Abstract
Background
In adults, the area under the concentration–time curve (AUC) divided by the minimum inhibitory concentration (MIC) is associated with better clinical and bacteriological response to vancomycin in patients with methicillin-resistant Staphylococcus aureus who achieve target AUC/MIC ≥ 400. This target is often extrapolated to pediatric patients despite the lack of similar evidence. The impracticalities of calculating the AUC in practice means vancomycin trough concentrations are used to predict the AUC/MIC.
Objective
This review aimed to determine the relationship between vancomycin trough concentrations and AUC/MIC in pediatric patients.
Methods
We searched the MEDLINE and Embase databases, the Cochrane Database of Systematic Reviews, and the Cochrane Central Register of Controlled Trials using the medical subject heading (MeSH) terms vancomycin and AUC and pediatric* or paediatric*. Articles were included if they were published in English and reported a relationship between vancomycin trough concentrations and AUC/MIC.
Results
Of 122 articles retrieved, 11 met the inclusion criteria. One trial reported a relationship between vancomycin trough concentrations, AUC/MIC, and clinical outcomes but was likely underpowered. Five studies found troughs 6–10 mg/l were sufficient to attain an AUC/MIC > 400 in most general hospitalized pediatric patients. One study in patients undergoing cardiothoracic surgery found a trough of 18.4 mg/l achieved an AUC/MIC > 400. Two oncology studies reported troughs ≥ 15 mg/l likely attained an AUC/MIC ≥ 400. In critical care patients: one study found a trough of 9 mg/l did not attain the AUC/MIC target; another found 7 mg/l corresponded to an AUC/MIC of 400.
Conclusions
Potential vancomycin targets varied based on the population studied but, for general hospitalized pediatric patients, troughs of 6–10 mg/l are likely sufficient to achieve AUC/MIC ≥ 400. For MIC ≥ 2 mg/l, higher troughs are likely necessary to achieve an AUC/MIC ≥ 400. More research is needed to determine the relationships between vancomycin trough concentrations, AUC/MIC, and clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Trough concentrations between 6 and 10 mg/l are likely sufficient to achieve an area under the concentration–time curve (AUC) divided by minimum inhibitory concentration (MIC) ≥ 400 in general hospitalized pediatric patients. |
Higher troughs may be required in critically ill, oncology, surgical, or adolescent pediatric patients to achieve an AUC/MIC target ≥ 400. |
Achievement of AUC/MIC target is highly dependent on the MIC of the isolated bacteria. Higher troughs may be empirically required in areas where MICs are commonly > 1 mg/l. |
More research is needed to determine the relation between vancomycin trough concentrations, AUC/MIC ratios, and clinical outcomes. |
1 Introduction
Vancomycin is a glycopeptide antibiotic that binds to the peptidoglycan precursor d-alanyl-d-alanine pentapeptide [1]. It disrupts cell wall synthesis by inhibiting the incorporation of monomers into the peptidoglycan chain [1]. Vancomycin has activity against Gram-positive organisms such as Staphylococcus, including methicillin-resistant S. aureus (MRSA), Streptococcus, and Enterococcus, although emerging resistance is a concern [2]. Vancomycin also has activity against Clostridium difficile and can be used to treat pseudomembranous enterocolitis if taken enterally [2]. Additionally, vancomycin is commonly used in pediatric patients to treat a variety of other organisms such as resistant S. pneumoniae or coagulase-negative S. aureus. In pediatrics, vancomycin is recommended by the Infectious Diseases Society of America (IDSA) for the treatment of infections caused by MRSA, which include complicated skin and soft tissue infections, bacteremia, infective endocarditis, pneumonia, osteomyelitis, septic arthritis, meningitis, and septic thrombosis [3].
Vancomycin is bactericidal and exhibits time-dependent killing, meaning that the time for which the concentration of the drug in the body is above the minimum inhibitory concentration (MIC) affects the antimicrobial effect [4]. In mouse models, the area under the concentration–time curve (AUC) divided by the MIC has been associated with the antimicrobial activity of vancomycin towards S. aureus [4]. Moise-Broder et al. [5] examined the use of vancomycin in adult patients with pneumonia caused by S. aureus and found significantly better clinical and bacteriological response to vancomycin in patients who achieved the target AUC/MIC > 400 than in those who did not. They also found that bacterial eradication occurred more rapidly when the AUC/MIC target was achieved [5]. Unfortunately, the same evidence does not exist for pediatric patients; therefore, a target of AUC/MIC > 400 is often extrapolated from adult patients [3].
The AUC can be calculated as dose/clearance using patient-specific parameters or population estimates such as the Rodvold et al. [6] equation or by other methods, such as those described by Pai et al. (Bayesian method and "simple" two-concentration analytic equations) [7]. In clinical practice, calculating the AUC/MIC is difficult, because determining the AUC with the trapezoidal rule requires multiple serum concentrations, which may be more difficult to obtain from children because of inherent limitations in the ability to obtain multiple blood samples. Given the impracticalities of calculating the AUC in clinical practice, it is recommended that clinicians monitor serum vancomycin trough concentrations as a predictive measure for AUC/MIC [3].
Various assay methods can be used to quantify vancomycin concentrations. The ideal analytic method must be sensitive, precise, accurate, and specific. To be useful in clinical practice, the assay should also yield quick results. A recent study by Shipkova et al. [8] revealed a very good between-system comparability of the concentrations measured. There were systematic deviations between certain systems when measuring vancomycin concentrations, but all results were within the acceptable limits for all systems [8]. While most MIC data are derived from the Etest, other automated MIC testing systems are commercially available and can yield different MIC results [8].
