Abstract
Purpose
Utilization of higher doses of vancomycin to achieve the trough concentrations of 15–20 mg/L for complicated infections has been recommended by the Infectious Diseases Society of America clinical practice guideline in recent years. Concerning this recommendation, several nomograms have been constructed targeting this optimal trough level range in different populations of patients. In this review, we have collected available nomograms targeting high trough serum levels of vancomycin, particularly comparing their advantages and limitations.
Method
The data were collected by searching Scopus, PubMed, Google scholar, Medline, and Cochrane database systematic reviews. The key words used as search terms were “vancomycin”, “high trough level”, “dosing nomogram”, “dosing strategy”, “neonates”, “critically ill”, “pediatrics”, and “hemodialysis”. We have included 17 related human studies published up to the date of this publication.
Results & conclusion
Most of the available nomograms have determined the doses according to body weight and renal function. Their initial predicting success rate were 44–76 % for non-critically ill patients, 42–84 % for critically ill patients, 54 % for one nomogram specially designed for hemodialysis patients, and 71 % for the only nomogram developed for neonates. Based on validation studies, in most of cases, using a vancomycin dosing nomogram significantly improved and accelerated achievement of target trough concentrations. However, it should be noted that there are limited data about patients’ clinical and microbiological outcomes and they are only validated in narrow groups of patients. Thus, their widespread application could not be encouraged for all patients before performing adequately powered, prospective randomized studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
For the last several decades, vancomycin has been the mainstay of therapy for methicillin-resistant Staphylococcus aureus (MRSA) infections in most of cases, and still remains the first-line choice [1–11].
Vancomycin has complicated pharmacokinetic characteristics that may be highly variable among different patients depending on their age, weight, concomitant diseases and especially renal function [1–3, 5, 8]. Thus, it is highly recommended to determine vancomycin doses individually and to monitor serum trough concentration as a surrogate marker instead of the area under the curve (AUC)/minimum inhibitory concentration (MIC), which is the best determinant of vancomycin efficacy. This is particularly important in conditions like prolonged vancomycin therapy (i.e., more than 48 to 72 h), renal impairment, pregnancy, burns, obesity, and also in critically ill patients to ensure that the therapeutic, nontoxic dose reaches the patients [5, 6, 9, 12, 13].
Previously, appropriate trough concentrations were considered to be between 5 and 15 mg/L, but due to the rising MIC values of MRSA for vancomycin, recommended trough concentrations have recently increased [9, 14].
A review published through a collaboration of the American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) recommended optimizing vancomycin dosing by use of actual body weight (15–20 mg/kg/dose IV administered every eight to 12 h) to achieve a trough concentration between 15 and 20 mg/L for complicated infections including endocarditis, osteomyelitis, meningitis, and hospital-acquired, health care-associated, or ventilator-associated pneumonia, and above 10 mg/L for other indications [15]. Targeting these higher trough concentrations increases the probability of achieving an AUC24/MIC ratio of 400 or higher, which is the best predictor of vancomycin efficacy [6, 9–11, 15, 16]. Moreover, it increases drug penetration to target sites, which may improve clinical outcomes [1, 2, 4]. However, inconsistent findings have been reported in some studies [4, 17–19].
Different nomograms have been developed to individualize pharmacokinetic monitoring of vancomycin. The use of a vancomycin dosing nomogram has been proven to be more cost-effective than conventional dosing. Moreover, it could increase the probability of achieving the target serum range, and requires minimal information about patients [5, 6, 18]. But several available vancomycin dosing nomograms, which are based on linear and non-linear regression, are complex models that need specialized pharmacokinetic information and costly computer programs with target trough serum concentrations of only 5–15 mg/L [20–24]. So, a necessity to develop novel nomograms targeting higher trough levels is thus identified [1–3, 5]. Now, some dosing nomograms have been designed to achieve these high trough levels.
In this review, we have collected available evidences about dosing nomograms for achieving vancomycin high trough levels in different populations, regarding their advantages and limitations.
Methods
The data were collected by searching the Scopus, PubMed, Google scholar, Medline, and Cochrane database systematic reviews. The key words used as search terms were “vancomycin”, “high trough level”, “dosing nomogram”, “dosing strategy”, “neonates”, “critically ill”, “pediatrics,” and “hemodialysis”.
Randomized clinical trials and prospective or retrospective observational human studies about vancomycin dosing nomogram development and validation for achieving high serum trough levels in different populations such as pediatrics, neonates, and critically and non-critically ill patients were included in this review. Twenty-one articles were retrieved in this field.
Irrelevant articles (basic experimental studies, non-English language reports, studies that did not include clinical end-point assessments, and case reports) were excluded. A total of 17 relevant human studies published up to the date of this publication were included for review.
Results
This study is reported according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guideline [25].
