Abstract
For a long time, pediatric heart failure (HF) with preserved systolic function (HFpEF) has been noted in patients with cardiomyopathies and congenital heart disease. HFpEF is infrequently reported in children and instead of using the HFpEF terminology the HF symptoms are attributed to diastolic dysfunction. Identifying HFpEF in children is challenging because of heterogeneous etiologies and unknown pathophysiological mechanisms. Advances in echocardiography and cardiac magnetic resonance imaging techniques have further increased our understanding of HFpEF in children. However, the literature does not describe the incidence, etiology, clinical features, and treatment of HFpEF in children. At present, treatment of HFpEF in children is extrapolated from clinical trials in adults. There are significant differences between pediatric and adult HF with reduced ejection fraction, supported by a lack of adequate response to adult HF therapies. Evidence-based clinical trials in children are still not available because of the difficulty of conducting trials with a limited number of pediatric patients with HF. The treatment of HFpEF in children is based upon the clinician’s experience, and the majority of children receive off-level medications. There are significant differences between pediatric and adult HFpEF pharmacotherapies in many areas, including side-effect profiles, underlying pathophysiologies, the β-receptor physiology, and pharmacokinetics and pharmacodynamics. This review describes the present and future treatments for children with HFpEF compared with adults. This review also highlights the need to urgently test new therapies in children with HFpEF to demonstrate the safety and efficacy of drugs and devices with proven benefits in adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pediatric heart failure with preserved ejection fraction (HFpEF) is an important clinical condition with high morbidity and mortality. |
The causes of HFpEF in children are heterogeneous and include cardiomyopathies (restrictive and hypertrophic), congenital heart disease (especially after Fontan), cancer therapy including radiotherapy and chemotherapy, human immunodeficiency virus infection, renal failure, obesity, and hereditary hemolytic anemia, among many etiologies. |
The etiologies, risk factors, clinical course, biomarkers, and treatments in children with HFpEF are different from adults. |
There is a lack of prospective randomized trials in children and no evidence-based guidelines or consensus statements on the therapeutic approach to HFpEF in children. |
1 Introduction
Heart failure can occur with preserved ejection fraction (HFpEF) or reduced ejection fraction (HFrEF). Heart failure (HF) with preserved ejection fraction is also known as diastolic HF, or HF with abnormal relaxation of the ventricular myocardium with primarily preserved left ventricular (LV) systolic function (LV ejection fraction [LVEF] > 50%) or mildly reduced LVEF [1]. The word ‘preserved’ was initially used to encompass all patients with an LVEF > 40%. A logical description of the HF syndrome where the LVEF is not severely reduced (< 40%), but the symptoms are disproportionate to mildly reduced EF, is to describe it as “HF with normal EF” [2]. Pediatric HFpEF although recognized as a component of cardiomyopathies and congenital heart diseases (CHD), has been less well understood and investigated. There are several reasons for this: (i) diastolic dysfunction is believed to overlap with systolic LV function [3], (ii) there is no single measure such as LVEF that adequately describes the diastolic function, and (iii) Doppler patterns that characterize diastolic function vary significantly with age and HR in children [4]. Despite these challenges, recently, there has been increasing recognition of HFpEF in children with cardiomyopathies [5, 6], single-ventricle physiology (Fontan) [7,8,9], following cardiac transplantation [10,11,12,13], sepsis [14,15,16], chronic renal disease [17,18,19,20], obesity [21], diabetes mellitus [22, 23], obstructive sleep apnea [24, 25], after anthracycline exposure for childhood cancer [26,27,28,29], hereditary hemolytic anemias [26, 27], human immunodeficiency virus infection [28, 29], and exposure to antiretroviral therapies [30, 31]. This review describes the present and future treatments for children with HFpEF compared with adults. Additionally, this review highlights the need to urgently test new therapies in children with HFpEF to demonstrate the safety and efficacy of drugs and devices proven beneficial in adults.
2 HFpEF in Children
A paradigm has been established in children and adults where diastolic function progresses from normal to impaired relaxation, with an intermediate phase of increased filling pressures, and ultimately progresses to HFpEF, which is well described in patients receiving chemotherapy [32, 33]. The diagnosis of HFpEF requires clinical symptoms and signs of HF and evidence of diastolic dysfunction with normal or mildly reduced LVEF. Unlike systolic function, assessment of diastolic function in children is challenging. Conventionally, Doppler parameters such as mitral valve inflow, pulmonary venous flow, LV systolic-to-diastolic duration ratio, myocardial performance indices, and left atrium (LA) size are used to assess LV diastolic dysfunction [34]. However, this conventional echocardiography and Doppler imaging parameters do not correlate well with invasive hemodynamics in pediatric HFpEF [35]. The LV diastolic function can be better determined by tissue Doppler imaging (TDI), evaluating the longitudinal movement at the mitral, tricuspid, and septal annulus levels, calculating early and late diastolic velocities (eʹ and aʹ, respectively), and comparing these with reference values for children [36]. However, evaluating diastolic function in neonates and younger children is still challenging because of maturational changes in diastolic parameters [37]. In 2016, American Society of Echocardiography guidelines for evaluation of diastolic dysfunction in adults included four variables: (1) e′ velocity, (2) E/e′ ratio, (3) LA volume indexed to body surface area, and (4) tricuspid regurgitation peak velocity [38]. The cut-off values of these four criteria are as follows: septal e′ < 7 cm/s or lateral e′ < 10 cm/s; average E/e′ > 14, LA volume indexed to body surface area > 34 cc/m2; and tricuspid regurgitation peak velocity > 2.8 m/s. However, the adult criteria are not validated and poorly correlate with LV filling pressure in children [39]. Despite these limitations, TDI has often been shown to be helpful in several studies in children to characterize LV diastolic dysfunction [40,41,42,43,44,45,46]. Speckle-tracking echocardiography and strain analysis, pressure-volume analysis, and cardiac magnetic resonance imaging are superior methods to determine diastolic dysfunction and are feasible in children [47,48,49,50]. Cardiac catheterization measurements of LV end-diastolic pressure (LVEDP) best diagnose HFpEF at rest and are more apparent after an intravenous fluid challenge. However, cardiac catheterization is an invasive procedure, and serial cardiac catheterization is not suitable for clinical surveillance for HFpEF in children.
2.1 Cardiomyopathy
Two common causes of HFpEF in children are hypertrophic and restrictive cardiomyopathies. Despite the lack of a standardized protocol or guidelines for TDI in children, it is helpful in pediatric patients to predict cardiac events in dilated cardiomyopathy [51, 52], characterize LV diastolic function in LV non-compaction cardiomyopathy [40, 41], estimate accurately LVEDP in hypertrophic [43, 44] and restrictive cardiomyopathies [45], and distinguish restrictive cardiomyopathy from constrictive pericarditis [46]. Speckle-tracking echocardiography and strain analysis provide a high-resolution real-time measure of LV contractility and relaxation in children and can differentiate between the athletic heart and hypertrophic cardiomyopathy [47].
