Abstract
Inflammatory bowel diseases are chronic disorders of the gastrointestinal tract that include Crohn’s disease (CD), ulcerative colitis (UC) and inflammatory bowel disease-unclassified (IBDU). The latter defines a subgroup of patients with clinical and endoscopic evidence of chronic colitis, without specific features of either CD or UC. These patients will possibly be re-classified as having UC or CD during the follow-up, although a significant percentage of them will keep the diagnosis of IBDU. IBDU is the rarest subtype of IBD, both in children and in adults, although it is twice as common among the pediatric population, especially in the younger ages. The diagnosis can only be made after a comprehensive diagnostic work-up, combining clinical history, physical and laboratory examination, upper and lower gastrointestinal endoscopy, with histology and imaging of the small bowel. The therapeutic strategy is borrowed from that of UC and CD, although recent data suggest that IBDU has a lower therapeutic burden with a generally mild disease course and a good response to mesalamine. Since there are only few published data on pediatric IBDU, and no guidelines on its management are available, this review aims at summarizing the most recent evidence for the diagnostic work-up with a specific focus on medical and surgical options in the treatment of IBDU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Inflammatory bowel disease-unclassified (IBDU) is a chronic disorder belonging to the inflammatory bowel diseases, characterized by an isolated colitis with no specific features of Crohn’s disease and ulcerative colitis. |
The diagnosis is based on a comprehensive diagnostic work-up, including evaluation of the upper and lower gastrointestinal tract. |
Based on the limited data available, IBDU seems to have a mild disease course, with a high response rate to mesalamine. Nevertheless, given the risk of misclassification, extreme caution must be taken when surgery is advised as a possible therapeutic option. |
1 Introduction
Inflammatory bowel diseases (IBDs) are chronic, relapsing, multifactorial disorders of the gastrointestinal tract, conventionally divided into three subtypes: Crohn’s disease (CD), ulcerative colitis (UC), and inflammatory bowel disease-unclassified (IBDU). The lattermost was introduced after the Montreal working group in 2005 and refers to patients with clinical and endoscopic evidence of IBD affecting the colon, without small bowel involvement, for whom a definitive diagnosis of either CD or UC is not possible [1]. The same working group reserved the original definition of indeterminate colitis (IC) by Ashley Price for those cases showing overlapping macroscopic and microscopic features of CD and UC in surgical specimens [1].
In children, the high frequency of atypical phenotypes of UC, like rectal sparing, backwash ileitis, skip lesions, cecal patch and gastric erosions [2], makes the diagnosis of an isolated colitis with no specific histological findings often challenging. Specifically, backwash ileitis, defined as the extension of macroscopic or histologic inflammation from the cecum to the most distal ileum, is a relatively common finding in pediatric UC, with a reported prevalence of 6–22% among patients with pancolitis [2]. Studies including patients with IBDU are mostly small and retrospective, and are unable to provide treatment conclusions [3]. This is related to the frequent exclusion of patients with IBDU from clinical trials due to both its relative rareness, and to previous uncertainties over its exact definition. The recently revised Porto criteria for IBD have added a specific indication for the diagnosis of IBDU in children [4], but there are no current available treatment guidelines for its management and it is debated whether clinicians should follow guidelines for CD or UC when treating IBDU patients [5, 6].
Thus, the aim of this article is to provide notes for clinical, pharmacological, and surgical management of IBDU as drawn from the most recent evidence, providing a diagnostic workup and suggesting a therapeutic algorithm.
2 Epidemiology
IBDU is the rarest subtype of IBD, both in children and in adults, although it is twice as common among the pediatric population. According to a recent meta-analysis, its prevalence at diagnosis is 13% in children (<18 years) and 6% in adults [7]. A recent study from the EUROKIDS registry by the Porto IBD working group of ESPGHAN (European Society for Paediatric Gastroenterology Hepatology and Nutrition) reported an initial diagnosis of IBDU in 265 of 3461 children with IBD (7.7%); interestingly, only half of them (48%) had undergone complete diagnostic workup [8]. This percentage reduced to 5.6% at the end of a 5-year follow-up, closer to the incidence rate described in other European cohorts [9–11]. Previous retrospective studies reported even higher rates of pediatric IBDU at diagnosis (up to 30%); one third of them were reclassified as UC or CD at follow-up [12].