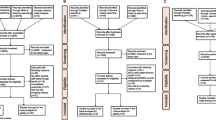
A flowchart presenting the process of initiation and selection of the studies is shown in Figure 1. In the final step of selection, 17 eligible articles were included in this systematic review, which are summarized in Table 1.
Vancomycin dosing nomograms: non-critically ill patients
Most of the available vancomycin dosing nomograms were developed for use in non-critically ill patients.
Thalakada et al. [3] performed a retrospective study on patients who received vancomycin from January 2008 to June 2010, and had achieved a trough level of 14.5–20.5 mg/L, in two Canadian hospitals (St. Paul’s & Vancouver General hospitals). They developed a vancomycin dosing nomogram for each hospital and finally an integrated nomogram by merging findings from both centers. Seventy-eight and 91 patients from St. Paul’s and Vancouver General hospitals, respectively, were assigned to the development group. Multiple linear regression with age, initial serum creatinine and actual body weight were used as variables to predict dosing intervals. As actual body weight was not significantly associated with the predicted dosing interval, they omitted it from the regression equation. A loading dose of 25 mg/kg and a maintenance dose of 15 mg/kg (maximum: 2500 mg/dose) with dosing intervals mentioned in Table 1 (suppl.) was the final recommendation of Thalakada et al. Nomogram validation was performed using the second group of patients for whom the nomogram correctly predicted the appropriate dosing interval to attain a target trough level. The integrated nomogram had a prediction success rate of 56 %. As q18h dosing is rarely prescribed in the clinical setting, the validation results were reanalyzed after its exclusion, which improved the prediction rate to 71 %. Application of this nomogram is limited by the narrow range of patients’ serum creatinine (0.5–1.6 mg/L) and by rounding the target trough level ranges [3].
A multicenter study was performed by Kullar et al. [5] prospectively in the United States to evaluate the effectiveness and safety of a newly designed vancomycin dosing nomogram, targeting trough levels of 15–20 mg/L (Table 2, suppl.). The nomogram was designed using standard vancomycin equations and population pharmacokinetic data derived from 704 patients from Detroit Medical Center using actual body weight and estimated creatinine clearance calculated by the Cockcroft-Gault equation. Two hundred patients received vancomycin based on this nomogram between June 2008 and November 2010, of which 58 % achieved the initial target trough of 15–20 mg/L and 83.8 % within a median of 2 days. They mentioned that the precision would improve if patients admitted to the intensive care unit (ICU) were excluded from analysis. Moreover, they proposed that administrating a loading dose could significantly reduce the time needed to achieve the targeted trough concentrations.
Nine patients (4.5 %) experienced nephrotoxicity, of which eight had trough serum concentrations ≥15 mg/L, but most of them (six patients) had received concomitant nephrotoxic drugs. It should be noted that the patient population was relatively young, weighed ≤110 kg, and had a stable renal function, which may have contributed to the low rate of nephrotoxicity occurrence [5].
Another protocol for vancomycin empiric dosing and monitoring was implemented at the University of Maryland Medical Center in May 2009. The protocol was incorporated into the computerized prescriber order entry (CPOE) system, which determined an initial vancomycin dose based on actual body weight (15 mg/kg/dose, maximum dose 2000 mg) and a dosing interval based on renal function (Table 3, suppl.). Target trough level was proposed at 10–20 mg/L. A retrospective study comparing pre- and post-implementation time periods was performed by Devabhakthuni et al. [26] on patients admitted to the internal ward that had received at least one dose of vancomycin (225 patients in each group). The number of patients who received an appropriate initial dose increased significantly in the post-implementation group (P < 0.001) but dosing interval appropriateness remained unchanged (P = 0.323). This may have been due to normal renal function of the majority of the patients in both groups, making a dosing interval of 12 h suitable for them. But it should be noted that appropriate initial trough concentration (44 % vs 45 %; P = 0.89), mean number of levels checked (1.9 vs 2.1; P = 0.56), and duration of therapy (4.9 vs 5.0; P = 0.77) were almost similar between the two groups. It should be noted that more patients with renal dysfunction were included in the pre-implementation group, but the appropriateness of the selected dosing interval was also significantly higher in this subgroup of patients in the pre-implementation group (61 % vs. 28 %, P < 0.001) [26].
Two vancomycin dosing nomograms were also designed in Winchester Medical Center by Wesner and his colleagues [9] for target trough levels of 10–15 mg/L and 15–20 mg/L, separately. The loading and maintenance doses are mentioned in Table 4, suppl. and dosing intervals in Table 5, suppl. [based on clearance of creatinine (ClCr)]. Then, for validating these two nomograms, patients older than 18 years, weighing ≤120 kg, for whom vancomycin was ordered from January 10 to October 31, 2011, were randomly included in treatment (novel nomogram; 221 patients) or control (traditional pharmacokinetic methods; 252 patients) groups as part of a prospective trial. Twenty percent of the patients in each group were critically ill. The average age of patients in the control group was significantly higher. More patients in the treatment group achieved appropriate trough levels (44 % vs. 33 %, P = 0.014), and subtherapeutic levels (<10 mg/L) were significantly less common in this group (P = 0.032). But no significant difference in trough levels > 20 mg/L occurrence was reported (P = 0.706). Comparing obese and non-obese patients, the nomogram accuracy was not considerably different between them (p = 0.19). The pharmacist’s impression about the nomogram significantly improved after this study, especially for its ease of use (p = 0.033) and time saving (p = 0.008), indicating that the reason for the nomogram's broad acceptance was not limited to its accuracy [9].
