Abstract
Heart failure is a highly morbid syndrome, recognized as a major cause of adult mortality. Heart failure in pediatric patients, whether in the setting of congenital or acquired heart disease, is similarly associated with high mortality and resource utilization. Understanding the clinical presentation, diagnosis, and initial stabilization of pediatric heart failure is paramount for any acute care clinician as it may mimic common childhood ailments like viral respiratory or gastrointestinal illnesses. Pediatric heart failure occurs in patients with palliated or unpalliated congenital heart disease, familial or acquired cardiomyopathy, acquired valve disease, and myocarditis. This review will focus on heart failure in pediatric patients with structurally normal hearts and will summarize what is known about patterns of presentation, etiologies, diagnostic evaluation, and the acute and chronic management of this highly morbid syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Definition and Etiology
Heart failure is a progressive clinical syndrome resulting from structural or functional cardiac abnormalities that lead to physiologic and neurohormonal changes [1, 2]. The resulting inadequate cardiac output and/or volume overload and elevated central venous pressure lead to failure to thrive, activity intolerance, and respiratory and gastrointestinal difficulties (Fig. 1). The etiologies of heart failure in the pediatric population are variable. Among all children admitted for decompensated heart failure, underlying congenital heart disease comprises the majority of patients [3]. In this setting, heart failure can develop from pressure overload, such as in left-sided obstructive lesions like valvar aortic stenosis or severe coarctation. Alternately, it can result from volume overload, as seen in patients with large left-to-right shunts, valvular dysfunction, or failed single ventricle physiology, or a combination of pressure and volume overload [4]. These patients may have preserved myocardial contractility, but inefficient blood flow and suboptimal ventricular loading conditions lead to an imbalance between oxygen demand and delivery. This impaired physiology may be further compounded by the presence of incessant or refractory atrial or ventricular arrhythmias. Delayed recognition and treatment of congenital heart disease may be more common in resource limited settings, and thus patients in low and middle income countries may initially present with heart failure.
The clinical manifestations and resultant end-organ injury caused by heart failure are mediated by elevated ventricular end-diastolic pressure and inadequate oxygen delivery. High right-sided filling pressure results in central venous congestion leading to hepatomegaly and intestinal edema; elevated left-sided filling pressure leads to pulmonary edema and associated respiratory symptoms. The combination of elevated central venous pressure and limited systemic oxygen delivery can lead to renal, hepatic, and splanchnic injury and maladaptive neuro-hormonal inflammatory activation. Reprinted from Eur J Heart Fail. 2017;19(7):821-836. Harjola VP, Mullens W, Banaszewski M, et al., Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management, with permission from Elsevier [42].
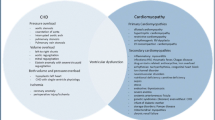
In patients with structurally normal hearts, dilated cardiomyopathy accounts for more than 50% of pediatric heart failure cases followed by hypertrophic cardiomyopathy in 42% [5]. Both dilated and hypertrophic cardiomyopathy may be primary or secondary to an underlying neuromuscular or metabolic condition. A broader differential for etiologies of heart failure in children is shown in Table 1.
In low- and middle-income countries, carditis and progressive valve disease secondary to rheumatic fever is a major contributor to heart failure in children and young adults [6]. Rheumatic fever is a complication of untreated group A streptococcal pharyngitis. The associated carditis most commonly affects the mitral valve, leading to mitral regurgitation, stenosis, or mixed disease, though the aortic and tricuspid valves may also be affected. Over time, this rheumatic heart disease (RHD) causes left atrial and ventricular dilatation, atrial arrhythmias, left ventricular failure, and secondary elevated pulmonary vascular resistance and right ventricular failure. Though the global incidence of RHD has fallen, it accounts for over 600,000 cases of severe heart failure and over 300,000 deaths annually worldwide (119,100 in India in 2015), with the largest number of deaths occurring in South Asia, Oceania, and central sub-Saharan Africa [7]. In India, the prevalence of clinical RHD in school-aged children is 0.36/1000 (95% CI: 0.1–0.7) and “silent” RHD detected by echocardiographic evaluation up to 7.7/1000 (95% CI 6.3, 9) [8]. These numbers highlight the need for RHD prevention, high index of suspicion, and early diagnosis, which may prevent progression to heart failure.