2.2 Congenital Heart Disease
In children, diastolic dysfunction associated with CHD can be due to three pathophysiologies: pressure overload, volume overload, and both pressure and volume overload [53]. After CHD surgery, ventricular geometry, especially the systemic right ventricle geometry, can be profoundly altered. The diastolic dysfunction in CHD may appear immediately after surgery, which can be transient or progress to HFpEF [54,55,56]. Assessment of diastolic dysfunction using echocardiography is complex in children with CHD because most parameters are affected by the patient’s age, HR, and type of cardiac defect [57, 58]. The interpretation of diastolic function in the context of CHD requires some understanding of the effects of the lesions themselves on Doppler echocardiographic parameters [59]. A multi-modality imaging approach combining different echocardiographic and cardiac magnetic resonance parameters with newer parameters such as diastolic strain rate may facilitate early diagnosis of HFpEF [60].
2.3 Other Acquired Cardiovascular Diseases
Comorbidities such as obesity, malnutrition, hyperlipidemia, diabetes, sepsis, and chronic renal disease drive LV remodeling and dysfunction in adults and children through a complex interaction with systemic inflammation, coronary microvascular endothelial dysfunction, and immune dysfunction [61, 62]. The latter affects LV diastolic dysfunction through macrophage infiltration, resulting in interstitial fibrosis. The inflammatory changes and endothelial dysfunction can produce reactive oxygen species, limiting nitric oxide (NO) bioavailability for adjacent cardiomyocytes. Limited NO bioavailability promotes the development of HFpEF by causing a deficiency in NO-cyclic guanosine monophosphate signaling, which may further alter ventricular mechanical properties [63, 64].
Adeniran et al. [65] studied the impaired calcium (Ca2+) homeostasis and sodium channel (INa) remodeling and reported a decreased systolic cytosolic Ca2+ level and elevated diastolic Ca2+ level inside the cardiomyocytes in a multilevel model for electro-mechanics of the LV in HFpEF. The cyclical changes in Ca2+ concentration within cardiomyocytes control cardiac contraction and relaxation cycles, and dysregulation of Ca2+ handling processes leads to systolic dysfunction, diastolic dysfunction, and adverse remodeling [66]. Selby et al. [67] carried out a study to evaluate tachycardia-induced relaxation abnormalities in the myocardium from adult patients with a normal LVEF. They observed incomplete relaxation with increased diastolic tension development at rising pacing rates, significantly elevated resting tone, and disproportionately high Ca2+ loads due to a reduced sarcolemmal Ca2+ extrusion reserve.
3 Treatment of HFpEF
3.1 Pharmacological Therapy
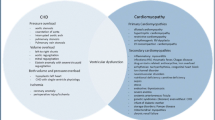
Numerous treatments exist for HFrEF, and most of these therapies may work in HFpEF with different doses and by different mechanisms. Because of the rarity and the heterogeneous nature of pediatric HFpEF, there are no clinical trials and research studies have not focused on this patient population. The treatment of HFpEF should begin with managing risk factors such as comorbidities, including reduction of weight, regular aerobic exercise, and control of hypertension, diabetes, and hyperlipidemia. The medical treatment of HFpEF in children can effectively target the underlying pathophysiologic mechanism based on the (limited) observations from the adult literature and summarized in Fig. 1. However, this approach may not work in children with HFpEF because there are differences in cellular and molecular signaling between failing pediatric and adult hearts. The following section focuses on the pharmacotherapies for HFpEF in children based on the clinical trials in adults but minimal pediatric studies.
Proposal for potential treatment targets in pediatric heart failure with preserved systolic function (HFpEF) based on limited data in children and studies in adult patients with HFpEF. ACE angiotensin-converting enzyme, ARB angiotensin receptor blocker, ARNI angiotensin receptor blocker-neprilysin inhibitor, AT1 angiotensin receptor 1, cGMP guanosine 3ʹ,5ʹ-cyclic monophosphate, CHD congenital heart disease, HMG-CoA hydroxymethylglutaryl coenzyme A, IL-6 interleukin-6, IL-1β interleukin-1-beta (a cytokine protein also known as lymphocyte activating factor), LA left atrium, LVEDP left ventricular end-diastolic pressure, NO nitric oxide, PDE phosphodiesterase, PKG protein kinase G (receptor for cGMP second messenger), RV right ventricle, sGC soluble guanylate cyclase, SGLT-2 sodium-glucose transport protein 2, SNS sympathetic nervous system, sST2 soluble suppression of tumorigenesis-2 (released in response to inflammatory stimuli and vascular congestion), TGF-β transforming growth factor-beta, TNF-α tumor necrosis factor-alpha
3.1.1 Diuretics
Despite a lack of robust evidence, diuretics have been the mainstay of HFpEF management and are recommended to relieve symptoms due to volume overload. The common pathway of HFpEF is elevated LVEDP, resulting in pulmonary venous congestion, which ultimately results in exercise intolerance and dyspnea. Loop diuretics, such as furosemide, torsemide, and bumetanide, are often used as first-line therapy to improve symptoms, maintain a euvolemic state, shifting the pressure-volume relation downward, relieve symptoms, and improve the quality of life [68]. Thiazide diuretics such as metolazone can be used as alternatives. However, diuretics must be used judiciously to find a balance in ventricular preload and afterload. Moreover, excess diuresis in patients with CHD after Fontan with diastolic dysfunction can result in a sudden drop in stroke volume and cardiac output. If always possible, long-term diuretics and fluid restriction are avoided because intravascular volume deficiency may further stimulate the neurohumoral axis, including the renin-angiotensin-aldosterone system (RAAS).
3.1.2 Spironolactone
The extracellular matrix in the myocardium is composed of fibrillary proteins (such as collagen and elastin), non-fibrillary proteins (such as aminoglycans, fibronectin, and laminin), and bioactive proteins such as transforming growth factor-β, matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases, and matricellular proteins. The homeostatic control of collagen is crucial for diastolic function. Spironolactone, an aldosterone antagonist, has decreased collagen synthesis by inhibiting fibroblast proliferation in animal models of experimental hypertension [69, 70]. These experimental data have shown promising results that spironolactone can improve myocardial relaxation. Spironolactone in a low non-diuretic dosage is beneficial preferentially as an anti-remodeling drug in children with HFpEF [54]. The beneficial effect of spironolactone may also be due to the afterload reduction, changes in serum electrolytes (potassium-sparing effect), and the reduction in LV mass [71, 72].
3.1.3 RAAS Antagonists
The RAAS plays an integral role in the pathogenesis of chronic HF and LV remodeling [73,74,75,76]. The RAAS can stimulate metalloproteinases and promote endothelial dysfunction, and it results in myocyte hypertrophy, fibrosis, and reduced ventricular compliance (stiffness) in experimental animals and clinical studies [77,78,79]. Ventricular hypertrophy, fibrosis, and resultant diastolic dysfunction increase myocardial oxygen consumption and imbalance myocardial oxygen supply and demand. Angiotensin II and aldosterone also can cause cardiomyocyte hypertrophy independent of hypertension-associated wall stress increase through upregulation of nicotinamide adenine dinucleotide phosphate (NADP) oxidase within the myocytes [78, 79]. Angiotensin-converting enzyme (ACE) inhibitors (e.g., enalapril, lisinopril, and perindopril) or angiotensin-II receptor blockers (e.g., losartan) along with aldosterone antagonists (e.g., spironolactone) block the activation of the RAAS and decrease adrenergic activity [80]. Angiotensin-converting enzyme inhibitors have the properties for reverse remodeling, reducing systemic vascular resistance, and improving vascular compliance [81]. The remodeling properties seem to be higher, especially with ACE inhibitors with an effective tissue penetration such as lisinopril [82, 83]. However, a large trial testing neurohormonal inhibition in infants and children with single-ventricle physiology failed to achieve positive outcomes [84]. Nonetheless, these drugs are commonly used to improve symptoms in children with HFpEF.