Lima et al. [7] designed a vancomycin dosing nomogram based on individualized pharmacokinetic parameters of 300 hospitalized patients by using a one-compartment model (Bauer method) in Brazil (Table 6, suppl.). The suggested loading dose was determined by multiplying total body weight by 25 mg, based on the IDSA guideline for MRSA therapy in seriously ill patients, and the maintenance dose was calculated based on total body weight and estimated creatinine clearance. This nomogram was accessible via the facility's Medtrak e-prescribing system. Based on MRSA vancomycin MIC distribution at this hospital, target peak and trough concentrations of vancomycin were selected as 50 and 17.5 mg/L, respectively. For patients on hemodialysis the recommended dosing interval was four days. This nomogram has not yet been endorsed for use in patients weighing >100 kg or <50 kg. This nomogram has not yet been validated [7].
Leu et al. [2] developed the first vancomycin dosing nomogram targeting high trough concentration in an Asian population. They selected six pharmacokinetic models, including the Matzke, Birt, Ambrose, Burton, revised Burton, and Bauer methods, and predicted a vancomyicn trough level for 45 patients retrospectively, based on pharmacokinetic parameters obtained from these models (Table 7, suppl.). By comparing the predicted and reported trough levels, they found that the Ambrose method was the best predictor in this population. So, they created two nomograms for targeting trough concentrations of 5–15 mg/L and 15–20 mg/L.
For evaluating the efficacy and safety of these nomograms, a study was performed on patients with MRSA-positive cultures receiving vancomycin based on conventional regimen (group A; n = 48) or nomogram (group B; n = 28). Vancomycin dosing based on the nomogram significantly improved the achievement of high trough levels in comparison with conventional dosing (41.2 % vs. 12.1 %, P = 0.019). The average duration of hospital stay was longer for group B, which may be due to factors like baseline conditions, co-morbidities, and concurrent drugs. However, the ICU stay rate was similar and the mortality rate was higher in group A. Moreover, there was no significant difference between the two groups [2].
As an AUC24/MIC ratio of 400 or higher is the best predictor of vancomycin efficacy, Michalets et al. [27] compared the outcome of patients that received vancomycin from 2005 to 2007 with a traditional dosing approach of 15–20 mg/kg/dose (N = 66) and patients who received vancomycin from 2007 to 2009 based on the AUC24/MIC nomogram in a teaching hospital, targeting AUC24/MIC ≥ 400 mg*h/L and trough level ≥ 15 mg/L (N = 67) (Table 8, suppl.). Seventy-six percent of patients in the nomogram group achieved the target AUC24/MIC, which was significantly higher than the traditional dosing group (P < 0.0001). Furthermore, the prevalence of trough levels < 15 mg/L was significantly lower in this group (P = 0.002) and fewer patients experienced nephrotoxicity (P = 0.034) [27].
So, it could be concluded that patient weight and creatinine clearance were the most significant parameters used in vancomycin dosing nomogram development in non-critically ill patients. Their initial predicting success rate was 44–76 %. This variation may be due to the different design of nomograms, specific inclusion and exclusion criteria for each study, and also the probable variations in quantification methods used in different centers for trough level measurement (which was not mentioned in most of the articles). Additionally, application of the majority of these nomograms is limited, as evidence for their clinical and microbiological outcome superiority in comparison with conventional dosing is whether not available or not significant. So, these nomograms should be validated in prospective, large, controlled studies before their widespread use is approved.
Based on available data, Michalets et al.'s nomogram is worthy of further evaluation, since in validation study it had a high achievement rate of AUC24/MIC > 400, which is the best predictor of vancomycin efficacy.
Vancomycin dosing nomograms: critically ill patients
Several recently published vancomycin dosing nomograms targeting high serum levels have primarily focused on non-critically ill patients with stable pharmacokinetic parameters. But in critically ill patients, physiologic changes, various degrees of organ dysfunction, and some of the received treatments for life support may alter pharmacokinetic parameters, resulting in unsuccessful antibiotic dosing. Critically ill patients show up to a two-fold increase in the volume of distribution of vancomycin due to fluid shifts, which increases drug clearance. Moreover, vascular permeability and variations in intravascular volume could exacerbate this increment. So, it is necessary to monitor vancomycin serum levels more accurately in this population [1, 28].