Incidence and Epidemiology
Heart failure requiring hospitalization occurs in 11,000–14,000 children annually in the United States, and cardiomyopathy affects 0.87–1.24/100,000 of children in large national cohorts [5, 9, 10]. Infants under one year are disproportionately affected, with an incidence of 8.34/100,000 and are at higher risk of death or transplant [5]. Pediatric heart failure comprises 6% of cardiac intensive care unit admissions, and nearly half of these are in patients without congenital heart disease [3]. Patients admitted with acute decompensated heart failure have a high risk of mortality (15%–18% in all comers with 11% in those with structurally normal hearts and 19% among those with congenital heart disease), transplant (20%), and readmission (13%–22%) [3, 11]. In addition, patients with acute decompensated heart failure have a high rate of complications, including arrhythmias, cardiac arrest, cerebral vascular events, and hepatic and renal failure [3, 12].
The burden of this highly morbid syndrome is increasing. In a Pediatric Health Information System (PHIS) database study, Shamszad et al. found a significant increase in the annual cardiomyopathy-related heart failure admissions during their 2005–2010 study period [12]. Among children with congenital heart disease from 2004 to 2015, there was a 74% increase in the rates of heart failure-related hospitalizations from 2004 to 2015 [13]. The cost associated with pediatric heart failure admissions is staggering [14]. In 2009, the estimated cost of pediatric heart failure hospital admissions in the United States alone totaled $1 billion [15]. Pediatric heart failure hospitalizations are significantly longer and more than twice as costly as in adults [16]. As patient complexity, multiorgan involvement, and use of advanced technologies such as ventricular assist devices (VADs) continue to grow, this cost will continue to rise.
Clinical Presentation
The presentation of heart failure in children without known congenital heart disease can be insidious and may mimic common childhood ailments. Symptoms vary based on age at presentation. In a study of nearly 100 children with newly diagnosed dilated cardiomyopathy, Hollander and colleagues found that though respiratory symptoms, including dyspnea, wheezing, cough, or respiratory distress were common in all age groups (affecting 59% of patients), gastrointestinal symptoms such as abdominal pain, nausea, vomiting, anorexia, and weight loss were the most common presenting symptoms in adolescents, reported in 65% of patients. In fact, gastrointestinal symptoms were the sole symptoms in 18% of this cohort [17]. Patients rarely presented with chest pain, cardiac arrest, and cardiogenic shock. In their review of 191 children with newly diagnosed heart failure, Puri and colleagues found that nearly half (49%, 94/191) were misdiagnosed on initial presentation. Common incorrect diagnoses included bacterial or viral infection and gastroenteritis [18]. As gastrointestinal complaints are exceedingly common in pediatric patients, a high index of suspicion is required in patients with persistent symptoms. A thorough family history including questions about cardiomyopathy, premature heart disease, need for heart transplant, ICD, or pacemaker, and unknown premature cardiac death are important questions to assess for risk of familial predisposition to cardiomyopathy.
Supplemental physical exam findings including persistent tachycardia, tachypnea, gallop rhythm, holosystolic regurgitant murmur from mitral regurgitation, hepatomegaly, impaired mental status, and poor perfusion should raise suspicion for a cardiac etiology. Laboratory and imaging data can further support the clinical diagnosis [1, 19]. Cardiac biomarkers such as BNP and troponin can be useful. A chest radiograph with cardiomegaly, pulmonary edema, or pleural effusions should also raise suspicion for cardiac etiology. EKG should be performed and assessed for sinus rhythm, the presence of atrial or ventricular arrhythmias, abnormal Q waves to suggest ischemia such as in ALCAPA, ST or T wave abnormalities to suggest strain pattern, or abnormal repolarization and low voltages to suggest myocardial edema. An echocardiogram can further support the clinical diagnosis. The clinical presentation, physical exam findings, suggested initial laboratory and imaging evaluation of a patient with suspected heart failure are summarized in Table 2.