The frequency of IBDU seems to be higher in younger age groups with a direct relationship between age and frequency of IBDU [8, 10, 12–16]. The same study from the EUROKIDS registry showed a significant correlation between the diagnosis of IBDU and lower median age; moreover, 34% of the children diagnosed with IBDU were younger than 10 years, whereas a lower proportion was observed among children diagnosed with CD and UC (19 and 26%, respectively) [8]. Similar results have been extrapolated from a large cohort of Italian children with IBD reporting an initial diagnosis of IBDU in 37 (7%) out of 506 patients, rising to 22% among children diagnosed under 5 years of age [13]. These results are in line with data from a large North American registry [10]. Very recently, a retrospective study of 62 patients with very-early-onset disease (before 2 years of age) reported a diagnosis of IBDU in 44 cases (71%) [14].
The atypical phenotype of pediatric IBD, along with the high rate of isolated colitis in early-onset disease [13–16], can partly explain the high frequency of IBDU in children. Moreover, an incomplete diagnostic workup (no evaluation of the small bowel and of the terminal ileum, no or incomplete esophagogastroduodenoscopy (EGD), or a combination of these) seems to occur more frequently among children diagnosed with IBDU compared with CD and UC cases [8]. The initial lack of diagnostic criteria and reevaluation during follow-up may possibly account for the initial higher rate of pediatric IBDU and its subsequent decline at the time of follow up [8, 16, 17]. Nevertheless, while reclassification occurs in about 80% of adults originally labeled as IBDU [18], approximately two thirds of children keep this diagnosis during the follow-up, particularly among younger age groups [8, 12, 16, 17].
No significant correlation with the duration of symptoms prior to the diagnosis has been reported, although a few studies showed a longer interval between onset of symptoms and diagnosis of CD and IBDU compared with UC [10].
3 Diagnosis
The diagnostic challenge in pediatric IBD occurs when an isolated colitis, without specific histological features (e.g., granulomas, transmural inflammation), is found at endoscopy. Plus, as already mentioned, pediatric IBD often presents with atypical features, making it difficult to distinguish between CD and UC, with a high risk of misclassification [4]. For instance, data from a large cohort of children originally diagnosed with UC and undergoing ileal pouch-anal anastomosis (IPAA) reported 14% of participants were reclassified as having CD after a 5-year follow-up [19]. Therefore, a complete diagnostic workup is required and, as stated by the recently revised Porto criteria, should be based on a combination of clinical history, physical and laboratory examination, endoscopy (EGD and ileocolonoscopy) with histological examination of all visualized bowel sections, and imaging of the small bowel. The latter can be deferred only after a diagnosis of typical UC is made [4]. The diagnostic workup in children and adolescents for the diagnosis of IBDU is presented in Fig. 1.
The diagnostic workup in children and adolescents for the diagnosis of IBDU. ASCA anti-Saccharomyces cerevisiae antibody, CD Crohn’s disease, CRP C-reactive protein, EGD esophagogastroduodenoscopy, ESR erythrocyte sedimentation rate, IBDU inflammatory bowel disease-unclassified, MRE magnetic resonance enterography, pANCA perinuclear antineutrophil cytoplasmic antibody, SB small bowel, SICUS small intestine contrast ultrasonography, UC ulcerative colitis, UGI upper gastrointestinal involvement, WCE wireless capsule endoscopy
3.1 Symptoms and Signs
Clinical features are not useful for the diagnosis of IBDU. Bloody diarrhea with nocturnal defecation and abdominal pain (with eventually rectal urgency and tenesmus in case of rectal involvement) are the classic symptoms of a left-sided or extensive colitis, non-specific to any of the forms of chronic colitis [18]. Growth impairment is not typical of UC, while it is more commonly reported in patients initially classified as UC or IBDU and who are re-diagnosed as CD during follow-up [20].
No difference in the rate of positive family history has been reported between IBDU, UC, and CD patients (overall rate 11.1%, range 11.4–9.9%) in the EUROKIDS cohort [8]. Nevertheless, since genetic/environmental influences have been proven to be stronger in CD than in UC [21], a positive familial history, especially for CD, might represent a useful clue to take into account when addressing the final diagnosis in patients displaying IBD colitis and no specific findings.