A vancomycin dosing nomogram for ICU admitted patients was designed based on actual body weight and eGFR calculated by the Modification of Diet in Renal Disease (MDRD) equation in Boston to achieve trough levels of 15–20 mg/L (Table 9, suppl.). They claimed that the MDRD formula provides the most accurate estimation of eGFR [1].
To compare patients’ outcomes before and after nomogram implementation, 57 and 60 patients were evaluated, respectively. In a three-month post-implementation evaluation, overall compliance with the nomogram was 49 %. The percentage of patients with trough level of 15–20 mg/L increased significantly from 19.3 to 41.67 % (P = 0.0099). However, a non-significant increase in the number of patients with trough levels >20 mg/L was also reported (11 vs. 18 patients). It should be mentioned that 22 % of patients in the post-implementation group had not received a loading dose but they achieved a higher mean initial trough level in comparison with patients who had received a loading dose (20.6 ± 6.9 vs. 17.3 ± 7.3 mg/L, P = 0.15). It may be due to the higher incidence of third-spacing occurrence in patients who received a loading dose or small sample size of the subgroup of patients who did not receive a loading dose. As they did not record the daily fluid status of patients, its impact on the results could not be evaluated.
There was no significant difference in nephrotoxicity occurrence between the two groups, but they still warranted close monitoring of patients for toxicity occurrence. Applicability of this nomogram is limited in patients with eGFR < 30 ml/min/1.732 or those weighing more than 150 kg or less than 40 kg [1].
In a critically ill population in the ICU ward of Alfred Hospital in Melbourne, Australia, Aubron et al. [28] prospectively evaluated a computer program for the prediction of vancomycin serum levels that is available for non-critically ill patients. Prediction of serum vancomycin concentration was carried out by MM-USCPACK program, based on a two-compartment model using a Bayesian estimation algorithm, individualized by taking into account height, weight, age, and GFR (evaluated by MDRD formula). Forty-eight patients admitted between February and May 2010 in the ICU requiring intravenous treatment with vancomycin were enrolled in the study (54 vancomycin treatments). The trough levels were higher than 15 mg/L in only 42 % of patients. The precision of prediction was acceptable (interquartile range 3.5–18.9 %) and the relative bias for all predictions was equal to −1.3 %. Obesity, unstable renal function, and severe illness, expressed by a systemic organ failure assessment (SOFA) score >11, were factors influencing the prediction [28].
It seems that Golenia et al.'s nomogram—after confirmation in a larger population of critically ill patients, especially by focusing on clinical outcome—could be applicable at least in non-obese patients with normal kidney function. However, given the high prevalence of acute kidney injury (AKI) in this population, it is necessary to design a nomogram for vancomycin dosing in critically ill patients with renal dysfunction.
Nomograms for continuous infusion of vancomycin in critically ill patients
Based on the time-dependent bactericidal activity of vancomycin, continuous infusion (CI) has been suggested frequently as an alternative for intermittent infusion especially in critically ill patients. Fast achievement of the target steady-state, lower inconsistency in drug exposure, simpler therapeutic drug monitoring, ease of administration, and lower rates of nephrotoxicity, costs, and mortality are the main advantages of CI [6, 29–38]. However, based on consensus review of ASHP, IDSA, and SIDP, the priority of CI versus intermittent infusion regarding treatment outcome is questioned except in patients with ventilator-associated pneumonia caused by MRSA [15]. Moreover, different factors, especially augmented renal clearance (ARC; defined as a creatinine clearance exceeding 130 ml/min for at least 8 h), which is common in critically ill patients, could still lead to a high clearance of hydrophilic antibiotics and subtherapeutic serum levels.
There are some dosing regimens for CI of vancomycin in ICU patients targeting high serum concentrations that should be evaluated accurately before their widespread use.
A 13-month single-center retrospective study was conducted by Baptista et al. [8] in Portugal on all ventilated, adult patients with severe sepsis or septic shock who received vancomycin (79 patients). They developed a formula for optimizing vancomycin dosing by evaluating the correlation of vancomycin clearance and 8 h clearance of creatinine. The target steady-state concentration (Css) was 20–30 mg/L. The nomogram was tested prospectively in a cohort study on 25 patients. The number of patients who achieved an adequate vancomycin serum level in the first 24 h was significantly higher in the post-implementation group (51 % vs. 84 %, P < 0.005). Thirty-six percent of patients the in pre-implementation group were cases of ARC, most of which (72 %) had not achieved target Css, but all of the ARC patients in the post-implementation group (10 patients) met it. Nephrotoxicity occurred in 6.3 % of the pre-implementation group patients and in none of the post-implementation group. Actually, this dosing nomogram significantly increased the achievement of therapeutic concentrations in the first 24 h of treatment, particularly in patients exhibiting ARC [8].