3.1.4 Beta-Blockers
Beta-blockers are often prescribed in HFpEF to treat comorbidities such as coronary artery disease and atrial fibrillation. Beta-blockers are thought to exert their action by reducing the impact of prolonged neurohormonal activation [85]. However, different types of β-blockers affect the HF phenotype in children differently from adults as in adult hearts, there is predominantly β1-receptor downregulation. In contrast, children have downregulation of both the β1-receptors and β2-receptors [86]. Beta-blockers decrease heart rate, improve LV diastolic filling, increase cardiac output, and prevent arrhythmias in hypertrophic cardiomyopathies with or without obstruction. Recently, a novel inhibitor of cardiac-specific myosin adenosine triphosphatase, mavacamten, has been shown to reduce LV outflow tract obstruction and improve myocardial relaxation by improving myocardial energetics [87, 88].
3.1.5 Calcium Channel Blockers
Although calcium channel blockers such as verapamil do not specifically improve diastolic function in the short term, they have improved diastolic filling during exercise in adults with HFpEF [89]. A significant increase in exercise capacity due to increased ventricular filling was observed after 5 weeks of therapy with verapamil compared with placebo, with no change in baseline systolic function and systolic blood pressure, in adults with HFpEF [90, 91]. Calcium channel blockers have also been found to reduce ventricular mass and improve LV relaxation in hypertrophic cardiomyopathies [91].
3.1.6 Inotropic Agents: Milrinone and Levosimendan
Milrinone, a phosphodiesterase-3 inhibitor, is commonly used in children for chronic HF because of its positive inotropic and lusitropic actions. Prophylactic intravenous use of high-dose milrinone after cardiac surgery in children led to a significant reduction in the prevalence of low cardiac output syndrome [92]. In clinical practice, milrinone is used commonly in pediatric patients with HFpEF and demonstrates symptomatic improvement [93].
Levosimendan is a calcium-sensitizing agent that binds to troponin C, enhancing its sensitivity to intracellular calcium, and has positive inotropic action. It also opens up the adenosine triphosphate-dependent potassium channels leading to smooth muscle relaxation, vasodilation, and decreased systemic vascular resistance. The hemodynamic effects of levosimendan include increased cardiac output and decreased filling pressure [94]. The drug causes an increase in contractility without an increase in myocardial oxygen demand and has lusitropic action on the myocardium. Prophylactic short-term administration of intravenous levosimendan led to mixed results in children who had undergone heart surgery in prior studies. Pediatric patients who received levosimendan are divided into two groups: the first group who received levosimendan as prophylaxis for low cardiac output in the post-operative period [95, 96] for whom there was no significant benefit of the drug; and the second group with end-stage HF and inotrope dependency [97, 98] showed improved status in terms of a decrease in additional inotrope requirements and hospital length of stay.
3.1.7 Angiotensin Receptor-Neprilysin Inhibitor
A combination of an angiotensin receptor blocker and neprilysin inhibitor has the advantage of concomitantly blocking a pro-fibrotic/pro-hypertrophic mechanism (angiotensin receptor blocker component, valsartan) while stimulating an anti-fibrotic/anti-hypertrophic mechanism (neprilysin inhibitor component, sacubitril) [99]. This combination drug has natriuretic and diuretic properties, better preserves renal function, better controls blood pressure than RAAS inhibitors, and improves ventricular-arterial coupling [100]. Consequently, an angiotensin receptor-neprilysin inhibitor provides better target organ protection than angiotensin receptor blocker therapy alone, including cardiac, vascular, and renal protection. The efficacy of sacubitril/valsartan was superior to valsartan alone in hospitalized adults with HFrEF [101]. Furthermore, sacubitril/valsartan favorably altered the extracellular matrix homeostasis and was expected to benefit adults with HFpEF [102]. However, the PARAGON-HF trial found that sacubitril/valsartan did not reduce mortality or hospitalization in adults with HFpEF [103]. In October 2019, the US Food and Drug Administration approved the use of sacubitril/valsartan in children aged > 1 year with symptomatic HFrEF [104]. The jury is still out regarding the role of sacubitril/valsartan in children with HFpEF.
3.1.8 Sodium-Glucose Cotransporter Type 2 Inhibitors
The DAPA-HF trial [105] and the EMPEROR-Reduced trial [106] showed sodium-glucose cotransporter type 2 inhibitors (dapagliflozin and empagliflozin) reduced the risk of worsening major cardiac events in adults with HFrEF irrespective of the presence of diabetes. The precise mechanism of sodium-glucose cotransporter type 2 inhibition in achieving the beneficial effects remains uncertain, although a modest reduction in central venous pressure has been demonstrated [107, 108]. One possible mechanism is empagliflozin increases natriuretic peptides and causes significant diuresis. The EMPULSE trial in hospitalized adults with acute decompensated HF either due to HFrEF or HFpEF and irrespective of diabetic status found decreased major cardiac adverse events and hospitalization over 90 days [109]. The experimental work suggests an antiapoptotic effect mediated via sarcolemmal sodium hydrogen cotransporter blockade [110]. Other benefits of the drug include reduced LV filling pressures and LV afterload, improved vascular function, myocardial efficiency by permitting fatty acid-based myocardial metabolism, and reduced oxidative stress and inflammatory cytokine production [108, 111]. No data are currently available for sodium-glucose cotransporter type 2 inhibitor use in children.
3.1.9 Nitric Oxide Donors
There is accumulating evidence indicating diastolic dysfunction is associated with a coronary vascular endothelial impairment through impaired NO production, increased NO degradation, and vascular smooth muscle hyporesponsiveness to NO [112]. Therefore, increasing NO-cyclic guanosine monophosphate GMP signaling by phosphodiesterase-5 inhibition would improve diastolic function by increasing NO in the coronary endothelium [113]. Nevertheless, the RELAX trial, which used sildenafil to treat adults with HFpEF, showed no significant improvement in exercise capacity or clinical status [114]. However, other studies show that NO improves LV relaxation, decreases filling pressure, and improves diastolic function in adults [115, 116]. In myocytes, when there is low cofactor tetrahydrobiopterin (BH4), NO synthase produces superoxide rather than NO. This situation is called NO synthase uncoupling and results in diastolic dysfunction independent of vascular uncoupling [117]. Additionally, in experimental models, supplementation with oral BH4 prevented or reversed the diastolic cardiac dysfunction [118]. Currently, there are no pediatric studies with NO modulators. Data from the animal models and trials in adults with HFpEF suggest a future role of NO modulators such as vericiguat in children with HFpEF [119].