3.2 Endoscopic and Histological Features
Upper gastrointestinal (GI) endoscopy and Ileocolonoscopy with biopsies taken from each segment should be performed in all patients with a chronic colitis. The macroscopic and histopathological definition of IBDU is not a ‘positive’ diagnosis but relies on the absence of a clear diagnostic pattern unequivocally suggestive of either CD or UC.
The Porto criteria provide some elements that can help exclude a diagnosis of UC in the presence of an untreated colitis at endoscopy, and lead to a diagnosis of CD (granulomas, deep serpentine ulcerations, cobblestoning or stenosis, perianal skin tags, evidence of significant small bowel inflammation, ileal inflammation with a normal cecum) or IBDU. The latter should be diagnosed when at least one of the elements (which rarely appear in UC) illustrated in Table 1 is present. Pediatric IBDU, as well as UC, commonly presents as an extensive disease, with pancolitis and extensive colitis frequently described [8, 10, 22]. Upper GI involvement is reported in up to one third of IBDU patients, especially in those with pancolitis [8]. There is no histological typical feature of IBDU, whose diagnosis is based on the absence of specific findings of both UC and CD [4, 18].
Repeated investigations, both by endoscopy and imaging, should be performed at follow-up, possibly allowing a change of the initial diagnosis of IBDU to either UC or CD [8].
3.3 Imaging, Biomarkers, and Genetics
3.3.1 Imaging
An accurate visualization of the small bowel, to detect any involvement that could lead to a different diagnosis, is recommended in all cases of IBDU [4]. According to the Porto IBD guidelines for the diagnosis of CD [4], magnetic resonance elastography (MRE) is the recommended diagnostic imaging modality for small bowel visualization, although a validation-based scoring system is not currently available for children [23, 24].
3.3.2 Biomarkers
Neither the acute phase C-reactive protein nor all the available fecal biomarkers (fecal calprotectin, lactoferrin) are useful for the differential diagnosis of subjects with IBD colitis [18]. Anti-Saccharomyces cerevisiae antibody (ASCA) and perinuclear antineutrophil cytoplasmic antibody (pANCA) are the two most widely studied immunological biomarkers. As shown by a meta-analysis of 60 studies (7860 IBD subjects and 3748 controls), combinations of these antibodies can help distinguish CD from UC with 40–50% sensitivity and 90% specificity, although their performance drops significantly when restricted to patients with colonic disease [25–29]. Very limited data are available about the serological profile of patients diagnosed with IBDU. In the largest cohort of IBDU patients examined so far, aiming at exploring the diagnostic utility of serological profile together with its ability to predict long-term outcomes and change of diagnosis in these patients, the most prevalent serologic profile was pANCA−/ASCA− (41%), followed by pANCA+/ASCA− and pANCA−/ASCA+ (34 and 17%, respectively). ASCA+/pANCA− profile, when present, was shown to have a high positive predictive value (PPV) (96%) and a specificity of 83% in differentiating IBDU from isolated Crohn’s colitis (CC). However, due to its low prevalence, its negative predictive value (NPV) dropped to 13%. A PPV of 94% was reported for pANCA+/ASCA− profile in differentiating UC from IBDU but, again, with low NPV (38%), sensitivity (65%), and specificity (66%) [30]. The same pANCA−/ASCA− profile was reported in 47 out of 97 (48.5%) adults with IBDU [31].
Serological markers may help predict a complicated disease behavior and the disease course in patients with IBDU as well. A disease progression or a high risk of complication after pouch surgery seems to be related to the presence of multiple antibodies [32]. Conversely, ASCA and pANCA seronegativity seems to predict the maintenance of a diagnosis of IBDU [30]. The adult study by Joossens et al. [31] reported similar results: while the seronegative group kept the diagnosis of IBDU in 85% of cases, among 26 IBDU patients classified at the time of diagnosis as ASCA+/pANCA−, eight were later diagnosed with CD and two with UC; contrarily, patients with a ASCA−/pANCA+ profile were more likely to be reclassified as UC [31].
No conclusive recommendation can be drawn from these data and the suboptimal performance of all these marker combinations hinders their contribution to clinical practice.