In a retrospective study performed by Ocapmos-Martinez et al. [39] 261 septic ICU patients received vancomycin as a loading dose of 15 mg/kg over 60–90 min, independent of kidney function, and then 30 mg/kg infused over 24 h for patients with normal kidney function, 20–30 mg/kg for patients with 50 < ClCr < 80 ml/min, and 10–20 mg/kg for patients with ClCr <50 ml/min, with a target serum level of 20–30 mg/L. The ClCr was calculated from 24-h urine collection and normalized to body surface area. Subtherapeutic levels had been reported in 53, 33, and 14 % of patients on days 1, 2, and 3, respectively. High ClCr and male sex were proposed as the variables most strongly associated with insufficient serum levels [39].
Jeurissen and colleagues [40] also proposed that a loading dose of 1000 mg followed by a daily continuous infusion of up to 3000 mg is necessary for achieving a serum level of 25 mg/L in septic ICU patients with normal renal function. They included 20 patients who received vancomycin by continuous infusion between April 2009 and April 2010. Vancomycin clearance calculated by measured vancomycin serum concentration was used for creation of the nomogram [40].
In another retrospective study, Saugel et al. [37] compared patients treated with continuous infusion (a loading dose of 1000–1250 mg, followed by a 60 mg/h continuous infusion with target serum level of 15–25 mg/L) with patients treated with intermittent infusion. This CI regimen was sufficient for achievement to target trough concentration, but it usually resulted in subtherapeutic levels on the first days of treatment [37].
So, based on these findings, they designed a CI regimen with a higher loading dose. Then, an observational study was conducted by Saugel et al. between June 2012 to February 2013 on 34 ICU admitted patients who needed vancomycin and received it as a loading dose of 20 mg/kg (during 180 min) followed by a continuous infusion (20–30 mg/kg, based on renal function, over 24 h). The targeted serum level was 20–30 mg/L (Figure 1, suppl.). The overall adherence to the guideline was 82 %. Forty-eight and 69 % of patients achieved a target level on the first and 7th days of treatment, respectively. However, they found supra-therapeutic serum levels especially on the second to fourth days of treatment [41]. It seems that the defined loading dose in the new regimen was too high.
In another study, Maarseveen et al. [42] designed a dosing nomogram for CI of vancomycin by simulation of CI using the PK software package MW/Pharm and a pharmacokinetic population model based on the historical population data of patients on intermittently dosed with vancomycin. This nomogram was developed based on eGFR calculated by MDRD formula (Table 10, suppl.). Vancomycin serum concentration of 15–20 mg/L at 24 h (C24) was set as the target level as well as an AUC ≥ 350 mg.h.L−1. Prospective validation performed on 35 general ward and 45 ICU patients showed 69 and 63 % achievement to target C24 and 80 and 72 % to target AUC, respectively. In this study patients weighing <50 kg or >100 kg and hemodialysis patients were excluded [42].
Therefore, considering all available findings on vancomycin dosing as CI, it seems that similar to with intermittent dosing it is difficult to suggest a dosing regimen that will reliably result in the therapeutic serum level in all patients and it is noteworthy that most studies evaluating this dosing regimen lack control groups. But based on available data, it can be hypothesized that these nomograms may be effective alternatives in comparison with current intermittent dosing procedure. Regarding the high achievement of target serum concentrations, it seems valuable to validate Baptista et al.'s nomogram in a larger population.
Vancomycin dosing nomograms: hemodialysis patients
One of the main causes of mortality in hemodialysis patients is infection, especially with Gram-positive bacteria penetrating from the dialysis site. Vancomycin is used in most of these cases but few data are available about its dosing in this population. Furthermore, regarding the drug's removal by hemodialysis, achieving the currently recommended high trough levels is more difficult in these patients.
One vancomycin dosing nomogram was introduced by Jeremiah et al. [43] in Darwin University, Australia, for hemodialysis patients in 2012. The initial dose of vancomycin is a loading dose of 25 mg/kg (maximum 2 g). Levels should be checked immediately at the beginning of the next dialysis session to guide further dosing according to the Table 11 (suppl.) (maximum administration rate 1 g/h). For evaluation of this nomogram, 25 hemodialysis patients that received 28 vancomycin courses were assessed retrospectively. A 25-mg/kg loading dose was administered in six patients but the others received a fixed dose of 1 g, and the mean trough level was significantly higher in the first group prior to the next dialysis session (16.9 vs 9.8 mg/L, P = 0.01). The maintenance dose was compatible with the nomogram in 97 % of cases. Trough levels of 15–20 mg/L were achieved in 54.4 % of patients [43]. This nomogram should be validated in a larger population prospectively and in comparison with a control group before its recommendation for general use is approved.
Vancomycin dosing nomograms: neonates
Late-onset sepsis (LOS) is a major cause of morbidity and mortality in neonates. Gram-positive bacteria are the most common pathogens (70 %) causing LOS, which usually have been treated with vancomycin for many years [24, 34]. An optimal dosing regimen for vancomycin in the neonate does not exist. Moreover, although no strong correlation between trough level and vancomycin efficacy is mentioned in neonates, adult target levels are usually used in this population. Due to various differences between neonates and adults in drug pharmacokinetic and pharmacodynamics, several different vancomycin dosing regimens are accessible in main pediatric handbooks and about 70 % of neonates only achieve subtherapeutic levels [44–48].