To achieve the target serum AUC/MIC > 400, the IDSA guidelines recommend targeting vancomycin trough concentrations of 15–20 mg/l in both adult and pediatric patients [3]. This recommendation is classified as a moderate-strength recommendation based on expert opinion [3]. However, the guidelines do acknowledge a lack of efficacy and safety data for this recommendation for pediatric patients and cite it as an area that requires additional research [3]. Additionally, target concentrations for both pediatric and adult patients will vary based on the site of infection due to differences in antibiotic penetration to the site of action. The concern with targeting higher trough concentrations is the increased risk of adverse drug reactions. McKamy et al. [9] found that higher vancomycin troughs, ≥ 15 mg/l, were more likely to be associated with nephrotoxicity. In addition to a lack of efficacy, a concern with targeting lower trough concentrations is the potential development of resistant organisms. A recent meta-analysis found that, in pediatric patients, vancomycin doses ≥ 60 mg/kg/day were more likely to attain a target trough of 10–20 mg/l and a target AUC/MIC > 400 compared with doses < 60 mg/kg/day, yet the relation between vancomycin troughs and the AUC/MIC remains relatively unclear [10]. The aim of this review is to describe the relationship between vancomycin serum trough concentrations and AUC/MIC in pediatric patients.
2 Methods
We conducted a literature search of the MEDLINE (1946–October 2017) and Embase (1974–October 2017) databases, the Cochrane Database of Systematic Reviews (CDSR), and the Cochrane Central Register of Controlled Trials (CENTRAL) with the medical subject headings (MeSH) vancomycin and AUC or area under the curve and pediatric* or paediatric*. Reference lists of included articles were reviewed for additional articles. Articles were included if they were in English and reported either a relation between vancomycin trough concentrations and AUC/MIC ratios in pediatric patients or between the mean or median vancomycin trough concentrations and the corresponding AUC/MIC ratio. We considered a relation to be a correlation coefficient, positive or negative predictive values, or estimated or predicted troughs that corresponded to a specific AUC/MIC, or vice versa. Narrative reviews, conference abstracts, case reports, case series, in vivo animal data, and in vitro data or studies involving vancomycin continuous infusions were excluded, as were articles that reported only an AUC without the AUC/MIC ratio and were not clear about the MIC used in the study. Finally, we also excluded articles that reported a trend in trough results and a trend in AUC/MIC ratios without reporting means, medians, or a relation between the results in such a manner that a meaningful association could be determined. All titles and abstracts were reviewed by one reviewer, and the full text was retrieved for potentially eligible articles. Two reviewers independently reviewed all full-text articles for inclusion, and all disagreements were resolved by a third reviewer. Data extracted from each study included design, population, outcomes, method used to calculate AUC, and vancomycin assay used.
3 Results
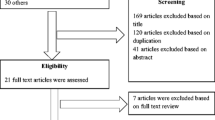
The literature search identified 122 articles. After duplicate results were excluded and titles and abstracts screened, 24 full texts were independently screened for inclusion in the review [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] (Fig. 1). The kappa score for inter-rater agreement during the full-text review was 0.6, which is considered a moderate level of agreement. A total of 11 articles met the inclusion criteria and were evaluated in this review [13, 14, 17, 20, 26,27,28,29, 31, 33, 34] (Table 1). Five studies reported the relationship between AUC/MIC and vancomycin trough concentrations in general hospitalized pediatric patients [17, 25,26,27, 33], one included pediatric patients undergoing cardiothoracic surgery (CTS) [13], two studied pediatric oncology patients [14, 31], two examined critically ill pediatric patients [20, 34], and one evaluated adolescent patients [27]. We excluded an additional four studies reporting trends in vancomycin trough and AUC/MIC ratios but not reporting a relationship between trough and AUC/MIC ratio and not reporting mean or median values for trough or AUC/MIC ratio [11, 21, 22, 28].
3.1 General Hospitalized Pediatric Patients
Frymoyer et al. [17] conducted a pharmacokinetic modeling and simulation analysis using three previously published pediatric vancomycin pharmacokinetic models and simulated 5000 hypothetical pediatric patients to determine the vancomycin serum trough concentration predictive of AUC/MIC > 400 based on currently recommended doses. They used a base patient who weighed 25 kg, with no age specified, and a base MIC of 1 mg/l. It is concerning that no age was specified, as pharmacokinetic parameters differ significantly based on a child’s age. They found that achieved AUC/MIC targets were highly dependent on the assumed MIC of the isolated MRSA bacteria. Overall, depending on the dose and interval, a trough between 7 and 10 mg/l was predicted to achieve an AUC/MIC > 400 in > 90% of simulated children. The authors concluded that goal troughs between 15 and 20 mg/l are likely unnecessary to achieve an AUC/MIC > 400 in the typical child.
Le et al. [25] conducted a cohort study and Monte Carlo simulation in 702 hospitalized pediatric patients with a median age of 6.6 years. A total of 1660 vancomycin concentrations were measured to determine the pharmacokinetic parameters of vancomycin in children using population-based modeling and to compare target attainment of AUC/MIC ≥ 400 and vancomycin trough concentrations ≥ 15 mg/l. They used MIC distributions based on MIC values of isolates from the two different hospitals, which ranged from 0.5 to 2.0 mg/l in hospital A and from 0.25 to 2.0 mg/l in hospital B. Their model demonstrated that a dose of 70 mg/kg/day achieved a target AUC/MIC ≥ 400 in 75–85% of subjects, depending on the hospital’s MIC distribution, and only 50% of children achieved a trough ≥ 15 mg/l. As the authors identified, a clinically significant finding was that AUC/MIC ~ 400 corresponded to trough ~ 8–9 mg/l for vancomycin doses between 60 and 70 mg/kg/day and that targeting a trough ≥ 15 mg/l may not be warranted.