3.3.3 Genetics
It is beyond the objective of this review to detail the recent advances in the genome-wide association studies (GWAS) in IBD due to their still-limited use in clinical practice. The number of confirmed IBD susceptibility loci has risen to 163, thus showing an important overlap in genetic risk factors between CD an UC [25]. In 2008, von Stein et al. identified a seven-gene panel with the ability to correctly classify IBD in >92% of cases, identifying nine out of ten changes in clinical diagnosis in a subset of 20 IBDU patients [33]. These data have been confirmed [34] but also refuted [35] in more recent studies and, although promising, are far from having an impact on disease management.
An integrated classification scheme involving clinical, serological, and genetic markers that could help indicate different IBD subtypes is warranted. Further prospective analyses are needed to demonstrate whether a combination of currently available serological and genetic markers might help in predicting the course of patients with colonic IBDU.
4 Management
Consensus guidelines of the European Crohn’s Colitis Organization (ECCO) and ESPGHAN have been recently reviewed and they provide useful evidence-based information on the medical management of pediatric CD and UC [5, 6]. No such guidelines are currently available for patients with IBDU and therefore their therapeutic management is predominantly drawn from clinical experience. Very recently, treatment options and outcomes of pediatric IBDU have been evaluated in a large cohort of patients with a diagnosis of IBDU (260 children with IBDU, 250 with CD and 287 with UC), showing a significantly lower treatment burden for patients with IBDU at follow-up, compared with those with CD and partly UC [22].
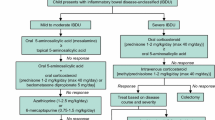
In the absence of specific IBDU treatment guidelines, some recommendations could possibly be borrowed from the strategies used to induce and maintain remission in UC and isolated CC. A proposed therapeutic algorithm is reported in Fig. 2.
4.1 Mesalamine
Oral 5-aminosalicyclic acid (5-ASA) is recommended as the first-line induction and maintenance therapy (alone or in combination with rectal therapy) for both adults and children with mild to moderate UC [25]. Mesalamine was the most common first-line treatment in patients with active IBDU (88%) included in the ESPGHAN cohort, with most of them maintaining remission at follow-up with this class of drugs [22]. Based on the limited data available, 5-ASA may be recommended as first-line therapy in children with IBDU.
4.2 Exclusive Enteral Nutrition
Exclusive enteral nutrition (EEN) is the primary recommended therapy for children with active CD but seems to be ineffective in UC [5, 6]. There are controversial results of its efficacy in isolated CC with only six studies (five on children) analyzing these patients separately from those with ileal disease, and none of them is conclusive [25]. There is no evidence supporting the use of EEN in treating children with IBDU.
4.3 Corticosteroids
Pediatric treatment guidelines recommend corticosteroid use for the induction of remission in mild to moderate UC not responding to oral 5-ASA. They show a short-term effectiveness, but long-term use is associated with significant adverse effects. Lower corticosteroids use was reported for patients with IBDU compared with UC (59 vs 71%) [22]; nevertheless, corticosteroids should be considered as the first-line treatment option for children with an extensive IBDU scarcely responsive to 5-ASA. Although there is no data on the efficacy of oral prolonged-released beclomethasone dipropionate in IBDU, a very recent systematic review and meta-analysis on its efficacy in UC showed superior efficacy versus oral 5-ASA in inducing clinical improvement of mild-to-moderate UC with a similar safety profile [36], suggesting a possible use in patients with IBDU as well.
4.4 Immunomodulators
Acknowledging ‘mucosal healing’ as the ultimate goal of IBD treatment and given the more aggressive nature of the disease in the pediatric population, thiopurines, azathioprine/6-mercaptopurine (AZA/6MP), and methotrexate have a leading role in the maintenance of remission of pediatric IBD. Only sparse data on the efficacy of immunomodulator therapy in IBDU are available. In 2008, a pediatric study reported similar rates of AZA use in children with IBDU, CD, and UC, with a delayed introduction of the drug in IBDU patients and a higher average use in isolated CC [37]. The 2016 study from the Porto IBD Working Group reported a significantly higher rate of immunomodulators, biologics, and surgery in CD compared with IBDU [22]. Based on the current evidence, we advise the introduction of AZA/6-MP in case of 5-ASA failure (at maximal dose), steroid dependency, or resistance. There is no convincing evidence for the regular use of methotrexate in patients with IBDU [38].
