rights and permissions
About this article
Cite this article
Thokala, P., Simpson, E.L., Tappenden, P. et al. Ledipasvir-Sofosbuvir for Treating Chronic Hepatitis C: A NICE Single Technology Appraisal—An Evidence Review Group Perspective. PharmacoEconomics 34, 741–750 (2016). https://doi.org/10.1007/s40273-016-0387-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-016-0387-y