Severity
There are multiple scales for assessing the severity of heart failure.The most widely used is the New York Heart Association (NYHA) functional class, which ranks severity from I–IV based on activity limitation. The Ross classification was developed for evaluation of heart failure in infants and included consideration of feeding difficulties [20]. An updated, modified Ross classification includes age-based vital signs norms, hepatomegaly, left ventricular ejection fraction, cardiac glycoside level, and atrioventricular valve regurgitation for pediatric patients of all ages [21]. These scales can be used to assess patients’ symptoms over time. They may also inform the decision to pursue advanced cardiac therapies or cardiac transplant [1] (Table 3).
A broader assessment divides patients into compensated versus decompensated heart failure. Those with decompensated heart failure present with signs of congestion (dyspnea, orthopnea, hypoxemia from pulmonary edema) due to elevated end-diastolic pressure or inadequate cardiac output and oxygen delivery (hypotension, poor perfusion, end organ injury) due to ventricular dysfunction or elevated systemic vascular resistance [22, 23]. Often, patients present with a combination of these findings and require immediate medical therapy. The Stanford acute heart failure symptom score is designed specifically for patients admitted with acute decompensated heart failure (Table 4). This score can be used to assess response to therapy and is associated with length of stay and in-hospital mortality. Patients with a peak score of 10–12 had a median LOS of 121 d and 50% mortality [24].
Management Considerations in Acute Decompensated Heart Failure
Initial Stabilization
Diuresis, respiratory support, and vasoactive inotropic infusions are the mainstays of initial stabilization of decompensated heart failure. Patients can be grouped into four hemodynamic profiles based on presence of congestion (wet/dry) and hypoperfusion (cold/warm) [25]. These profiles correlate with patient outcomes, with cold and wet patients having the highest mortality, and can be used to guide therapy (Fig. 2). Upon presentation, all patients should undergo rapid cardiopulmonary assessment, placement of reliable intravenous access, and initial assessment of acid–base status and end-organ function (BUN/creatinine, hepatic function panel, arterial lactate). A baseline BNP or NT-proBNP should be measured and can be an adjunct in assessing response to therapy [1]. Troponin I level should be obtained if myocarditis is suspected. The initial diagnostic evaluation should also assess for potentially treatable causes of heart failure, including anemia and thyroid abnormalities.
Physiologic profile of acute heart failure with suggested disposition and management strategies for consideration. Adapted with permission from Elsevier from J Am Coll Cardiol. 2003;41(10):1797-1804., Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure [25]
Patients with decompensated heart failure should be cared for in an intensive care unit and monitored with continuous cardiorespiratory monitors given the risk of rapid physiologic changes and malignant arrhythmias. If possible, central venous and peripheral arterial access should be obtained for monitoring of central venous pressure, venous oxygen saturation (SVO2), and arterial blood pressure. Near-infrared tissue spectroscopy (NIRS) may also be useful in monitoring cerebral and renal tissue oxygenation and may be used as a proxy of oxygen extraction [26, 27].
Diuresis
Diuretic therapy should be administered in patients with evidence of volume overload [1]. Though not associated with change in mortality, diuretic therapy can provide symptomatic improvement by decreasing peripheral and pulmonary edema. This, in turn, may improve oxygenation and decrease work of breathing, improving the balance of oxygen demand and delivery. Reducing volume overload has the additional benefit of lowering central venous pressure and, if systemic arterial pressure is maintained, improving renal perfusion pressure. Loop diuretics like furosemide and bumetanide are the first-line agents and can be administered as intermittent bolus or continuous infusions. Continuous infusion may be superior in achieving weight reduction, augmented urine output, and BNP reduction without an increased risk of electrolyte disturbance or acute kidney injury [28]. Thiazide diuretics, like chlorothiazide are routinely used as second-line agents. Tolvaptan, a vasopressin-receptor antagonist, is an effective option in patients with hyponatremia and diuretic resistance, even in the setting of renal failure [29].