4.5 Biologic Therapies
Anti-tumor necrosis factor alpha (anti-TNFα) agents are effective therapies in the induction and maintenance of remission of pediatric IBD [5, 6]. Data on infliximab efficacy and safety in pediatric IBDU are limited, with few pediatric and adult case series published so far (some referred to IC) [39–42]. A 2003 open-label multicenter study reported lower rates of response to infliximab in patients with IC than UC (50 vs 89%). A 70% response rate to infliximab has been reported in a retrospective study on patients with IC [41].
In the European pediatric population, patients with CD received more biologics compared with those with IBDU and UC (34 vs 12% with IBDU and 17% with UC), whereas similar rates of biological therapy were found between IBDU and UC [22]. No comparable data are available for adalimumab.
4.6 Surgical Treatments
Medical therapy represents the first-line choice in the management of IBDU. However, abdominal colectomy and IPAA is performed in UC and IBDU as well. Indeed, while it has already been demonstrated that patients with isolated CC receiving IPAA have a much higher rate of pouch complications and pouch loss than patients with UC [43], results on IPAA outcomes in IBDU patients suggest that their functional outcome, pouch survival rates, and quality of life are equivalent to patients with UC [19, 44]. In a recent prospective study on 149 children and adult patients with IBDU treated with IPAA, 22% were reclassified as CD during a 3-year follow-up after ileostomy closure. This rate increased to 32% in patients with a disease onset prior to 18 years of age, and to 40% in those with a disease onset between 18 and 20 years of age, allowing identification of younger age at disease onset as the only clinical predictor of the development of CD after an IPAA [19]. These data underline the particular relevance of the differential diagnosis between UC and CD, and we strongly suggest caution in surgically treating patients labeled as IBDU.
5 Outcomes and Conclusions
The high frequency of atypical features in pediatric IBD makes the diagnosis of IBDU relatively common in this age group. The few available data suggest that this IBD subtype may deserve an individualized approach and patients should be counseled on all the possible disease outcomes. Moreover, genetic, serologic, and clinical variables at the diagnosis could help in predicting the long-term evolution of the disease. Although the current data suggest IBDU to have a milder disease course with a lower medication burden than the other IBD subtypes, the lack of prospective large studies and robust evidence make the management of these patients difficult, particularly in the long term. A complete diagnostic work-up at follow-up should be performed in all patients in order to ascertain the final diagnosis, regardless of the disease course. New widely recognized diagnostic criteria specifically conceived for IBDU could help in identifying subsequent patients to include in randomized control trials in order to draw up validated guidelines for their management.
References
Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Towards an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(suppl A):5–36.
Levine A, de Bie CI, Turner D, Cucchiara S, Sladek M, Murphy MS, et al. Atypical disease phenotypes in pediatric ulcerative colitis: 5-year analyses of the EUROKIDS Registry. Inflamm Bowel Dis. 2013;12:370–7.
Romano C, Famiani A, Gallizzi R, Comito R, Ferrau V, Rossi P. Indeterminate colitis: a distinctive clinical pattern of inflammatory bowel disease in children. Pediatrics. 2008;122:e1278–81.
Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806.
Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8:1179–207.
Turner D, Levine A, Escher JC, Griffiths AM, Russell RK, Dignass A, et al. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr. 2012;55:340–61.
Prenzel F. Uhlig HH Frequency of indeterminate colitis in children and adults with IBD—a metaanalysis. J Crohns Colitis. 2009;3:277–81.
Winter DA, Karolewska-Bochenek K, Lazowska-Przeorek I, Lionetti P, Mearin ML, Chong SK, et al. Pediatric IBD-unclassified is less common than previously reported; results of an 8-year audit of the EUROKIDS registry. Inflamm Bowel Dis. 2015;21:2145–53.
Auvin S, Molinié F, Gower-Rousseau C, Brazier F, Merle V, Grandbastien B, et al. Incidence, clinical presentation and location at diagnosis of pediatric inflammatory bowel disease: a prospective population-based study in northern France (1988–1999). J Pediatr Gastroenterol Nutr. 2005;41:49–55.
Castro M, Papadatou B, Baldassare M, Balli F, Barabino A, Barbera C, et al. Inflammatory bowel disease in children and adolescents in Italy: data from the pediatric national IBD register (1996–2003). Inflamm Bowel Dis. 2008;14:1246–52.