Ploessl et al. [27] sought to validate a pharmacokinetic model in a pediatric hospital and to determine correlation between AUC/MIC and trough vancomycin concentrations in 40 hospitalized pediatric patients with positive S. aureus cultures and a median age of 8.5 years. The mean vancomycin trough was 11 ± 5.5 mg/l, and mean AUC/MIC was 489 ± 133. There was no statistically significant difference between mean trough concentrations or doses in subjects with an AUC/MIC < 400 compared with those with an AUC/MIC ≥ 400 (p = 0.6262). The MIC was the only significantly different parameter between those who achieved an AUC/MIC ≥ 400 and those who did not. All patients in the AUC/MIC ≥ 400 group had an MIC of 1 mg/l. When all 40 subjects were included, there was no correlation between AUC/MIC and trough vancomycin concentrations (r = 0.29; r2 = 0.082; p = 0.07). When an MIC of 1 mg/ml was assumed to calculate the AUC/MIC ratio, there was a positive correlation between AUC/MIC and trough concentrations (r = 0.44; r2 = 0.19; p = 0.00046). As estimated by the authors, to achieve an AUC/MIC = 400, the corresponding trough is likely < 10 mg/l. One limitation of this study was that the authors did not report a power calculation; therefore, the potential exists that no difference was found between the mean trough concentration or doses in those who attained an AUC/MIC < 400 compared with those with an AUC/MIC ≥ 400 because the study was not powered to detect a difference.
Kishk et al. [33] conducted a retrospective chart review of 36 pediatric patients aged between 2 months and 18 years with positive S. aureus blood cultures who were treated with vancomycin. There were no significant differences in the admitting diagnoses between the two groups. They used four different methods to calculate the AUC (Table 1). Their primary objective was to assess the likelihood of different dosing intervals attaining an AUC/MIC > 400 and to determine the associated serum vancomycin concentrations. They found that the trapezoidal lower limit method yielded the highest percentage of patients achieving an AUC/MIC > 400 and that, based on this method, an AUC/MIC of 400 corresponds to a trough of 10.98 mg/l. They also looked at clinical outcomes for all patients who received vancomycin for longer than 24 h (N = 29). There was no statistically significant difference in hospital length of stay (19.6 vs. 23.5 days; p = 0.11), intensive care unit (ICU) length of stay (13.8 vs. 15.6 days; p = 0.41), or time to first negative blood culture (1 vs. 2 days; p = 0.36) between those who achieved an AUC/MIC ≥ 400 and those with an AUC/MIC < 300, respectively. There were no reports of vancomycin-associated nephrotoxicity in either group. The authors concluded that the likelihood of achieving an AUC/MIC > 400 was highly variable depending on the method used to calculate the AUC, and an AUC/MIC of 400 may correlate with a vancomycin trough of 11 mg/l.
Nassar et al. [26] conducted an observational cohort study to evaluate the pharmacokinetic parameters of vancomycin in 51 hospitalized children (median age 5 years) of different weight groups and to calculate dosing regimens to achieve therapeutic goals. In total, 64 vancomycin trough concentrations were drawn in children receiving a standard regimen of 20 mg/kg twice daily. The mean vancomycin trough for all children (n = 64 episodes of infection and corresponding vancomycin troughs from 51 children) was 3.36 ± 2.58 mg/l, and the mean AUC/MIC for all children with evidence of a Gram-positive infection (n = 18 episodes of infection) was 314 ± 186. An AUC/MIC was calculated for all children, assuming three different MIC values (0.5, 1, and 2 mg/l); the authors concluded that achieving an AUC/MIC > 400 was only feasible if the MIC was ≤ 0.5 mg/l in all weight groups. No difference was seen between weight groups for any MIC value. As the authors pointed out, there was no clinically significant difference in vancomycin trough concentrations or pharmacokinetic parameters between different weight groups. However, a power calculation was not reported, and the potential exists that the trial was underpowered.
3.2 Pediatric Cardiothoracic Surgery Patients
Benefield et al. [12] conducted an observational cohort study of 54 hospitalized pediatric patients with a mean age of 1.1 years; 27 underwent CTS and 27 did not. The study goal was to compare the vancomycin trough concentrations of CTS and non-CTS patients and to assess the likelihood of attaining an AUC/MIC target of ≥ 400. Mean trough concentrations and calculated AUC/MIC in CTS patients was compared with those for non-CTS patients with assumed MICs of 0.5, 1.0, or 2.0 mg/l (Table 1). Mean trough values between the CTS and non-CTS groups were significantly different (CTS 18.4 mg/l vs. non-CTS 8.8 mg/l; p < 0.01), but there was no difference in AUC/MIC values between the two groups for any of the MIC values (Table 1). The authors concluded that the higher trough concentrations were achieved in CTS patients, and pharmacokinetic parameters were highly variable in CTS patients. This study also demonstrated that attaining troughs of 18 mg/l achieved the AUC/MIC target of > 400 when the MIC was ≤ 1 mg/l.