3.1.10 Ranolazine
Ranolazine is a partial fatty acid oxidation inhibitor that shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation. Because glucose oxidation requires less oxygen than the oxidation of fatty acids, ranolazine can help maintain myocardial function in times of ischemia. Ranolazine has shown some promise as a treatment for diastolic dysfunction in adults. In the mice model of hypertension-induced diastolic dysfunction, ranolazine reversed diastolic dysfunction, probably resulting from direct effects on myofilaments [120]. Ranolazine inhibits the ryanodine receptor decreasing the late I(Na+) current and lowering cellular Na+ and Ca2+ levels during diastole to improve active relaxation and passive diastolic compliance [121]. Infusion of ranolazine in adults with HFpEF resulted in a modest decrease in LVEDP in the randomized, RALI-diastolic HF trial [122]. Unfortunately, ranolazine has not yet been evaluated in children with HFpEF.
3.1.11 Hydroxymethylglutaryl Coenzyme A Reductase Inhibitors
Metabolic comorbidities such as diabetes and hyperlipidemia are presumed to worsen the severity of HFpEF through a cascade of events from systemic inflammation, perturbing the physiology of the endothelium and the perivascular environment, and immune dysfunction that ultimately converges to myocardial fibrosis in both children and adults [61, 123]. The hydroxymethylglutaryl coenzyme A reductase inhibitors (statins) decrease reactive nitrogen species and reactive oxygen species derived from NADP oxidases, balance endothelial redox, and restore NO bioavailability, independently of low-density lipoprotein lowering in adults with HFpEF [124]. Statins are found to reduce all-cause mortality in adults with HFpEF regardless of the serum cholesterol level and presence of coronary artery disease [125,126,127]. Limited data support the efficacy of hydroxymethylglutaryl coenzyme A inhibitors such as atorvastatin or rosuvastatin in pediatric heart transplant recipients because of their anti-inflammatory properties in addition to lowering cholesterol [128].
3.1.12 Other Anti-Inflammatory Drugs
Recently, experimental models of HFpEF have demonstrated compelling evidence for bidirectional interaction between metabolic stress and chronic inflammation, resulting in alterations in systemic and cardiac immune responses that have been shown to participate in HFpEF pathophysiology [61]. There is also evidence of elevated circulating inflammatory biomarkers such as interleukin-1, C-reactive protein, tumor necrosis factor-α, and soluble suppression of tumorigenesis-2 in HFpEF. Inflammatory cells express transforming growth factor β, interferon-γ, Galectin-3, connective tissue growth factor, and ACE, promoting the conversion of fibroblasts to myofibroblasts and collagen deposition [129]. Anti-inflammatory agents (such as anakinra and canakinumab) and anti-fibrotic agents (such as pirfenidone) have been found helpful in adults with HFpEF [130, 131], but no pediatric studies or data are currently available.
3.2 Device Therapy in HFpEF
Several types of atrial shunts, LV expanders, simulation-based therapies, and mechanical circulatory support devices are currently under development to target one or more of the symptoms in patients with HFpEF [132]. Although most of these solutions have shown promising results in clinical or preclinical studies, no device-based therapy has yet been approved to treat patients with HFpEF.
3.2.1 Atrial Shunt Devices
Atrial shunt devices are designed to lower LA pressure by connecting the LA to other cardiac chambers or the aorta [133]. Many atrial shunt devices, the V-Wave shunt (V-Wave Ltd., Agoura Hills, CA, USA), and the transcatheter atrial shunt system (Edwards Lifesciences, Irvine, CA, USA) are currently under investigation and have shown promising results [134]. A transcatheter interatrial left-to-right shunt in adults with HFpEF has been shown to offset the high LA pressure that develops in HFpEF [135, 136]. Trials with interatrial shunt device outcomes have demonstrated the safety of these devices, with increased exercise tolerance, quality of life, and a trend toward decreased hospitalizations and HF symptoms [137,138,139]. The Occlutech Atrial Flow Regulator is a self-expandable nitinol mesh braided into two flat discs, which can be introduced percutaneously. The atrial shunt created can have various diameters (6, 8, and 10 mm) and is designed to allow an inter-atrial bidirectional flow [140]. In the future, it will be helpful to do clinical trials of these devices in children with HFpEF.
3.2.2 CardioMEMS Device
In patients with HFpEF, post-capillary pulmonary hypertension is common. Continuous monitoring of hemodynamics through an implanted device such as a CardioMEMS device (St Jude Medical, Saint Paul, MN, USA) allows for assessing LV filling pressure and appropriate administration of diuretics [141]. The CHAMPION trial in adult patients with HF (20–23% with LVEF ≥ 40%) found reduced hospitalizations with this device by alerting physicians to high pulmonary pressures and directing subsequent changes to medicines [142].
3.2.3 Cardiac Synchronization Therapy
Atrioventricular conduction disturbances are often seen in the setting of chronic HF. These conduction disturbances produce suboptimal ventricular filling due to atrioventricular dyssynchrony. Cardiac synchronization therapy has improved symptoms and reduced mortality in adult patients with HFpEF and electrical or mechanical dyssynchrony [143, 144]. Targeting LV dyssynchrony with an implanted cardiac resynchronization device may be helpful. Other experimental devices, such as cardiac contractility modulation devices, including baroreceptor activation therapy and the BAROSTIM NEO system (CVRx, Inc., Minneapolis, MN, USA), have also been tried to improve atrioventricular dyssynchrony [145, 146].
3.2.4 Left-Ventricular Expander Devices
In HFpEF, there is diastolic dysfunction and elevated LVEDP. Left-ventricular expanders devices such as ImCardia® (CorAssist Cardiovascular Ltd, Haifa, Israel) and the CORolla® TAA (CorAssist Cardiovascular Ltd) are under clinical evaluation in adults. These volume expander devices store elastic energy during systole and transfer it to the LV wall during diastole, thereby improving early diastolic refill, an active relaxation phase of the cardiac cycle [147]. The CORolla® TAA has advantages over ImCardia® in that a minimally invasive implantation procedure installs it. There is an ongoing trial of CORolla® TAA evaluating its safety, feasibility, and efficacy in adult patients with HFpEF (NCT02499601) [148].
3.2.5 Miscellaneous Devices
Renal denervation, a catheter-based radiofrequency ablation of the renal sympathetic nerves, has effectively lowered systolic and diastolic blood pressure and decreased LV mass, thereby improving diastolic function [149,150,151]. However, a recent trial in adults with HFpEF did not confirm a beneficial effect of renal denervation on diastolic function or quality of life [152].
3.2.6 Mechanical Circulatory Support
Mechanical circulatory support is the mainstay of advanced therapy for patients with HFrEF. Because of the success of mechanical circulatory support in the management of children and adults with HFrEF, the devices such as Synergy Micro-Pump (Circulite, Inc., Hackensack, NJ, USA), the left atrial assist device, and the CorePuls (Corpuls, Kaufering, Germany) valveless design is under development for the treatment of HFpEF. However, the long-term safety of these devices still requires evaluation because of concerns regarding the increased risks of atrial arrhythmias, paradoxical embolism, and right HF. A simulation study connected a valveless volume displacement support pump to the ventricle and was driven in co-pulsation [153, 154]. The valveless pulsatile pump increased stroke volume by 30–45% and normalized hemodynamics in selected HFpEF conditions, especially with a small LV volume and markedly elevated end-systolic pressure-volume relationship.
4 Gene Therapy
While new drug and device-based therapies have improved outcomes over the past several decades, patients with HFpEF continue to experience a low quality of life, a high likelihood of being hospitalized, and a marked reduction in survival. Several preclinical studies [155, 156] suggest that gene therapy targeting sarco(endo)plasmic reticulum calcium-adenosine triphosphatase 2a improves myocyte contraction and diastolic function. Gene therapy could be a potential therapeutic means in children with single-ventricle CHD in the future.