Müller KE, Lakatos PL, Arató A, Kovács JB, Várkonyi Á, Szűcs D, et al. Incidence, Paris classification, and follow-up in a nationwide incident cohort of pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;57:576–82.
Carvalho RS, Abadom V, Dilworth HP, Thompson R, Oliva-Hemker M, Cuffari C. Indeterminate colitis: a significant subgroup of pediatric IBD. Inflamm Bowel Dis. 2006;12:258–62.
Aloi M, Lionetti P, Barabino A, Guariso G, Costa S, Fontana M, et al. Phenotype and disease course of early-onset pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:597–605.
Oliva-Hemker M, Hutfless S, Al Kazzi ES, Lerer T, Mack D, LeLeiko N, et al. Clinical presentation and five-year therapeutic management of very early-onset inflammatory bowel disease in a large north American cohort. J Pediatr. 2015;167:527–32.
Kammermeier J, Dziubak R, Pescarin M, Drury S, Godwin H, Reeve K, et al. Phenotypic and genotypic characterisation of inflammatory bowel disease presenting before the age of 2 years. J Crohns Colitis. 2017;11:60-69.
Heyman MB, Kirschner BS, Gold BD, Ferry G, Baldassano R, Cohen SA, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35–40.
Turunen P, Kolho KL, Auvinen A, Iltanen S, Huhtala H, Ashorn M. Incidence of inflammatory bowel disease in Finnish children, 1987–2003. Inflamm Bowel Dis. 2006;12:677–83.
Tontini GE, Vecchi M, Pastorelli L, Neurath MF, Neumann H. Differential diagnosis in inflammatory bowel disease colitis: state of the art and future perspectives. World J Gastroenterol. 2015;7(21):21–46.
Alexander F, Sarigol S, DiFiore J, Stallion A, Cotman K, Clark H, et al. Fate of the pouch in 151 pediatric patients after ileal pouch anal anastomosis. J Pediatr Surg. 2003;38:78–82.
Mamula P, Telega GW, Markowitz JE, Brown KA, Russo PA, Piccoli DA, et al. Inflammatory bowel disease in children 5 years of age and younger. Am J Gastroenterol. 2002;97:2005–10.
Annese V, Andreoli A, Astegiano M, Campieri M, Caprilli R, Cucchiara S, et al. Clinical features in familial cases of Crohn’s disease and ulcerative colitis in Italy: a GISC study. Italian Study Group for the Disease of Colon and Rectum. Am J Gastroenterol. 2001;96:2939–45.
Aloi M, Birimberg-Schwartz L, Buderus S, Hojsak I, Fell JM, Bronsky J, et al. Treatment Options and Outcomes of Pediatric IBDU Compared with Other IBD Subtypes: A Retrospective Multicenter Study from the IBD Porto Group of ESPGHAN. Inflamm Bowel Dis. 2016;22:1378–83.
Rimola J, Ordás I, Rodriguez S, García-Bosch O, Aceituno M, Llach J, et al. Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011;17:1759–68.
Ruemmele FM, Hyams JS, Otley A, Griffiths A, Kolho KL, Dias JA, et al. Outcome measures for clinical trials in paediatric IBD: an evidence-based, expert-driven practical statement paper of the paediatric ECCO committee. Gut. 2015;64:438–46.
Subramanian S, Ekbom A, Rhodes JM. Recent advances in clinical practice: a systematic review of isolated colonic Crohn’s disease: the third IBD? Gut. 2017;66:362-381.
Ruemmele FM, Targan SR, Levy G, Dubinsky M, Braun J, Seidman EG. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998;115:822–9.
Vandewalle-El Khoury P, Colombel JF, Joossens S, Standaert-Vitse A, Collot M, Halfvarson J, et al. Detection of antisynthetic mannoside antibodies (ASigmaMA) reveals heterogeneity in the ASCA response of Crohn’s disease patients and contributes to differential diagnosis, stratification, and prediction. Am J Gastroenterol. 2008;103:949–57.
Lakatos PL, Papp M, Rieder F. Serologic antiglycan antibodies in inflammatory bowel disease. Am J Gastroenterol. 2011;106:406–12.
Reese GE, Constantinides VA, Simillis C, Darzi AW, Orchard TR, Fazio VW, et al. Diagnostic precision of anti-Saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Am J Gastroenterol. 2006;101:2410–22.