Continuous infusion of vancomycin could be an alternative for the usual intermittent infusion in neonates in order to accelerate achievement of target levels. Different regimens with or without a loading dose, adjusted based on weight, age, and/or renal function, have been proposed.
In a study conducted in three hospitals in France, the probability of reaching the target concentration in neonates with three different dosing regimens was assessed and vancomycin dosing was optimized using NONMEM software [34]. Patient-tailored optimized dosing regimens were evaluated in a prospective study. One hundred sixteen neonates receiving vancomycin as CI between November 2010 and June 2011 were included. They administered a loading dose of 10–15 mg/kg in two hospitals and no loading dose in the third one, and the recommended maintenance doses are summarized in Table 12 suppl. The target trough range was proposed as 15–25 mg/L. From 116 enrolled neonates, only 41.4 % of these patients achieved therapeutic trough levels and in 4.3 % of cases the trough level was <10 mg/L. Most of the subtherapuetic levels were observed in the hospital not using a loading dose, supporting its necessity given the long half-life of vancomycin in neonates.
Pharmacokinetic analysis was performed using the non-linear modeling program NONMEM VI, taking into account birth and current weight, postnatal age (PNA), and serum creatinine to fit a one-compartment model. Then, 100 simulated trials were carried out to optimize the dosing, which demonstrated good predictive performance for the developed models. Clinical validation of this optimized regimen was tested in a prospective study on 58 neonates. Most of the patients (70.7 %) achieved the goal trough level and just 15.5 % had subtherapeutic concentration [34].
Based on Zhao et al.'s findings, it seems that this nomogram could be applicable in a NICU setting. However, it should first be validated in a large prospective clinical trial in comparison with conventional dosing before its widespread use is approved.
Vancomycin dosing nomograms: pediatrics
Based on recent guidelines, the recommended dosing of vancomycin for achieving trough levels of 15–25 mg/L in children with normal kidney function is 15 mg/kg every 6 h [10]. Unlike infancy and early childhood, little is known about the pharmacokinetic differences of teenagers and adults. This uncertainty makes it difficult to choose an appropriate vancomycin dosing regimen in teens. Vancomycin nomograms for adults may be applicable for older teens, as after puberty body mass and ClCr become similar to adults, but they should be evaluated in this population. Gillon et al. [14] assessed two nomograms designed for adult patients (Table 13 and 14, suppl.) in children older than 10 years, weighing ≥40 kg with normal renal function in a pediatric hospital in the USA. The targeted trough level range was proposed as 10–20 mg/L. Evaluating 165 trough level from 120 patients, 53 trough levels were in the desired range. Thirty-eight patients were enrolled in the final analysis. The mean daily dose prescribed to achieve a desired trough level (53 mg/kg/day) was significantly higher than the predicted dose by nomogram A (36 mg/kg/day) (P < 0.001) and lower than the predicted dose of nomogram B (73 mg/kg/day) (P < 0.001). Evaluating patient groups separately, the mean daily dose required for achieving the target trough level was 50 mg/kg/day in healthy children. Doses predicted by the nomogram were more appropriate for oncology patients [14]. So, application of nomograms developed in adult patients in pediatrics may cause some errors in vancomycin dosing and thus they are not usable before accurate evaluation and validation in this population.
Conclusion
Available data show that using a vancomycin dosing nomogram can be a useful tool for achieving high target trough concentrations in a greater percentage of patients earlier in the course of treatment. However, there are limited data about clinical and microbiological outcomes related to dosing based on these nomograms. So far, they are only validated in specific groups of patients, so they should be used with caution in the general population and clinical judgment should not be completely replaced with nomograms. In addition, some of these nomograms have not been validated or are only validated based on retrospective studies with small sample sizes that are not adequate for concluding their efficacy and safety. Adequately powered, prospective randomized studies are needed to confirm the superiority of each nomogram. Finally, it should be noted that although most of these nomograms significantly improved achievement of target trough levels, the percentage of target level achievement has been between 40 and 70 % in most of cases, which is not ideal, and thus it seems necessary to continue development of more accurate nomograms for vancomycin dosing.