5 Conclusions
Assessment of diastolic function should be part of a routine echocardiographic examination in children. A multi-modality imaging approach will undoubtedly improve the diagnosis of pediatric HFpEF in the future. Currently, β-blockers, ACE inhibitors, aldosterone antagonists, and diuretics are the most frequently used drugs for symptomatic relief in pediatric HFpEF. The majority of HFpEF medications proven effective in adults are not approved for use in children. Some children receive off-level medicines for the treatment of HFpEF. Using adult HF drugs with the dose scaled based on children’s body weight without knowing the pharmacokinetics and pharmacodynamics in children is dangerous. Moreover, data indicate a significant difference between children and adults with HFpEF. Therefore, there is an urgent need to develop disease-specific therapies in children with HFpEF. Pharmacokinetic and pharmacodynamic studies of newer drugs proven effective in adults in small cohorts of pediatric patients with HFpEF can provide safety and efficacy information on newer drugs. In the absence of the possibility of large-scale trials occurring soon in children with HFpEF, small observational studies are the only way forward to advance the treatment for HFpEF in children.
References
McDonagh TA, Metra M, Adamo M, ESC Scientific Document Group, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726.
Campbell RT, Petrie MC, McMurray JJV. Redefining heart failure phenotypes based on ejection fraction. Eur J Heart Fail. 2018;20:1634–5.
Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16.
Recher M, Botte A, Soquet J, Baudelet JB, Godart F, Leteurtre S. Assessment of left ventricular diastolic function in pediatric intensive care patients: a review of parameters and limitations compared with those for adults. World J Pediatr. 2021;17:21–30.
McMahon CJ, Nagueh SF, Pignatelli RH, et al. Characterization of left ventricular diastolic function by tissue Doppler imaging and clinical status in children with hypertrophic cardiomyopathy. Circulation. 2004;109:1756–62.
Friedberg MK, Silverman NH. The systolic to diastolic ratio in children with heart failure secondary to restrictive cardiomyopathy. J Am Soc Echocard. 2006;19:1326–31.
Budts W, Ravekes WJ, Danford DA, Kutty S. Diastolic heart failure with Fontan circulation: a review. JAMA Cardiol. 2020;5:590–7.
Alsaied T, Moore RA, Lang SM, et al. Myocardial fibrosis, diastolic dysfunction and elevated liver stiffness in the Fontan circulation. Open Heart. 2020;7: e001434.
Hui W, Abd El Rahman MY, Schuck R, et al. Diastolic asynchrony and myocardial dysfunction in patients with univentricular heart after Fontan operation. J Echocardiogr. 2013;11:130–7.
Kao AC, Trigt PV, Shaeffer-McCall GS, et al. Allograft diastolic dysfunction and chronotropic incompetence limit cardiac output response to exercise two to six years after heart transplantation. J Heart Lung Transplant. 1995;14:11–22.
Tallaj JA, Kirklin JK, Brown RN, et al. post-heart transplant diastolic dysfunction is a risk factor for mortality. J Am Coll Cardiol. 2007;50:1064–9.
Korang-Asante A, Fickey M, Boucek MM, et al. Diastolic performance assessed by tissue Doppler after pediatric heart transplantation. J Heart Lung Transplant. 2004;23:865–72.
Kindel SJ, Law YM, Chin C, et al. Improved detection of cardiac allograft vasculopathy: a multi-institutional analysis of functional parameters in pediatric heart transplant recipients. J Am Coll Cardiol. 2015;66:547–57.
Blanco J, Muriel-Bombin A, Sagredo V, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicenter study. Crit Care. 2008;12:R158.
Jain A, Sankar J, Anubhuti A, Yadav DK, Sankar MJ. Myocardial dysfunction in children with “sepsis” “with” and “without shock”: a prospective observational study. J Trop Pediatr. 2018;64:501–9.
Vallabhajosyula S, Pruthi S, Shah S, Wiley BM, Mankad SV, Jentxer JC. Basic and advanced echocardiographic evaluation of myocardial dysfunction in sepsis and septic shock. Anaesth Intensive Care. 2018;46:13–24.
Mitsnefes MM, Kimball TR, Border WL, et al. Impaired left ventricular diastolic function in children with chronic renal failure. Kidney Int. 2004;65:1461–6.
Mitsnefes MM. Cardiovascular complications of pediatric chronic kidney disease. Pediatr Nephrol. 2008;23:27–39.
Unger ED, Dubin RF, Deo R, et al. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2016;18:103–12.
Doyon A, Haas P, Erdem S, et al. Impaired systolic and diastolic left ventricular function in children with chronic kidney disease: results from the 4C Study. Sci Rep. 2019;9:11462.
Mehta SK, Holliday C, Hayduk L, et al. Comparison of myocardial function in children with body mass indexes 25 versus those < 25 kg/m2. Am J Cardiol. 2004;93:1567–9.
Casagrande SS, Menke A, Linder B, Osganian SK, Cowie CC. Cardiovascular risk factors in adolescents with prediabetes. Diabet Med. 2018. https://doi.org/10.1111/dme.13661.
Procar-Almela M, Codoner-Franch P, Tuzon M, Navarro-Solera M, Carrasco-Luna J, Ferrando J. Left ventricular diastolic function and cardiometabolic factors in obese normotensive children. Nutr Metab Cardiovasc Dis. 2015;25:108–15.
Amin RS, Kimball TR, Kalra M, et al. Left ventricular function in children with sleep-disordered breathing. Am J Cardiol. 2005;95:801–4.
Hui W, Slorach C, Guerra V, et al. Effect of obstructive sleep apnea on cardiovascular function in obese youth. Am J Cardiol. 2019;123:341–7.
Seliem MA, Al-Saad HI, Bou-Holaaigah IH, Khan MN, Palileo MR. Left ventricular diastolic dysfunction in congenital chronic anemias during childhood as determined by comprehensive echocardiographic imaging including acoustic quantification. Eur J Echocardiogr. 2002;3:103–10.
Allen KY, Jones S, Jackson T, et al. Echocardiographic screening of cardiovascular status in pediatric sickle cell disease. Pediatric Cardiol. 2019;40:1670–8.
Lipshultz SE, Miller TL, Wilkinson JD, et al. Cardiac effects in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents: a view from the United States of America. J Int AIDS Soc. 2013;16:18597.
Perez-Atayde AR, Kearney DI, Bricker JT, P2C2 HIV Study Group, et al. Cardiac, aortic, and pulmonary arteriopathy in HIV-infected children: the prospective P2C2 HIV multicenter study. Pediatr Dev Pathol. 2004;7:61–70.
Antony I, Kannichamy V, Banerjee A, Gandhi AB, Valaiyaduppu Subas S, Hamid P. An outlook on the impact of HIV infection and highly active antiretroviral therapy on the cardiovascular system: a review. Cureus. 2020;12: e11539.
Casaretti L, Paolillo S, Formisano R, et al. Metabolic and cardiovascular effects of combined antiretroviral therapy in patients with HIV infection: systematic review of literature. Monaldi Arch Chest Dis. 2011;76:175–82.
Nicol M, Baudet M, Cohen-Solal A. Subclinical left ventricular dysfunction during chemotherapy. Card Fail Rev. 2019;5:31–6.
Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–48.
Singh GK, Holland MR. Diastolic dysfunction in pediatric cardiac patients: evaluation and management. Curr Treat Options Cardiovasc Med. 2010;12:503–17.
Dragulescu A, Mertens L, Friedberg MK. Interpretation of left ventricular diastolic dysfunction in children with cardiomyopathy by echocardiography: problems and limitations. Circ Cardiovasc Imaging. 2013;6:254–61.
Eidem BW, McMahon CJ, Cohen RR, et al. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. 2004;17:212–21.
Schmitz L, Xanthopoulos A, Koch H, Lange PE. Doppler flow parameters of left ventricular filling in infants: how long does it take for the maturation of the diastolic function in a normal left ventricle to occur? Pediatr Cardiol. 2004;25:482–91.
Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314.
Margossian R, Sleeper LA, Pearson GD, Pediatric Heart Network Investigators, et al. Assessment of diastolic function in single-ventricle patients after the Fontan procedure. J Am Soc Echocardiogr. 2016;29:1066–73.
McMohan CJ, Pignatelli RH, Naguesh SF, et al. Left ventricular non-compaction cardiomyopathy in children: characterization of clinical status using tissue Doppler-derived indices of left ventricular diastolic relaxation. Heart. 2007;93:676–81.
Niemann M, Liu D, Hu K, et al. Echocardiographic quantification of regional deformation helps to distinguish isolated left ventricular non-compaction from dilated cardiomyopathy. Eur J Heart Failure. 2012;14:155–61.
Nagueh SF, Lakkis NM, Middleton KJ, et al. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation. 1999;99:254–61.
Geske JB, Sorajja P, Nishimura RA, et al. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation. 2007;116:2702–8.
Haland TF, Edvardsen T. The role of echocardiography in management of hypertrophic cardiomyopathy. J Echocardiogr. 2020;8:77–85.
Ryan TD, Madueme PC, Jefferies JL, et al. Utility of echocardiography in the assessment of left ventricular diastolic function and restrictive physiology in children and young adults with restrictive cardiomyopathy: a comparative echocardiography-catheterization study. Pediatr Cardiol. 2017;38:381–9.
Butz T, Piper C, Langer C, et al. Diagnostic superiority of a combined assessment of the systolic and early diastolic mitral annular velocities by tissue Doppler imaging for the differentiation of restrictive cardiomyopathy from constrictive pericarditis. Clin Res Cardiol. 2010;99:207–15.
Afonso L, Kondur A, Simegn M, et al. Two- dimensional strain profiles in patients with physiological and pathological hypertrophy and preserved left ventricular systolic function: a comparative analyses. BMJ Open. 2012;2: e001390.
Margossian R, Schwartz ML, Prakash A, et al. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study). Am J Cardiol. 2009;104:419–28.
Chowdhury SM, Butts RJ, Hlavacek AM, et al. Echocardiographic detection of increased ventricular diastolic stiffness in pediatric heart transplant recipients: a pilot study. J Am Soc Echocardiogr. 2018;31:342-8.e1.
Matsubara D, Chang J, Kauffman HL, et al. Longitudinal assessment of cardiac outcomes of multisystem inflammatory syndrome in children associated with COVID-19 infections. J Am Heart Assoc. 2022;19: e023251.
Rihal CS, Nishimura RA, Hatle LK, et al. Systolic and diastolic dysfunction in patients with a clinical diagnosis of dilated cardiomyopathy: relation to symptoms and prognosis. Circulation. 1994;90:2772–9.
Dujardin KS, Tei C, Yeo T, et al. Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82:1071–6.
Eidem BW, McMohan CJ, Ayres NA. Impact of chronic left ventricular preload and afterload on Doppler tissue imaging velocities; a study in congenital heart disease. J Am Soc Echocardiogr. 2005;18:830–8.
Masutani S, Saiki H, Kurishima C, Ishido H, Tamura M, Senzaki H. Heart failure with preserved ejection fraction in children: hormonal imbalance between aldosterone and brain natriuretic peptide. Circ J. 2013;77:2375–82.
Andrade AC, Jerosch-Herold M, Wegner P, et al. Determinants of left ventricular dysfunction and remodeling in patients with corrected Tetralogy of Fallot. J Am Heart Assoc. 2019;8(17):e009618.48.
Klitsie LM, Hazekamp MG, Roest AA, et al. Tissue Doppler imaging detects impaired biventricular performance shortly after congenital heart defect surgery. Pediatr Cardiol. 2013;34:630–8.
Harada K, Tamura M, Yasuoka K, Toyono M, Takada G. A comparison of tissue Doppler imaging and velocities of transmitral flow in children with elevated left ventricular preload. Cardio Young. 2001;11:261–8.
Vassalos A, Lilley S, Young D, et al. Tissue Doppler imaging following pediatric cardiac surgery: early patterns of change and relationship to outcome. Interact Cardiovasc Thoracic Surg. 2009;9:173–7.
Panesar D, Burch M. Assessment of diastolic function in congenital heart disease. Front Cardiovasc Med. 2017;4:5.
Mawad W, Friedberg MK. The continuing challenges of evaluating diastolic function by echocardiography in children: developing concepts and newer modalities. Curr Opin Cardiol. 2017;32:93–100.
Schiattarella GG, Alcaide P, Condorelli G, et al. Immunometabolic mechanisms of heart failure with preserved ejection fraction. Nat Cardiovasc Res. 2022;1:211–22.
Lipshultz SE. Ventricular dysfunction clinical research in infants, children, and adolescents. Prog Pediatr Cardiol. 2000;12:1–28.
van Heerebeek L, Hamdani N, Falcao-Pires I, et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–9.
Schiattarella GG, Altamirano F, Tong D, et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature. 2019;568:351–6.
Adeniran I, Maclver DH, Hancox JC, Zhang H. Abnormal calcium homeostasis in heart failure with preserved ejection fraction is related to reduced contractile function and incomplete relaxation: an electromechanically detailed biophysical modeling study. Front Physiol. 2015;6:78. https://doi.org/10.3389/fphys.2015.00078.
Peana D, Doeier TL. Cardiomyocyte Ca2+ homeostasis as a therapeutic target in heart failure with reduced and preserved ejection fraction. Curr Opin Pharmacol. 2017;33:17–26.
Selby DE, Palmer BM, LeWinter MM, Meyer M. Tachycardia-induced diastolic dysfunction and resting tone in myocardium from patients with a normal ejection fraction. J Am Coll Cardiol. 2011;58:147–54.
Price JF, Younan S, Cabrera AG, et al. Diuretic responsiveness and its prognostic significance in children with heart failure. J Cardiac Fail. 2019;25:941–7.
Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium: fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–65.
Silvestre JS, Heymes C, Oubénaissa A, et al. Role of cardiac aldosterone in post-infarction ventricular remodeling in rats. Arch Mal Coeur Vaiss. 1999;92:991–6.
Degre S, Detry JM, Unger P, Cosyns J, Brochet C, Kormos N. Effects of spironolactone-altizide on left ventricular hypertrophy. Acta Cardiol. 1998;53:261–7.
Edelmann F, Wachter R, Schmidt AG, et al. Effects of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection function: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–91.