Birimberg-Schwartz L, Wilson DC, Kolho KL, Karolewska-Bochenek K, Afzal NA, Spray C, et al. pANCA and ASCA in Children with IBD-Unclassified, Crohn’s Colitis, and Ulcerative Colitis—A Longitudinal Report from the IBD Porto Group of ESPGHAN. Inflamm Bowel Dis. 2016;22:1908–14.
Joossens S, Reinisch W, Vermeire S, Sendid B, Poulain D, Peeters M, et al. The value of serologic markers in indeterminate colitis: a prospective follow-up study. Gastroenterology. 2002;122:1242–7.
Prideaux L, De Cruz P, Ng SC, Kamm MA. Serological antibodies in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2012;18:1340–55.
von Stein P, Lofberg R, Kuznetsov NV, Gielen AW, Persson JO, Sundberg R, et al. Multigene analysis can discriminate between ulcerative colitis, Crohn’s disease, and irritable bowel syndrome. Gastroenterology. 2008;134:1869–81.
Janczewska I, Kapraali M, Saboonchi F, Nekzada Q, Wessulv Å, Khoshkar J, et al. Clinical application of the multigene analysis test in discriminating between ulcerative colitis and Crohn’s disease: a retrospective study. Scand J Gastroenterol. 2012;47:162–9.
Bjerrum JT, Nyberg C, Olsen J, Nielsen OH. Assessment of the validity of a multigene analysis in the diagnostics of inflammatory bowel disease. J Intern Med. 2014;275:484–93.
Manguso F, Bennato R, Lombardi G, Riccio E, Costantino G, Fries W. Efficacy and Safety of Oral Beclomethasone Dipropionate in Ulcerative Colitis: A Systematic Review and Meta-Analysis. PLoS One. 2016;15(11):e0166455.
Mossop H, Davies P, Murphy MS. Predicting the need for azathioprine at first presentation in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2008;47:123–9.
Willot S, Noble A, Deslandres C. Methotrexate in the treatment of inflammatory bowel disease: an 8-year retrospective study in a Canadian pediatric IBD center. Inflamm Bowel Dis. 2011;17:2521–6.
Kelsen JR, Grossman AB, Pauly-Hubbard H, Gupta K, Baldassano RN, Mamula P. Infliximab therapy in pediatric patients 7 years of age and younger. J Pediatr Gastroenterol Nutr. 2014;59:758–62.
Gornet JM, Couve S, Hassani Z, Delchier JC, Marteau P, Cosnes J, et al. Infliximab for refractory ulcerative colitis or indeterminate colitis: an open-label multicentre study. Aliment Pharmacol Ther. 2003;15(18):175–81.
Papadakis KA, Treyzon L, Abreu MT, Fleshner PR, Targan SR, Vasiliauskas EA. Infliximab in the treatment of medically refractory indeterminate colitis. Aliment Pharmacol Ther. 2003;1(18):741–7.
Caspersen S, Elkjaer M, Riis L, Pedersen N, Mortensen C, Jess T, Sarto P, et al. Infliximab for inflammatory bowel disease in Denmark 1999–2005: clinical outcome and follow-up evaluation of malignancy and mortality. Clin Gastroenterol Hepatol. 2008;6:1212–7.
Brown CJ, Maclean AR, Cohen Z, Macrae HM, O’Connor BI, McLeod RS. Crohn’s disease and indeterminate colitis and the ileal pouch-anal anastomosis: outcomes and patterns of failure. Dis Colon Rectum. 2005;48:1542–9.
Dayton MT, Larsen KR, Christiansen DD. Similar functional results and complications after ileal pouch-anal anastomosis in patients with indeterminate vs ulcerative colitis. Arch Surg. 2002;137:690–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
G. D’Arcangelo and M. Aloi declare no financial relationships with a commercial entity producing health-related products and or services related to this article. No honorarium, grant, or other form of payment was given to anyone to write and produce the manuscript.
Rights and permissions
About this article
Cite this article
D’Arcangelo, G., Aloi, M. Inflammatory Bowel Disease-Unclassified in Children: Diagnosis and Pharmacological Management. Pediatr Drugs 19, 113–120 (2017). https://doi.org/10.1007/s40272-017-0213-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-017-0213-9