Respiratory Support
Patients with acute decompensated heart failure often require respiratory support to improve oxygenation and treat respiratory distress. Supplemental oxygen should be administered for hypoxemia to optimize oxygen delivery. Oxygen therapy should be used cautiously in patients with shunt-dependent single ventricle physiology, large left-to-right shunts, or unrepaired runoff lesions in whom it can contribute to excessive pulmonary blood flow at the expense of systemic perfusion.
Noninvasive positive-pressure ventilation can have a significant hemodynamic benefit in patients with heart failure. Increased alveolar distending pressure can redistribute pulmonary edema and reduce lung and airway resistance, thus improving oxygenation and reducing work of breathing [30]. The muscles of respiration consume a significant portion of delivered oxygen in patients with respiratory distress. Thus, reducing work of breathing favorably impacts the balance of oxygen demand and delivery [31]. Finally, positive-pressure ventilation reduces left ventricular afterload and improves cardiac output by reducing left ventricular transmural pressure and wall stress [31]. Patients who do not respond to noninvasive positive pressure ventilation may require intubation and mechanical ventilation [1]. The change from negative- to positive-pressure ventilation can be provocative in patients with cardiogenic shock due to reduction in preload associated with positive pressure ventilation and the risk of hypotension during induction of anesthesia. In patients at high risk of acute hemodynamic deterioration, intubation may be performed with extracorporeal membrane oxygenator (ECMO) support on standby.
Inotropy and Afterload Reduction
Patients with impaired systolic ventricular function may require inotropic support and afterload reduction to improve myocardial contractility, left ventricular stroke volume (SV), and cardiac output (CO). Choice of agent depends on the patient’s underlying heart disease, comorbid organ system dysfunction, and pathophysiologic state. Catecholaminergic agents like dopamine and dobutamine both increase SV and CO, but dobutamine results in reduced pulmonary capillary wedge pressure (PCWP) via arterial and venous vasodilation, which makes it more appealing in patients with congestive symptoms [32]. Milrinone, a phosphodiesterase III inhibitor, increases intracellular cyclic AMP and has a hemodynamic profile similar to dobutamine. It has been shown to reduce the incidence of low cardiac output syndrome in neonates after congenital heart surgery [33]. Milrinone delivers more effective afterload reduction and improved coronary perfusion pressure compared to dobutamine with less effect on heart rate and myocardial oxygen demand [32, 34]. Due to its long half-life and renal clearance, milrinone should be used cautiously in patients with renal failure. Levosimendan, a calcium-sensitizing agent, also increases CO and SV while reducing PCWP and may improve survival in adults with acute decompensated heart failure, but is not approved by the United States Food and Drug Administration [32].
Hypotensive patients with ventricular dysfunction may require epinephrine, which at low doses (< 0.05 µg/kg/min) reduces SVR without venous vasodilation [1]. At higher doses, epinephrine increases SVR and myocardial oxygen demand. It should be used cautiously in patients with myocarditis as it is potentially arrhythmogenic [23].
Inotropic infusions are not routinely recommended for patients with hypertrophic or restrictive cardiomyopathy and preserved ventricular function. In these patients, increased inotropy or chronotropy may limit ventricular filling time and decrease SV and CO. Vasodilators are not recommended for patients with hypertrophic cardiomyopathy and left-sided obstructive lesions as they can lead to reduced coronary perfusion pressure and may exacerbate left ventricular outflow tract obstruction [23].
Management of Heart Failure in Children with Congenital Heart Disease
There is limited data to inform the medical management of heart failure in children with heart disease, and the approach to therapy is similar to that for patients with structurally normal hearts [4]. However, surgical correction or palliation should be pursued early in patients with hemodynamically significant congenital heart lesions (e.g., reimplantation of left coronary artery in ALCAPA, relief of AS, coarctation repair, VSD closure). Mechanical support and heart transplant evaluation should be considered early for congenital heart disease patients who are not candidates for surgical or interventional palliation [35].