References
Golenia BS, Levine AR, Moawad IM, Yeh DD, Arpino PA (2013) Evaluation of a vancomycin dosing nomogram based on the Modification of Diet in Renal Disease equation in intensive care unit patients. J Crit Care 28(5):710–716
Leu WJ, Liu YC, Wang HW, Chien HY, Liu HP, Lin YM (2012) Evaluation of a vancomycin dosing nomogram in achieving high target trough concentrations in Taiwanese patients. Int J Infect Dis 16(11):e804–e810
Thalakada R, Legal M, Lau TT, Luey T, Batterink J, Ensom MH (2012) Development and validation of a novel vancomycin dosing nomogram for achieving high-target trough levels at 2 canadian teaching hospitals. Can J Hosp Pharm 65(3):180–187
Hall RG 2nd, Giuliano CA, Haase KK, Hazlewood KA, Frei CR, Forcade NA et al (2012) Empiric guideline-recommended weight-based vancomycin dosing and mortality in methicillin-resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Infect Dis 12:104
Kullar R, Leonard SN, Davis SL, Delgado G Jr, Pogue JM, Wahby KA et al (2011) Validation of the effectiveness of a vancomycin nomogram in achieving target trough concentrations of 15–20 mg/L suggested by the vancomycin consensus guidelines. Pharmacotherapy 31(5):441–448
Avent ML, Vaska VL, Rogers BA, Cheng AC, van Hal SJ, Holmes NE et al (2013) Vancomycin therapeutics and monitoring: a contemporary approach. Intern Med J 43(2):110–119
Lima TM, Elias SC, Estrela RC, Cardoso FL (2014) Implementation of vancomycin dosing nomogram in an elecreonic describing system: an innovative tool in antibiotic stewardship. Braz J Pharm Sci 50(3):567–572
Baptista JP, Roberts JA, Sousa E, Freitas R, Deveza N, Pimentel J (2014) Decreasing the time to achieve therapeutic vancomycin concentrations in critically ill patients: developing and testing of a dosing nomogram. Crit Care 18(6):654
Wesner AR, Brackbill ML, Coyle LL, Kidd RS (2013) Prospective trial of a novel nomogram to achieve updated vancomycin trough concentrations. Interdiscip Perspect Infect Dis. doi:10.1155/2013/839456
Liu C, Bayer A, Cosgrove SE et al (2011) Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292
Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A (2012) Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol 68(9):1243–1255
Mueller M, Pena A, Derendorf H (2004) Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: kill curves versus MIC. Antimicrob Agents Chemother 48(2):369–377
Marquis KA, DeGrado JR, Labonville S, Kubiak DW, Szumita PM (2015) Evaluation of a pharmacist-directed vancomycin dosing and monitoring pilot program at a Tertiary Academic Medical Center. Ann Pharmacother 49(9):1009–1014
Gillon JE, Cassat JE, Di Pentima MC (2014) Validation of two vancomycin nomograms in patients 10 years of age and older. J Clin Pharmacol 54(1):35–38
Rybak M, Lomaestro B, Rotschafer JC et al (2009) Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98
Elyasi S, Khalili H, Dashti-Khavidaki S, Emadi-Koochak H, Mohammadpour A, Abdollahi A (2014) Elevated vancomycin trough concentration: increased efficacy and/or toxicity? Iran J Pharm Res 13(4):1241–1247
Ackerman BH, Guilday RE, Reigart CL, Patton ML, Haith LR (2013) Evaluation of the relationship between elevated vancomycin trough concentrations and increased efficacy and/or toxicity. J Burn Care Res 34(1):e1–e9
Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A (2006) High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 166(19):2138–2144
Jeffres MN, Isakow W, Doherty JA, McKinnon PS, Ritchie DJ, Micek ST et al (2006) Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest 130(4):947–955
Moellering RC Jr, Krogstad DJ, Greenblatt DJ (1981) Vancomycin therapy in patients with impaired renal function: a nomogram for dosage. Ann Intern Med 94:343–346
Matzke GR, McGory RW, Halstenson CE, Keane WF (1984) Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother 25:433–437
Karam CM, McKinnon PS, Neuhauser MM, Rybak MJ (1999) Outcome assessment of minimizing vancomycin monitoring and dosing adjustments. Pharmacotherapy 19:257–266
Lake KD, Peterson CD (1988) Evaluation of a method for initiating vancomycin therapy: experience in 205 patients. Pharmacotherapy 8:284–286
Thomson AH, Staatz CE, Tobin CM, Gall M, Lovering AM (2009) Development and evaluation of vancomycin dosage guidelines designed to achieve new target concentrations. J Antimicrob Chemother 63(5):1050–1057
Moher D, Shamseer L, Clarke M, Ghersi D et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) statement. Syst Rev 2015:4(1). doi:10.1186/2046-4053-4-1
Devabhakhthuni S, Gonzales JP, Tata AL, Lee S, Shah P, Offurum AI, Gulati M (2012) Evaluation of vancomycin dosing and monitoring in adult medicine patients. Hosp Pharm 47(6):451–459