3.3 Pediatric Oncology Patients
In a retrospective analysis of vancomycin trough serum measurements in pediatric oncologic/hematologic patients hospitalized in the ICU, da Silva et al. [30] aimed to evaluate suitable dosing regimens for this population. They obtained 61 vancomycin concentrations in 31 patients with a mean age of 7 years. MICs ranged from 0.5 to 1.5 mg/l, with a mode value of 1.0 mg/l, which was used in the AUC/MIC ratio calculations. Of the 61 vancomycin levels obtained, the authors found an AUC/MIC > 400 in all instances (n = 23) when the trough was > 15 mg/l, whereas a trough < 15 mg/l was associated with an AUC/MIC > 400 in 11 instances and with an AUC/MIC ≤ 400 in 27 instances. Therefore, trough concentrations > 15 mg/l had a positive predictive value (PPV) of 100% and a negative predictive value of 71% for this population, assuming an MIC of 1 mg/l. The authors suggested that higher than usual doses of vancomycin may be required to treat pediatric patients with oncologic/hematologic diseases.
Seixas et al. [29] conducted an observational cohort study in 94 hospitalized pediatric oncology patients (median age 7.28 years) admitted to the ICU or stem cell transplant ward. They took 256 vancomycin concentrations with the aim of obtaining vancomycin pharmacokinetic and pharmacodynamic data in this population. They also conducted a Monte Carlo simulation with 10,000 virtual patients. Mean vancomycin trough was 15.6 ± 12.4 mg/l. Attainment of the AUC/MIC target was highly dependent on the MIC (Table 1). An AUC/MIC ≥ 400 was attained for only 37 (14.4%) vancomycin trough concentrations that were < 15 mg/l. Multivariate logistic regression analysis found that vancomycin trough concentrations ≥ 20 mg/l had a significant impact on an increased risk of developing nephrotoxicity, with an odds ratio of 17.83 (95% confidence interval [CI] 3.28–96.6). The authors determined that current doses of vancomycin do not reach the therapeutic target for critically ill oncology patients, particularly if isolates have an MIC > 1 mg/l. Generalizability of the study findings is limited because of uncertainty about whether the patient ages matched the ages simulated, actual MICs were not provided, and large standard deviation values likely indicated that vancomycin trough values were not normally distributed (thus, it would have been more appropriate to report the median rather than the mean trough values).
3.4 Critically Ill Pediatric Patients
De Cock et al. [32] conducted a prospective observational study of critically ill children admitted to the ICU, with the aim of evaluating plasma protein binding and target attainment rates of vancomycin therapy in this population. They included 188 samples from 32 patients and assumed an MIC of 1 mg/l. The median serum creatinine was 0.22 mg/dl. A total of 12 patients (57%) achieved a target AUC/MIC ≥ 400 (Table 1). Spearman’s rank correlation coefficient for the relationship between vancomycin trough concentrations and AUC/MIC was 0.85. The authors concluded that a trough concentration of ~ 7 mg/l corresponded to an AUC/MIC of 400.
Giachetto et al. [18] conducted an observational cohort study of hospitalized children admitted to the ICU, with the aim of determining pharmacokinetic parameters for vancomycin in critically ill children and to estimate AUC/MICs for S. aureus, which is achieved with recommended vancomycin dosages of 40 mg/kg/day for non-central nervous system (CNS) infections and 60 mg/kg/day for CNS infections. Patients with renal dysfunction were excluded, and the mean clearance was 2.47 ml/kg/min. On days 1 and 3, vancomycin trough concentrations were obtained, and AUC/MICs were calculated using MIC values of 1 and 2 mg/l (Table 1). Actual MIC values were not reported, but the authors reported that most isolates had MIC values between 1 and 2 mg/l. On day 1, results were available for 18 children: the mean trough was 7.7 mg/l with a mean AUC/MIC of 364 if the MIC was 1 mg/l and 188 if the MIC was 2 mg/l. Only nine children reached target AUC/MIC > 400 when the MIC was 1 mg/l, and only one child reached target when the MIC was 2 mg/l. On day 3, vancomycin trough concentrations were available for 15 children (Table 1): The mean trough was 7.8 mg/l with a mean AUC/MIC of 364 if the MIC was 1 mg/l and 181.9 if the MIC was 2 mg/l. Only seven of the 15 patients achieved the AUC/MIC target of > 400 if the MIC was 1 mg/l and only one achieved target if the MIC was 2 mg/l. The authors concluded that the recommended doses of vancomycin did not achieve therapeutic serum concentrations or an AUC/MIC > 400.
3.5 Adolescent Patients
Lanke et al. [24] sought to evaluate vancomycin pharmacokinetics in an adolescent population by conducting an observational cohort study in 463 hospitalized patients with median age of 15.6 years who had 1107 vancomycin concentrations drawn. They then used a pharmacokinetic model to determine dosing strategies that predicted target AUC/MIC ≥ 400 and target trough concentration to achieve that value. No isolate had an MIC > 1 mg/l. The population pharmacokinetic model predicted that a dose of 60 mg/kg/day was necessary to achieve an AUC/MIC ≥ 400 in > 90% of subjects. Authors found that vancomycin trough concentrations between 10 and 12.5 mg/l were highly predictive of achieving a target AUC/MIC of ≥ 400 (Table 1). The authors recommended that lower trough concentrations are adequate to achieve an AUC/MIC ≥ 400 and personalized dosing regimens, rather than a standard 60 mg/kg/day, should be considered.