Mechanical Support and Transplant
Advanced cardiac therapies, including ECMO and temporary or durable VAD support, should be considered early in patients who do not respond to medical therapy. Cardiac transplant evaluation should be initiated for patients with refractory heart failure who require continuous inotropic therapy or have persistent symptoms at rest, growth failure, severely reduced activity tolerance, or intractable life-threatening arrythmias. Given the limited therapeutic options and risk of developing fixed pulmonary vascular disease, transplant should also be considered in patients with restrictive cardiomyopathy and reversible elevation in pulmonary vascular resistance. Patients with severe or irreversible multiorgan system failure and fixed elevated PVR are not candidates for heart transplant [35].
Additional Considerations
Patients with heart failure should undergo baseline EKG at admission and have continuous telemetry monitoring. Tachycardia-induced cardiomyopathy is a potentially reversible process and should be considered in the differential diagnosis of dilated cardiomyopathy and heart failure. Arrhythmias are common in patients with heart failure, especially in those with myocarditis. Arrhythmias that persist after electrolyte repletion and are poorly tolerated may warrant antiarrhythmic therapy [1]. Additionally, patients with cardiomyopathy and moderate-to-severe left ventricular dysfunction, and/or risk factors for sudden cardiac death may warrant implantation of an implantable cardioverter defibrillator (ICD). Those with severe ventricular dysfunction, bundle branch block pattern on EKG, and significantly prolonged QRS duration may also benefit from cardiac resynchronization therapy [1].
Finally, many centers use anticoagulation in patients with an LV ejection fraction < 30%, especially if they have indwelling central venous access, given their higher risk for thrombosis and thromboembolic complications.
Diagnostic Evaluation
After their initial stabilization, the patients admitted with acute decompensated heart failure should undergoa thorough diagnostic evaluation, including imaging, laboratory, and genetic testing. Transthoracic echocardiography (TTE) provides both a structural and functional assessment of the heart. All pediatric patients with heart failure should be assessed for structural heart disease such as left-sided obstructive lesions, atrioventricular valve regurgitation, and coronary abnormalities which may be treatable. Additionally, left ventricular dimensions and wall thickness, as well as, biventricular systolic and diastolic functions can be measured by TTE and followed serially [1]. Anomalous left coronary artery from the pulmonary artery (ALCAPA) is a treatable cause of ischemic cardiomyopathy, which usually presents with dilated cardiomyopathy in infancy. ALCAPA can be suggested by classic EKG and echocardiographic findings, including echo bright papillary muscles, dilated right coronary artery, mitral regurgitation, and an abnormal color jet seen flowing into the pulmonary arteries [36]. If ALCAPA is suspected, cardiac catheterization with coronary angiography is diagnostic.
Endomyocardial biopsy is the diagnostic gold-standard in patients with suspected myocarditis. Increasingly, cardiac MRI is used as a noninvasive imaging modality to detect myocarditis based on the Lake Louise criteria assessment of myocardial inflammation and late gadolinium enhancement suggestive of scarring [37].
Genetic Evaluation
Genetic testing is recommended in patients with dilated, restrictive, and hypertrophic cardiomyopathies and those with arrhythmogenic right ventricular and left-ventricular noncompaction cardiomyopathies [1]. The choice of genetic testing panel should be informed by a pediatric heart failure specialist. Testing of first-degree relatives is generally recommended if a pathologic mutation is identified.
Chronic Heart Failure Management
Once medically stabilized, patients can be transitioned to an enteral heart failure regimen. There is limited evidence for chronic therapy in pediatric heart failure, and recommendations are based largely on adult data. Oral heart failure regimens are aimed at reducing maladaptive neurohormonal activation (renin–angiotensin–aldosterone) which mediate sodium and water retention, cardiac fibrosis, and over time, ventricular dilation, and dysfunction [38]. Regimens consist of beta blockade (carvedilol, metoprolol, bisoprostol), angiotensin-converting enzyme (ACE) inhibition (captopril, enalapril, lisinopril) or angiotensin-receptor blockade (ARB) for the patients intolerant of ACEs including losartan and mineralocorticoid antagonism (spironolactone, eplerenone) [1, 23, 38]. Newer therapies are of increasing interest in pediatric patients with heart failure. Ivabradine, a funny channel (If) inhibitor which slows heart rate by slowing sinoatrial-node depolarization is associated with improved left ventricular ejection fraction in children [39]. The neprilysin inhibitor sacubitril combined with valsartan has a significant mortality benefit in adults and has been approved for use in pediatric heart failure, with results of the PANORAMA-HF trial pending [40, 41]. Additional agents for treating chronic heart failure, including sodium-glucose cotransporter 2 (SGLT-2) inhibitors have recently been included as part of adult guideline–directed medical management of heart failure, and their use in pediatric patients with heart failure and obesity or insulin resistance is actively being explored.