Michalets EL, Pounders SJ, Hollis SJ, Sutherland S (2011) Outcomes associated with AUC24/MIC nomogram dosing of vancomycin. Ann Pharmacother 45(5):687–689
Aubron C, Corallo CE, Nunn MO, Dooley MJ, Cheng AC (2011) Evaluation of the accuracy of a pharmacokinetic dosing program in predicting serum vancomycin concentrations in critically ill patients. Ann Pharmacother 45(10):1193–1198
Blot SKD, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, Roberts JA (2014) Does contemporary vancomycin dosing achieve therapeutic targets in a heterogeneous clinical cohort of critically ill patients? Data from the multinational DALI Study. Crit Care 18:R99
Wysocki M, Delatour F, Faurisson F, Rauss A, Pean Y, Misset B, Thomas F, Timsit JF, Similowski T, Mentec H, Mier L, Dreyfuss D (2001) Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 45:2460–2467
Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N (2012) Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother 67:17–24
Rello J, Sole-Violan J, Sa-Borges M, Garnacho-Montero J, Munoz E, Sirgo G, Olona M, Diaz E (2005) Pneumonia caused by oxacillin-resistant Staphylococcus aureus treated with glycopeptides. Crit Care Med 33:1983–1987
Hutschala D, Kinstner C, Skhirdladze K, Thalhammer F, Muller M, Tschernko E (2009) Influence of vancomycin on renal function in critically ill patients after cardiac surgery: continuous versus intermittent infusion. Anesthesiology 111:356–365
Zhao W, Lopez E, Biran V, Durrmeyer X, Fakhoury M, Jacqz-Aigrain E (2013) Vancomycin continuous infusion in neonates: dosing optimisation and therapeutic drug monitoring. Arch Dis Child 98(6):449–453
Roberts JA, Lipman J, Blot S et al (2008) Better outcomes through continuous infusion of time-dependent antibiotics to critically ill patients? Curr Opin Crit Care 14:390–396
Pea F, Furlanut M, Negri C et al (2009) Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in critically ill patients. Antimicrob Agents Chemother 53:1863–1867
Saugel B, Nowack MC, Hapfelmeier A et al (2013) Continuous intravenous administration of vancomycin in medical intensive care unit patients. J Crit Care 28:9–13
De Waele JJ, Danneels I, Depuydt P et al (2013) Factors associated with inadequate early vancomycin levels in critically ill patients treated with continuous infusion. Int J Antimicrob Agents 41:434–438
Ocampos-Martinez E, Penaccini L, Scolletta S et al (2012) Determinants of early inadequate vancomycin concentrations during continuous infusion in septic patients. Int J Antimicrob Agents 39:332–337
Jeurissen A, Sluyts I, Rutsaert R (2011) A higher dose of vancomycin in continuous infusion is needed in critically ill patients. Int J Antimicrob Agents 37:75–77
Saugel B, Gramm C, Wagner JY, Messer M, Lahmer T, Meidert AS et al (2014) Evaluation of a dosing regimen for continuous vancomycin infusion in critically ill patients: an observational study in intensive care unit patients. J Crit Care 29(3):351–355
Maarseveen EM, Bouma A, Touw DJ, Neef C et al (2014) Design and prospective validation of a dosing instrument for continuous infusion of vancomycin: within-population approach. Eur J Clin Pharmacol 70:1353–1359
Jeremiah CJ, Wills C, Bayly A, Perry GJ, Davis JS, Tong SY et al (2014) Vancomycin dosing nomogram for haemodialysis patients. Nephrology 19(8):513–514
Vandendriessche A, Allegaert K, Cossey V, Naulaers G, Saegeman V, Smits A (2014) Prospective validation of neonatal vancomycin dosing regimens is urgently needed. Curr Ther Res Clin Exp 76:51–57
Young TE (2011) Neofax, 24th edn. Thomson Reuters, Montvale
Tschudy M, Arcara KM (2012) The Harriet lane handbook, 19th edn. Elsevier Mosby, Philadelphia
Sanford JP (2013) Sanford guide to antimicrobial therapy 2012–2013, 23th Edition of Belgian/Luxemburg version Sperryville, J.C. Sanford
(2011) Neonatal formulary. Drug use in pregnancy and the first year of life. 6th edn, BMJ books Wiley-Blackwell
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors of the manuscript have no competing interests to report with respect to this work.
Funding
None
Ethical approval
Not required
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1
(DOCX 19 kb)
Table 2
(DOCX 19 kb)
Table 3
(DOCX 18 kb)
Table 4
(DOCX 18 kb)
Table 5
(DOCX 18 kb)
Table 6
(DOCX 19 kb)
Table 7
(DOCX 20 kb)
Table 8
(DOCX 31 kb)
Table 9
(DOCX 19 kb)
Table 10
(DOCX 18 kb)
Table 11
(DOCX 18 kb)
Table 12
(DOCX 19 kb)
Table 13
(DOCX 20 kb)
Table 14
(DOCX 20 kb)
Figure 1
(DOCX 368 kb)
Rights and permissions
About this article
Cite this article
Elyasi, S., Khalili, H. Vancomycin dosing nomograms targeting high serum trough levels in different populations: pros and cons. Eur J Clin Pharmacol 72, 777–788 (2016). https://doi.org/10.1007/s00228-016-2063-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2063-8