3.6 Excluded Studies
Four studies reported trends in vancomycin trough and AUC/MIC ratios but did not report mean or median trough values or mean AUC/MIC ratios or discuss the relation between the two parameters and were therefore excluded from review [11, 21, 22, 28]. Hahn et al. [21] conducted a retrospective cohort study in hospitalized pediatric patients and reported the number of patients who achieved AUC/MICs above and below 400 based on their trough value. They reported a correlation between trough concentrations to AUC but did not explicitly report the MIC used or assumed and therefore did not meet our inclusion criteria. They found that 17% of subjects with a trough > 10 mg/l did not achieve an AUC/MIC > 400, whereas 52% of subjects with a trough < 15 mg/ml attained an AUC/MIC > 400 [21]. Hwang et al. [22] conducted a retrospective cohort study in hospitalized children and reported the proportion of children who attained various trough ranges and those who attained an AUC/MIC ≥ 400 based on various MIC values. Although they also reported a correlation between AUC and trough concentrations, they did not report a correlation between AUC/MIC and trough concentrations. This study found that trough concentrations of 10–15 mg/l correlated with an AUC of 400 [22]. Rainkie et al. [28] conducted a retrospective cohort study in hospitalized pediatric patients and reported mean trough concentrations and AUC/MIC values for different age groups and used two different methods to calculate the AUC. However, they did not report overall mean trough concentrations or overall mean AUC/MIC ratios. Finally, Hadi et al. [11] conducted a retrospective cohort study and Monte Carlo simulation in pediatric oncology patients. They found that a dose of 80 mg/kg/day divided every 6 h achieved a mean AUC of 479 mg × h/l, which correlated to a mean trough of 10 mg/l [11]. This study did not report AUC/MIC ratios or the mean MIC used and therefore did not meet the inclusion criteria for our review.
Two studies were excluded from the review as they did not explicitly report the AUC/MIC ratio or the mean, median, or assumed MIC [13, 14]. Chhim et al. [13] conducted a retrospective cohort study of pediatric patients with suspected invasive streptococcal infections. The median trough attained for 40 mg/kg/day dosing was 8.6 mg/l, with a median AUC of 288 mg × h/l. The median trough attained for 60 mg/kg/day dosing was 10.6 mg/l, with a median AUC of 279 mg × h/l. The authors reported that 95% of MRSA isolates had an MIC ≤ 1 mg/l but did not report mean or median MIC or the AUC/MIC ratio. This study calculated the AUC using the vancomycin dose divided by clearance. Demirjian et al. [14] conducted a randomized controlled trial investigating a conventional vancomycin dosing regimen with versus without a loading dose in pediatric patients. The median trough concentration before the third dose was 9 mg/l. The median AUC was 446.5 mg × h/l in the loading dose group and 434 mg × h/l in the conventional dosing group. The authors did not report the mean, median, or assumed MIC or the AUC/MIC ratios. This study calculated the AUC using SAAM II (http://www.saam.com/) modeling software. The results of these two studies highlight the wide disparity in AUC values when different methods are used to calculate the AUC.
4 Discussion
Guidelines recommend targeting vancomycin troughs between 15 and 20 mg/l for serious MRSA infections in pediatric patients, although they also state that limited evidence is available to support this recommendation [3]. Targeting an AUC/MIC ≥ 400 has been found to be associated with positive clinical outcomes in adult patients, and thus this target is extrapolated to pediatric patients [3]. Our review has found that it may not be necessary to target troughs as high as 15–20 mg/l in pediatric patients to achieve the target AUC/MIC ≥ 400. If the MIC is 1 mg/l, a target trough of 6–10 mg/l may be sufficient to achieve an AUC/MIC > 400 in a general hospitalized pediatric patient. However, significant variability exists between different pediatric populations and AUC/MIC target attainment. This is likely due to differences in the pharmacokinetic parameters of different pediatric patients, such as those who are critically ill, post-operative, or receiving chemotherapy, and the changes that occur as children age. Pharmacokinetics vary significantly with age; therefore, neonates, children, and adolescents are all very different populations. Additionally, all studies included in this review examined vancomycin concentrations in the serum and therefore cannot be extrapolated to infections such as osteomyelitis or meningitis where there are significant differences in penetration to the site of infection.
4.1 General Hospitalized Pediatric Patients
In studies that included all general hospitalized pediatric patients [17, 25,26,27, 33], four found that low trough targets were required to achieve target AUC/MIC ≥ 400, and one study simply found that a lower vancomycin trough concentration did not achieve the AUC/MIC target. The lower target troughs ranged from > 3.36 to 11 mg/l [17, 25,26,27, 33]. Three of these trials included only a very small number of general hospitalized patients [26, 27, 33]. However, the two larger modeling studies yielded similar results: troughs between 6–10 and 8–9 mg/l were adequate to achieve an AUC/MIC > 400 [17, 25]. Overall, based on results of these trials, it appears that trough targets between 6 and 10 mg/l should be sufficient to attain an AUC/MIC in the majority of the general hospitalized pediatric population.