Böckmann I, Lischka J, Richter B, et al. FGF23-mediated activation of local RAAS promotes cardiac hypertrophy and fibrosis. Int J Mol Sci. 2019;20:4634.
Lu M, Qin Q, Yao J, Sun L, Qin X. Induction of LOX by TGF-beta1/Smad/AP-1 signaling aggravates rat myocardial fibrosis and heart failure. IUBMB Life. 2019;71:1729–39.
Brilla CG, Rupp H, Funck R, Maisch B. The renin-angiotensin-aldosterone system and myocardial collagen matrix remodeling in congestive heart failure. Eur Heart J. 1995;Suppl. O:107–9.
Ramirez Gil JF, Delcayre C, Robert V, et al. In vivo left ventricular function and collagen expression in aldosterone/salt-induced hypertension. J Cardiovasc Pharmacol. 1998;32:927–34.
Zhang WW, Zheng RH, Bai F, et al. Steroidogenic acute regulatory protein/aldosterone synthetase mediates angiotensin II-induced cardiac fibrosis and hypertrophy. Mol Biol Rep. 2020;47:1207–22.
Gang C, Qiang C, Xiangli C, Shifen P, Chong S, Lihong L. Puerarin suppresses angiotensin II-induced cardiac hypertrophy by inhibiting NADPH oxidase activation and oxidative stress-triggered AP-1 signaling pathways. J Pharm Sci. 2015;18:235–48.
Stas S, Whaley-Connell A, Habibi J, et al. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin–angiotensin–aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology. 2007;148:3773–80.
Brunner-La Rocca HP, Vaddadi G, Esler MD. Recent insight into therapy of congestive heart failure: focus on ACE inhibition and angiotensin-II antagonism. J Am Coll Cardiol. 1999;33:1163–73.
Brilla CG, Maisch B, Rupp H, Funck R, Zhou G, Weber KT. Pharmacological modulation of cardiac fibroblast function. Herz. 1995;20:127–34.
Chrysant SG. Vascular remodeling: the role of angiotensin-converting enzyme inhibitors. Am Heart J. 1998;135:S21-30.
Saha SA, Molnar J, Arora RR. Tissue ACE inhibitors for secondary prevention of cardiovascular disease in patients with preserved left ventricular function: a pooled meta-analysis of randomized placebo-controlled trials. J Cardiovasc Pharmacol Ther. 2007;12:192–204.
Hsu DT, Zak V, Mahony L, Pediatric Heart Network Investigators, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–40.
Giardini A, Formigari R, Bronzetti G, et al. Modulation of neurohormonal activity after treatment of children in heart failure with carvedilol. Cardiol Young. 2003;13:333–6.
Miyamoto SD, Stauffer BL, Nakano S, et al. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J. 2014;35:33–41.
Mamidi R, Li J, Doh CY, et al. Impact of the myosin modulator mavacamten on force generation and cross-bridge behavior in a murine model of hypercontractility. J Am Heart Assoc. 2018;7(17): e009627.
del Rio CL, Ueyama Y, Baker DC, et al. In vivo cardiac effects of mavacamten (MYK-461): evidence for negative inotropy and improved compliance. Circulation. 2017;136(Suppl. 1):A20593.
Setaro JF, Zaret BL, Schulman DS, Black HR, Soufer R. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981–6.
Betocchi S, Chiariello M. Effects of calcium antagonists on left ventricular structure and function. J Hypertens Suppl. 1993;11:S33–7.
Iliceto S. Left ventricular dysfunction: which role for calcium antagonists? Eur Heart J. 1997;18 Suppl. A:A87-91.
Hoffman TM, Wernovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002.
Price JF, et al. Outpatient continuous parenteral inotropic therapy as bridge to transplantation in children with advanced heart failure. J Card Fail. 2006;12(2):139–43.
Rossano JW, Cabrera AG, Jefferies JL, Naim M, Humlicek T. Pediatric cardiac intensive care society 2014 consensus statement: pharmacotherapies in cardiac critical care chronic heart failure. Pediatr Crit Care Med. 2016;17:S20-34.
Lechner E, Hofer A, Leitner-Pender G, et al. Levosimendan versus milrinone in neonates and infants after corrective open-heart surgery: a pilot study. Ped Crit Care Med. 2012;13:542–8.
Wang A, Cui C, Fan Y, et al. Prophylactic use of levosimendan in pediatric patients undergoing cardiac surgery: a prospective randomized controlled trial. Crit Care. 2019;23:428.
Namachivayam P, Crossland DS, Butt WW, Shekherdemian LS. Early experience with levosimendan in children with ventricular dysfunction. Pediatr Crit Care Med. 2006;7:445–8.
Egan JE, Clarke AJB, Williams S, et al. Levosimendan for low cardiac output: a pediatric experience. J Intensive Care Med. 2006;21:183–7.
Bayes-Genis A, Barallat J, Richards AM. A test in context: neprilysin: function, inhibition, and biomarker. J Am Coll Cardiol. 2016;68:639–53.
Gori M, D’Elia E, Senni M. Sacubitril/valsartan therapeutic strategy in HFpEF: clinical insights and perspectives. Int J Cardiol. 2019;281:158–65.
Vaduganathan M, Claggett BL, Desai AS, et al. Prior heart failure hospitalization, clinical outcomes, and response to sacubitril/valsartan compared with valsartan in HFpEF. J Am Coll Cardiol. 2020;75:245–54.
Cunningham JW, Claggett BL, O’Meara E, Prescott MF, Pfeffer MA, Shah SJ, et al. Effect of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFpEF. J Am Coll Cardiol. 2020;76:503–14.
Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–20.
FDA approves Entresto for pediatric heart failure patients. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207620s013lbl.pdf. Accessed 13 Oct 2021.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008.
Packer M, Anker SD, Butler J, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and reduced ejection fraction: the EMPEROR-reduced trial. Circulation. 2021;143:326–36.
Januzzi JL Jr, Ibrahim NE. Understanding the mechanistic benefit of heart failure drugs matters. J Am Coll Cardiol. 2020;76:2752–4.
Omar M, Jensen J, Frederiksen PH, et al. Effect of empagliflozin on hemodynamics in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2020;76:2740–51.
Lee MT, George J, Shahab H, Hermel M, Rana JS, Virani SS. Highlights of cardiovascular disease studies presented at the 2021 American Heart Association Scientific Sessions. Curr Atheroscler Rep. 2022;24:1–12. https://doi.org/10.1007/s11883-022-00985-0.
Iborra-Egea O, Santiago-Vacas E, Yurista SR, et al. Unraveling the molecular mechanism of action of empagliflozin in heart failure with reduced ejection fraction with or without diabetes. JACC Basic Transl Sci. 2019;4:831–40.
Nassif ME, Kosiborod M. Effects of sodium-glucose cotransporter type 2 inhibitors on heart failure. Diabetes Obes Metab. 2019;Suppl. 2:19–23.
Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71.
Takimoto E, Belardi D, Tocchetti C, et al. Compartmentalization of cardiac beta-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation. 2007;115:2159–67.
Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. (RELAX) trial: rationale and design. JAMA. 2013;309:1268–77.
Borbely A, van der Velden J, Papp Z, et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–81.
Rochette L, Lorin J, Zeller M, et al. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol Ther. 2013;140:239–57.