Conclusions
Heart failure is a major cause of morbidity and mortality in pediatric patients. A high index of suspicion is required for timely diagnosis. Careful medical stabilization, diagnostic evaluation, and transition to a chronic heart failure regimen are necessary to optimize outcomes in this fragile population.
References
Kirk R, Dipchand AI, Rosenthal DN, et al. The international society for heart and lung transplantation guidelines for the management of pediatric heart failure: executive summary. J Hear Lung Transplant. 2014;33:888–909.
Hsu DT, Pearson GD. Heart failure in children part I: History, etiology, and pathophysiology. Circ Hear Fail. 2009;2:63–70.
Lasa JJ, Gaies M, Bush L, et al. Epidemiology and outcomes of acute decompensated heart failure in children. Circ Hear Fail. 2020;13:e006101.
Hinton RB, Ware SM. Heart failure in pediatric patients with congenital heart disease. Circ Res. 2017;120:978–94.
Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–55.
Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet. 2012;379:953–64.
Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377:713–22.
Saxena A, Desai A, Narvencar K, et al. Echocardiographic prevalence of rheumatic heart disease in Indian school children using World Heart Federation criteria – A multi site extension of RHEUMATIC study (the e-RHEUMATIC study). Int J Cardiol. 2017;249:438–42.
Rossano JW, Kim JJ, Decker JA, et al. Prevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the united states:a population-based study. J Card Fail. 2012;18:459–70.
Andrews RE, Fenton MJ, Ridout DA, Burch M. British congenital cardiac association. New-onset heart failure due to heart muscle disease in childhood: a prospective study in the United Kingdom and Ireland. Circulation. 2008;117:79–84.
Moffett BS, Humlicek TJ, Rossano JW, Price JF, Cabrera AG. Readmissions for heart failure in children. J Pediatr. 2016;177:153-8.e3.
Shamszad P, Hall M, Rossano JW, et al. Characteristics and outcomes of heart failure-related intensive care unit admissions in children with cardiomyopathy. J Card Fail. 2013;19:672–7.
Burstein DS, Shamszad P, Dai D, et al. Significant mortality, morbidity and resource utilization associated with advanced heart failure in congenital heart disease in children and young adults. Am Heart J. 2019;209:9–19.
Nandi D, Lin KY, O’Connor MJ, et al. Hospital charges for pediatric heart failure-related hospitalizations from 2000 to 2009. Pediatr Cardiol. 2016;37:512–8.
Nandi D, Rossano JW. Epidemiology and cost of heart failure in children. Cardiol Young. 2015;25:1460–8.
Wittlieb-Weber CA, Lin KY, Zaoutis TE, et al. Pediatric versus adult cardiomyopathy and heart failure-related hospitalizations: A value-based analysis. J Card Fail. 2015;21:76–82.
Hollander SA, Addonizio LJ, Chin C, et al. Abdominal complaints as a common first presentation of heart failure in adolescents with dilated cardiomyopathy. Am J Emerg Med. 2013;31:684–6.
Puri K, Singh H, Denfield SW, et al. Missed diagnosis of new-onset systolic heart failure at first presentation in children with no known heart disease. J Pediatr. 2019;208:258-64.e3.
Maher KO, Reed H, Cuadrado A, et al. B-type natriuretic peptide in the emergency diagnosis of critical heart disease in children. Pediatrics. 2008;121:1484–8.
Ross RD, Bollinger RO, Pinsky WW. Grading the severity of congestive heart failure in infants. Pediatr Cardiol. 1992;13:72–5.
Ross RD. The ross classification for heart failure in children after 25 years: A review and an age-stratified revision. Pediatr Cardiol. 2012;33:1295–300.