4.2 Pediatric Cardiothoracic Surgery Patients
The Benefield et al. [12] trial was the only study to specifically look at a CTS pediatric population. Based on their trial data, we can only conclude that vancomycin troughs of 18.4 mg/l should be sufficient to achieve an AUC/MIC > 400 in this population if the MIC of isolated bacteria is < 2 mg/l. However, based on the high AUC/MIC ratios attained with troughs of 18.4 mg/l, it is unlikely that troughs this high are necessary to achieve an AUC/MIC > 400. It is also important to note the significant pharmacokinetic variations between the CTS and non-CTS populations. Despite achieving a significantly higher trough in CTS patients, the AUC/MIC ratios did not differ much between the CTS and non-CTS populations. It is therefore likely that higher trough concentrations are required in this unique population to achieve a target AUC/MIC. An observational trial specifically looking at the trough targets in pediatric CTS patients compared with the AUC/MIC ratios in those specific patients with clinically significant outcomes, such as mortality, duration of hospitalization, or adverse effects, would significantly improve our ability to determine a specific vancomycin trough target in this population.
4.3 Pediatric Oncology Patients
Two studies specifically included only pediatric oncology patients [29, 30]. Based on the current literature, if the MIC is 1 mg/l, a trough ≥ 15 mg/l will likely attain a target AUC/MIC ≥ 400 in this population. Seixas et al. [29] suggested that higher troughs or alternative antimicrobial therapy may be warranted if isolated bacteria has an MIC > 1 mg/l. Unfortunately, neither study looked at the relationship between troughs < 15 mg/l and attainment of an AUC/MIC ≥ 400. It might be feasible to target a trough < 15 mg/l in this population if the MIC is ≤ 1 mg/l, although that would need to be examined further in a clinical trial. An adequately powered observational trial investigating the vancomycin troughs obtained in pediatric oncology patients and their specific AUC/MIC ratios with clinically significant outcomes, such as mortality, hospitalization, and vancomycin toxicities, and that include vancomycin troughs < 15 mg/l would be helpful in making specific target trough concentrations in this unique population.
4.4 Critically Ill Pediatric Patients
Critical care patients have unique pharmacokinetic parameters. Studies have found that the vancomycin volume of distribution is increased and clearance may be altered in critically ill pediatric patients [34]. Our review identified two studies that specifically looked at children admitted to the ICU [18, 32], but their results seem to conflict. One study suggested that higher troughs were required in critically ill pediatric patients compared with general hospitalized patients to reach a target AUC/MIC of > 400 and that troughs of at least 9 mg/l in critically ill pediatric patients appear to be insufficient [18]. In contrast, the other study found that a trough of 7 mg/l corresponded to an AUC/MIC of 400 [32]. Interestingly, both trials were observational cohort studies and included a similar number of children of similar ages. An adequately powered observational trial examining vancomycin troughs in critically ill pediatric patients and their specific AUC/MIC ratios with clinically significant outcomes, such as mortality, hospitalization, and vancomycin toxicities, and includes troughs > 9 mg/l would be useful in determining specific vancomycin troughs required to adequately treat this fragile population.
4.5 Adolescents
One study specifically examined the relation between vancomycin troughs and AUC/MIC target attainment in adolescents aged 12–18 years [24]. Trough concentrations between 10 and 12.5 mg/l were highly predictive of achieving a target AUC/MIC ≥ 400 [24]. Therefore, in adolescent populations, we might need to target slightly higher vancomycin trough concentrations of between 10 and 12.5 mg/l compared with the general pediatric population. Although this is still lower than the current recommended target of 15–20 mg/l, the lack of trials with clinically significant outcomes such as mortality, hospitalization, or vancomycin toxicities means the clinical significance remains unknown.
4.6 Minimum Inhibitory Concentrations
Attainment of an AUC/MIC ≥ 400 target was highly dependent on the MIC of isolated bacteria. The five studies that examined different MIC values did not report the target attainment when the MIC was ≥ 2 mg/l [12, 17, 26, 27, 29]. Three studies did not report differences in AUC/MIC target attainment based on different MIC values [24, 25, 30]. Finally, one trial found that, based on troughs of 7.8 mg/l, neither MIC group—1 or 2 mg/l—attained an AUC/MIC target of ≥ 400, although the MIC 2 mg/l group had lower AUC/MIC ratios [18]. These results support the IDSA guideline recommendation to consider using an alternative agent for infections caused by bacteria with an MIC ≥ 2 mg/l [3] as it is unlikely the target AUC/MIC ≥ 400 will be achieved. Additionally, centers may also consider targeting higher initial trough concentrations based on local MIC values or testing the MIC if MRSA is isolated as the potential pathogen to aid in predicting achievement of the AUC/MIC target.
Six studies [12, 17, 18, 26, 29, 32] assumed hypothetical MICs for isolates. One study [30] used the mode MIC from isolates in their study to calculate the AUC/MIC. Seixas et al. [29] assumed various MICs for their AUC/MIC calculations, but they also reported MIC results of actual isolates. Hypothetical MICs may not have been reflective of the MICs of true pathogens in patients in these trials; therefore, the results must be interpreted with caution and should not be extrapolated to pathogens with higher MICs.
Only one trial included in this review looked at clinical outcomes for efficacy in attaining trough or AUC/MIC targets [33]. They found no difference in attaining an AUC/MIC ≥ 400 compared with < 400 in terms of hospital or ICU length of stay or time to first negative blood culture. There were no reports of vancomycin-induced nephrotoxicity, and no other adverse events were reported. This was a very small trial, and no power calculation was reported, so it was likely underpowered for clinical outcomes. One trial found that vancomycin troughs ≥ 20 mg/l were associated with nephrotoxicity [29]. This is an area requiring further research as we are currently extrapolating weak evidence from adult patients to pediatric patients to support an AUC/MIC target ≥ 400. Further trials of pediatric patients receiving dialysis or extracorporeal membrane oxygenation (ECMO) are required because these patients have unique pharmacokinetic parameters and were typically excluded from the trials included in this review. Finally, no trial that demonstrated that lower targets may be considered to attain an AUC/MIC target of ≥ 400 took into account the risk of antimicrobial resistance with potentially lower vancomycin targets. This is another potential area of further research in this era of emerging antimicrobial resistance.