Jeong EM, Monasky MM, Gu L, et al. Tetrahydrobiopterin improves diastolic dysfunction by reversing changes on myofilament properties. J Mol Cell Cardiol. 2013;56:44–54.
Alkaitis MS, Crabtree MJ. Recoupling the cardia nitric oxide synthases: tetrahydrobiopterin synthesis and recycling. Curr Heart Fail Rep. 2012;9:200–10.
Follmann M, Ackerstaff J, Redlich G, et al. Discovery of the soluble guanylate cyclase stimulator vericiguat (BAY 1021189) for the treatment of chronic heart failure. J Med Chem. 2017;60:5146–61.
De Angelis A, Cappetta D, Piegari E, et al. Long-term administration of ranolazine attenuates diastolic dysfunction and adverse myocardial remodeling in a model of heart failure with preserved ejection fraction. Int J Cardiol. 2016;217:69–79.
Lovelock JD, Monasky MM, Jeong EM, et al. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Reas. 2012;110:841–50.
Maier LS, Layug B, Karwatowska-Prokopczuk E, et al. Ranolazine for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Fail. 2013;1:115–22.
Lipshultz SE, Messiah SE, Miller TL. (Editors). Pediatric metabolic syndrome: comprehensive clinical review and related health issues. Springer-Verlag London Ltd., London, 2012; p. 1–378
Antoniades C, Bakogiannis C, Leeson P, et al. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation. 2011;124:335–45.
Nochioka K, Sakata Y, Miyata S, et al. Prognostic impact of statin use in patients with heart failure and preserved ejection fraction: a report from the CHART-2 study. Circ J. 2015;79:574–658.
Marume K, Takashio S, Nagai T, et al. Effect of statins on mortality in heart failure with preserved ejection fraction without coronary artery disease: report from the JASPER Study. Circ J. 2019;83:357–67.
Oikawa T, Sakata Y, Nochioka K, et al. Prognostic impact of statin intensity in heart failure patients with ischemic heart disease: a report from the CHART-2 Study. J Am Heart Assoc. 2018;7: e007524.
de Ferranti SD, Steinberger J, Ameduri R, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association. Circulation. 2019;139:e603–4.
Aoki T. Failure with preserved ejection fraction (HFpEF) patients: HFpEF as a manifestation of systemic disease. Circ J. 2019;83:277–8.
Graziani F, Lillo R, Crea F. Rationale for the use of pirfenidone in heart failure with preserved ejection fraction. Front Cardiovasc Med. 2021;8: 678530.
Van Tassell BW, Arena R, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol. 2014;113:321–7.
Rosalia L, Ozturk C, Shoar S, et al. Device-based solutions to improve cardiac physiology and hemodynamics in heart failure with preserved ejection fraction. JACC Basic Transl Sci. 2021;6(9–10):772–95. https://doi.org/10.1016/j.jacbts.2021.06.002.
Burkoff D, Maurer MS, Joseph SM, et al. Left atrial decompression pump for severe heart failure with preserved ejection fraction. JACC Heart Fail. 2015;3:275–82.
Søndergaard L, Reddy V, Kaye D, et al. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail. 2014;16:796–801.
Hasenfuß G, Hayward C, Burkhoff D, REDUCE LAP-HF Study Investigators, et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet. 2016;387:1298–304.
Emani S, Burkhoff D, Lilly SM. Interatrial shunt devices for the treatment of heart failure. Trends Cardiovasc Med. 2021;31:427–32.
Feldman T, Mauri L, Kahwash R, et al. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–62.
Kaye DM, Hasenfuß G, Neuzil P, et al. One-year outcomes after transcatheter insertion of an interatrial shunt device for the management of heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9: e003662.
Shah SJ, Feldman T, Ricciardi MJ, et al. One-year safety and clinical outcomes of a transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction in the reduce elevated left atrial pressure in patients with heart failure (REDUCE LAP-HF I) trial: a randomized clinical trial. JAMA Cardiol. 2018;3:968–77.
Gupta A, Bailey SR. Update on devices for diastolic dysfunction: options for a no option condition? Curr Cardiol Rep. 2018;20:85. https://doi.org/10.1007/s11886-018-1027-2.
Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–44.
Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomized trial. Lancet. 2016;387:453–61.
Vardas PE, Auricchio A, Blanc JJ, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy. The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Europace. 2007;9:959–98.
Penicka M, Bartunek J, Trakalova H, et al. Cardiac resynchronization therapy for the causal treatment of heart failure with preserved ejection fraction: insight from a pressure-volume loop analysis. Eur J Heart Fail. 2010;12:634–6.
Morris DA, Vaz Perez A, Blaschke F, Eichstadt H, Ozcelik C, Haverkamp W. Myocardial systolic and diastolic consequences of left ventricular mechanical dyssynchrony in heart failure with normal left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging. 2012;13:556–67.
Tschöpe C, Kherad B, Klein O, et al. Cardiac contractility modulation: mechanisms of action in heart failure with reduced ejection fraction and beyond. Eur J Heart Fail. 2019;21:14–22.
Feld Y, Dubi S, Reisner Y, et al. Energy transfer from systole to diastole: a novel device-based approach for the treatment of diastolic heart failure. Acute Card Care. 2011;13:232–42.
Corolla® TAA for heart failure with preserved ejection fraction (HFpEF) and diastolic dysfunction (DD). October 8, 2020. ClinicalTrials.gov identifier: NCT02499601. https://clinicaltrials.gov/ct2/show/NCT02499601. Accessed 18 Oct 2021.
Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–9.
Mahfoud F, Urban D, Teller D, et al. effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multicentre cardiovascular magnetic resonance imaging trial. Eur Heart J. 2014;35:2224–31.
Schirmer SH, Sayed MMYA, Reil J-C, et al. Atrial remodeling following catheter-based renal denervation occurs in a blood pressure and heart rate-independent manner. JACC Cardiovasc Interv. 2015;8:972–80.
Patel HC, Rosen SD, Hayward C, et al. Renal denervation in heart failure with preserved ejection fraction (RDT-PEF): a randomized controlled trial. Eur J Heart Fail. 2016;18:703–12.
Granegger M, Dave H, Knirsch W, Thasmen B, Schweiger M, Hobler M. A valveless pulsatile pump for the treatment of heart failure with preserved ejection fraction: a simulation study. Cardiovasc Eng Techn. 2019;10:69–79.
Landesberg A, Shenhav A, Shofty R, et al. Effects of synchronized cardiac assist device on cardiac energetics. Ann N Y Acad Sci. 2006;1080:466–78.
Tate CA, Helgason T, Hyek MF, et al. SERCA2a and mitochondrial cytochrome oxidase expression are increased in the hearts of exercise-trained old rats. Am J Physiol. 1996;271:H68-72.
Schmidt U, del Monte F, Miyamoto MI, et al. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca2+-ATPase. Circulation. 2000;101:790–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflict of interest/competing interests
Bibhuti Das has no potential conflicts of interest that might be relevant to the contents of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for Publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
BBD conceptualized, wrote, and revised this article.
Rights and permissions
About this article
Cite this article
Das, B.B. Therapeutic Approaches in Heart Failure with Preserved Ejection Fraction (HFpEF) in Children: Present and Future. Pediatr Drugs 24, 235–246 (2022). https://doi.org/10.1007/s40272-022-00508-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00508-z