Vellore S., York JL, Gazit AZ. Acute Decompensated Heart Failure. In: Wheeler D., Wong H., Shanley T (eds). Pediatric Critical Care Medicine. Springer; London. 2014. p. 497–508.
Del Castillo S, Shaddy RE, Kantor PF. Update on pediatric heart failure. Curr Opin Pediatr. 2019;31:598–603.
Almond CS, Chen S, Dykes JC, et al. The stanford acute heart failure symptom score for patients hospitalized with heart failure. J Hear Lung Transplant. 2020;39:1250–9.
Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–804.
Green MS, Sehgal S, Tariq R. Near-infrared spectroscopy: the new must have tool in the intensive care unit? Semin Cardiothorac Vasc Anesth. 2016;20:213–24.
Ghanayem NS, Hoffman GM. Near infrared spectroscopy as a hemodynamic monitor in critical illness. Pediatr Crit Care Med. 2016;17:S201–6.
Ng PC. Effect of stress on the hypothalamic-pituitary-adrenal axis in the fetus and newborn. J Pediatr. 2011;158:e41–3.
Higashi K, Murakami T, Ishikawa Y, et al. Efficacy and safety of tolvaptan for pediatric patients with congestive heart failure. multicenter survey in the working group of the japanese society of PEdiatric circulation and hemodynamics (J-SPECH). Int J Cardiol. 2016;205:37–42.
Nadar S, Prasad N, Taylor RS, Lip GY. Positive pressure ventilation in the management of acute and chronic cardiac failure: a systematic review and meta-analysis. Int J Cardiol. 2005;99:171–85.
Bronicki A, Cardiopulmonary R. interactions in children with heart failure. Curr Cardiol Rev. 2016;12:104–6.
Tume SC, Schwartz SM, Bronicki RA. Pediatric cardiac intensive care society 2014 consensus statement: pharmacotherapies in cardiac critical care treatment of acute heart failure. Pediatr Crit Care Med. 2016;17:S16–9.
Hoffman TM, Wemovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002.
Cavigelli-Brunner A, Hug MI, Dave H, et al. Prevention of low cardiac output syndrome after pediatric cardiac surgery: a double-blind randomized clinical pilot study comparing dobutamine and milrinone. Pediatr Crit Care Med. 2018;19:619–25.
Canter CE, Shaddy RE, Bernstein D, et al. Indications for heart transplantation in pediatric heart disease: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young; the Councils on Clinical Cardiology, Cardiovascular Nursing, and Cardiovascular Surgery and Anesthesia; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;115:658–76.
Li RJ, Sun Z, Yang J, et al. Diagnostic Value of transthoracic echocardiography in patients with anomalous origin of the left coronary artery from the pulmonary artery. Medicine (Baltimore). 2016;95:e3401.
Law YM, Lal AK, Chen S, et al. American heart association pediatric heart failure and transplantation committee of the council on lifelong congenital heart disease and heart health in the young and stroke council. Diagnosis and management of myocarditis in children: a scientific statement from the American heart association. Circulation. 2021;144:e123–35.
Schranz D, Voelkel NF. “Nihilism” of chronic heart failure therapy in children and why effective therapy is withheld. Eur J Pediatr. 2016;175:445–55.
Bonnet D, Berger F, Jokinen E, Kantor PF, Daubeney PEF. Ivabradine in children with dilated cardiomyopathy and symptomatic chronic heart failure. J Am Coll Cardiol. 2017;70:1262–72.
McMurray JJ, Packer M, Desai AS, et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004.
Loss KL, Shaddy RE, Kantor PF. Recent and upcoming drug therapies for pediatric heart failure. Front Pediatr. 2021;9:681224.
Harjola VP, Mullens W, Banaszewski M, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 2017;19:821–36.
Author information
Authors and Affiliations
Contributions
FM and DB: Manuscript preparation and revision. FM will act as the guarantor for this paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mille, F., Burstein, D. Diagnosis and Management of Pediatric Heart Failure. Indian J Pediatr 90, 492–500 (2023). https://doi.org/10.1007/s12098-022-04433-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-022-04433-4