Several limitations of this review deserve mention. First, we did not assess the quality of the included studies, so we cannot be certain that only high-quality studies were included. Second, we included only trials published in English, so we may have excluded trials that otherwise met the inclusion criteria. Finally, we did not contact authors for unpublished information.
Heterogeneity between the studies also prevented our ability to meta-analyze the data. Different methods of calculating the AUC, determining MICs, and quantitating vancomycin concentrations contributed to the heterogeneity. In calculating the AUC, serial concentration measurement and applying the trapezoidal rule would obviously yield the most accurate results; however, this is difficult, if not impossible, in pediatric patients. Patient-individualized data should be more accurate than population estimates, yet studies have not shown a definitive benefit of using one over the other. Regardless of whether a relationship exists between trough and AUC/MIC, further research is warranted to show a predictive effect of trough and AUC/MIC on patient outcomes.
5 Conclusions
Guidelines recommend targeting vancomycin troughs of 15–20 mg/l for serious MRSA infections to attain serum AUC/MIC ≥ 400 in both pediatric and adult patients, despite a lack of evidence for these targets in pediatrics. In our review, only one trial reported a relation between vancomycin trough concentrations, AUC/MIC, and clinical outcomes in pediatric patients, and it was likely underpowered. Our results suggest that vancomycin target trough concentrations might not need to be as high as recommended in the guidelines to achieve a target AUC/MIC ≥ 400. Results varied based on the populations studied, but—for a general hospitalized pediatric patient—troughs between 6 and 10 mg/l are likely sufficient to achieve an AUC/MIC ≥ 400 if the MIC is ≤ 1 mg/l. For critically ill pediatric patients, troughs > 9 mg/l are likely necessary to attain an AUC/MIC target ≥ 400, but further research is required in this specific population. If the MIC of isolated bacteria is ≥ 2 mg/l, higher troughs are likely necessary to achieve an AUC/MIC ≥ 400, although specific recommendations cannot yet be made. More research is needed in specific populations of pediatric patients to determine the relation between vancomycin trough concentrations, AUC/MIC, and clinical outcomes.
References
Reynolds P. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis. 1989;8:943–50.
Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42:S5–12.
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52.
Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. clinical infectious diseases [Internet]. 2006;42:S35–9. https://cid.oxfordjournals.org/lookup/doi/10.1086/491712.
Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–42.
Rodvold KA, Blum RA, Fischer JH, et al. Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob Agents Chemother. 1988;32:848–52.
Pai MP, Neely M, Rodvold KA, Lodise T. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50–7.
Shipkova M, Petrova DT, Rosler AE, Orth M, Engelmayer J, Wieland E, et al. Comparability and imprecision of 8 frequently used commercially available immunoassays for therapeutic drug monitoring. therapeutic drug monitoring [Internet]. 2014;36:433–41. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00007691-201408000-00003.
McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr [Internet]. Mosby, Inc.; 2011;158:422–6. https://dx.doi.org/10.1016/j.jpeds.2010.08.019.
da Silva Alves GC, da Silva SD, Frade VP, Rodrigues D, Baldoni A de O, de Castro WV, et al. Determining the optimal vancomycin daily dose for pediatrics: a meta-analysis. Eur J Clin Pharmacol [Internet]. 2017. https://springerlink.bibliotecabuap.elogim.com/10.1007/s00228-017-2306-3.
Hadi OA, Al Omar S, Nazer LH, Mubarak S, Le J. Vancomycin pharmacokinetics and predicted dosage requirements in pediatric cancer patients. J Oncol Pharm Pract [Internet]. 2015;22:448–53. http://opp.sagepub.com/content/22/3/448?etoc.
Benefield EC, Hagemann TM, Allen HC, Farmer K, Burton ME, Chavez-Bueno S, et al. Vancomycin dosing and pharmacokinetics in postoperative pediatric cardiothoracic surgery patients. J Pediatr Pharmacol Therapeut [Internet]. 2016;21:66–74. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4778698&tool=pmcentrez&rendertype=abstract.
Chhim RF, Arnold SR, Lee KR. Vancomycin dosing practices, trough concentrations, and predicted area under the curve in children with suspected invasive staphylococcal infections. J Pediatr Infect Dis Soc. 2013;2:259–62.
Demirjian A, Finkelstein Y, Nava-Ocampo A, Arnold A, Jones S, Monuteaux M, et al. A randomized controlled trial of a vancomycin loading dose in children. Pediatr Infect Dis J [Internet]. 2013;32:1217–23. http://www.ncbi.nlm.nih.gov/pubmed/23817340.
Frymoyer A, Hersh AL, Benet LZ, Guglielmo BJ. Current recommended dosing of vancomycin for children with invasive methicillin-resistant Staphylococcus aureus infections is inadequate. Pediatr Infect Dis J [Internet]. 2009;28:398–402. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3101254&tool=pmcentrez&rendertype=abstract.
Frymoyer A, Hersh AL, Coralic Z, Benet LZ, Joseph Guglielmo B. Prediction of vancomycin pharmacodynamics in children with invasive methicillin-resistant Staphylococcus aureus infections: a Monte Carlo simulation. Clin Therapeut [Internet]. Excerpta Medica Inc.; 2010;32:534–42. https://dx.doi.org/10.1016/j.clinthera.2010.03.005.
Frymoyer A, Guglielmo BJ, Hersh AL. Desired vancomycin trough serum concentration for treating invasive methicillin-resistant Staphylococcal infections. Pediatr Infect Dis J [Internet]. 2013;32:1077–9. http://www.ncbi.nlm.nih.gov/pubmed/23652479.
Giachetto G, Telechea H, Speranza N, Oyarzun M, Nanni L, Menchaca A. Vancomycin pharmacokinetic–pharmacodynamic parameters to optimize dosage administration in critically ill children. Pediatr Crit Care Med. 2011;12:e250–4.
Gomez DS, Campos EV, De Azevedo RP, Da Silva JM, Ferreira MC, Sanches-Giraud C, et al. Individualised vancomycin doses for paediatric burn patients to achieve PK/PD targets. Burns. 2013;39:445–50.
Hahn A, Frenck RWF Jr, Zou Y, Vinks AA. Validation of a pediatric population pharmacokinetic model for vancomycin. Ther Drug Monit. 2015;37:413–6.
Hahn A, Frenck RWF Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37:619–25.
Hwang D, Chiu NC, Chang L, Peng CC, Huang DTN, Huang FY, et al. Vancomycin dosing and target attainment in children. J Microbiol Immunol Infect [Internet]. Elsevier Taiwan LLC; 2015;6–11. https://dx.doi.org/10.1016/j.jmii.2015.08.027.
Janssen EJH, Välitalo PAJ, Allegaert K, De Cock RFW, Simons SHP, Sherwin CMT, et al. Towards rational dosing algorithms for vancomycin in neonates and infants based on population pharmacokinetic modeling. Antimicrob Agents Chemother. 2016;60:1013–21.
Lanke S, Yu T, Rower JE, Balch AH, Korgenski EK, Sherwin CM. AUC-guided vancomycin dosing in adolescent patients with suspected sepsis. J Clin Pharmacol. 2016;57:77–84.
Le J, Bradley JS, Murray W, Romanowski GL, Tran TT, Nguyen N, et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J [Internet]. 2013;32:e155–63. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006454-201304000-00015.
Nassar L, Hadad S, Gefen A, Shachor-Meyouha Y, Mashiach T, Krivoy NKI. Prospective evaluation of the dosing regimen of vancomycin in children of different weights. Basic Clin Pharmacol Toxicol [Internet]. 2011;109:132–3. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=70623258.
Ploessl C, White C, Manasco K. Correlation of a vancomycin pharmacokinetic model and trough serum concentrations in pediatric patients. Pediatr Infect Dis J [Internet]. 2015;34:e244-7. http://www.ncbi.nlm.nih.gov/pubmed/26121203.
Rainkie D, Ensom MHH, Carr R. Pediatric assessment of vancomycin empiric dosing (PAVED): a retrospective review. pediatric drugs [Internet]. Springer International Publishing; 2015;17:245–53. https://dx.doi.org/10.1007/s40272-015-0122-8.
Seixas GTF, Araujo OR, Silva DCB, Arduini RG, Petrilli AS. Vancomycin therapeutic targets and nephrotoxicity in critically ill children with cancer. J Pediatr Hematol/Oncol [Internet]. 2016;38:e56–62. http://www.ncbi.nlm.nih.gov/pubmed/26558810.
da Silva DC, Seixas GT, Araujo OR, Arduini RG, Carlesse FA, Petrilli AS. Vancomycin serum concentrations in pediatric oncologic/hematologic intensive care patients. Braz J Infect Dis. 2012;16:361–5.
Zhang H, Wang Y, Gao P, Hu J, Chen Y, Zhang L, et al. Pharmacokinetic characteristics and clinical outcomes of vancomycin in young children with various degrees of renal function. J Clin Pharmacol. 2016;56:740–8.
De Cock PAJG, Desmet S, De Jaeger A, Biarent D, Dhont E, Herck I, et al. Impact of vancomycin protein binding on target attainment in critically ill children: back to the drawing board? J Antimicrob Chemother [Internet]. 2016;dkw495. https://academic.oup.com/jac/article-lookup/doi/10.1093/jac/dkw495.
Kishk OA, Lardieri AB, Heil EL, Morgan JA. Vancomycin AUC/MIC and corresponding troughs in a pediatric population. J Pediatr Pharmacol Therapeut [Internet]. 2017;22:41–7. https://www.jppt.org/doi/10.5863/1551-6776-22.1.41.
Thakkar N, Salerno S, Hornik CP, Gonzalez D. Clinical pharmacology studies in critically ill children. Pharmaceut Res [Internet]. 2016;1–18. https://dx.doi.org/10.1007/s11095-016-2033-y.
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ST and KC have no conflicts of interest. MHHE has held grants (not related to the article) from Medisca Pharmaceutique, Inc. in the past 36 months.
Rights and permissions
About this article
Cite this article
Tkachuk, S., Collins, K. & Ensom, M.H.H. The Relationship Between Vancomycin Trough Concentrations and AUC/MIC Ratios in Pediatric Patients: A Qualitative Systematic Review. Pediatr Drugs 20, 153–164 (2018). https://doi.org/10.1007/s40272-018-0282-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-018-0282-4