Abstract
Ventricular arrhythmias are common and can modify the risk profile in patients with advanced heart failure. Guideline-directed medical therapy as well as arrhythmia control are warranted to optimize outcomes and prevent sudden cardiac death. Antiarrhythmic therapy may result in undesired toxicities which can further complicate management. We present a case of an advanced heart failure patient with recurrent ventricular tachycardia and ventricular fibrillation that was refractory to guideline-directed medical therapy and resulted in him being considered for advanced heart failure therapies. We describe the clinical course of antiarrhythmic therapy and the resultant complication of clinically significant thyroid dysfunction. We also summarize our review of the literature regarding the treatment of ventricular arrhythmias in advanced heart failure and the management of antiarrhythmic therapy-induced thyroid dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ventricular arrhythmias are common in advanced heart failure patients and portend a poor prognosis |
Antiarrhythmic therapy options are limited in advanced heart failure and treatment can be further complicated by intolerance or side effects |
Sudden cardiac death prevention in the setting of ventricular arrhythmias and heart failure may warrant device implantation and/or advanced heart failure therapies |
Introduction
Ventricular Arrhythmias (VAs) are one of the several clinical variables that portend a poor heart failure (HF) prognosis and have a direct correlation with recurrent hospitalization and increased rates of sudden cardiac death (SCD) in patients with cardiomyopathy [1]. Certain familial/inherited cardiomyopathies have an increased predilection for VAs and SCD; namely Lamin A [2], long QT [3], and arrhythmogenic right ventricular cardiomyopathy (ARVC) [4, 5]. The most clinically prevalent form of VAs is premature ventricular complexes (PVCs), documented in 70-95% of patients, followed by non-sustained ventricular tachycardia (VT) in 50-80% of patients. Conversely, sustained VT or ventricular fibrillation (VF) occurs in 5% of patients, but they account for the majority of SCD cases [6]. Antiarrhythmic therapy (AT) and/or implantable cardioverter defibrillator (ICD) placement for the prevention of SCD is commonly utilized in these patients. Guideline-directed medical therapy (GDMT) can reduce the risk of VAs and SCD, but AT is still frequently required. We present a case of an advanced HF patient with recurrent VT and VF that was refractory to GDMT which resulted in him being considered for advanced HF therapies [1]. We describe the clinical course of AT and the resultant complication of clinically significant thyroid dysfunction. We also summarize our review of the literature regarding the treatment of VAs in advanced HF and the management of AT-induced thyroid dysfunction.
Methodology
We report the case of a patient in the United Arab Emirates (UAE) with advanced HF secondary to familial dilated cardiomyopathy (DCM). The patient suffered from recurrent VT and VF that were complicated by thyroid dysfunction, mainly thyrotoxicosis. Written informed consent was obtained from the patient and included in the study.
We conducted a thorough literature search through several databases including PubMed, Google Scholar, and MEDLINE using the terms ‘heart failure’, ‘ventricular arrhythmias, ‘thyrotoxicosis’, and ‘Amiodarone’. Relevant papers were reviewed and cited to include eligible data in our case report.
Case report
A 21-year-old male with chronic systolic HF due to DCM presented to the emergency room with syncope. He was first diagnosed with DCM at the age of 11, and his family history is significant for two siblings who carried the diagnosis of DCM but succumbed to SCD while undergoing therapy. Prior to this presentation, he was followed in the advanced HF clinic at our institution and was tolerating optimal doses of GDMT. His echocardiogram showed a severely dilated left ventricle with severely reduced left ventricular systolic function (LVEF 27%; normal LVEF being 55% [7]). He had suffered recurrent VT and VF in the past and had undergone placement of a transvenous ICD. After ICD implantation, he had further episodes of appropriate ICD shocks for recurrent VAs. As such, he was initiated on AT with amiodarone 100 mg once daily, for secondary prevention of SCD. This amiodarone-based AT, which continued for eleven months, succeeded in reducing his VAs burden, albeit with resultant thyroid dysfunction, namely Amiodarone-Induced Thyrotoxicosis (AIT); initial hypothyroidism (treated with replacement therapy with levothyroxine 50 mcg daily) which then transitioned to hyperthyroidism (treated with cessation of replacement therapy and initiation of carbimazole 5 mg three times daily).
Prior to his current presentation with syncope, he had self-discontinued his thyroid suppression therapy with carbimazole, shortly after initiation. Electronic interrogation of his ICD demonstrated several defibrillation discharges to abort recurrent monomorphic unstable VT, and a review of his cardiac telemetry monitoring in the emergency room showed recurrent monomorphic VT, in short bursts. The patient was otherwise hemodynamically stable with no evidence of acute HF decompensation or volume overload. At the same visit, a thyroid panel was done and showed suppressed Thyroid Stimulating Hormone (TSH) with an elevation of both free thyroxine (FT4) and free triiodothyronine (FT3) which is consistent with overt hyperthyroidism. TSH receptor antibodies and thyroid peroxidase antibodies were negatives, suggesting more toward AIT rather than Graves’s disease. His ICD was reprogrammed to allow for rapid anti-tachycardia pacing (ATP), which is a device-based therapy for rapid VAs, without resolving to recurrent defibrillation therapy.
Amiodarone was discontinued and was initiated on an extensive regimen for his AIT. Carbimazole was restarted at a higher dose of 15 mg three times daily. Also, a non-selective β-blocker (propranolol 80 mg twice daily) and IV hydrocortisone 25 mg three times daily were initiated. VAs, in the form of non-sustained VT, persisted and thus the β-blocker dose was changed from 80 mg twice daily to 80 mg three times daily, and IV lidocaine of 1 mg/min was added as an alternative form of AT. Subsequent thyroid function tests (TFTs) showed a reduction in serum levels of FT3 but no reduction in FT4. Therefore, the doses of carbimazole and hydrocortisone were further increased to 20 mg three times daily and 50 mg three times daily, respectively. Additionally, cholestyramine 4 mg twice daily was prescribed. The patient subsequently self-discontinued the cholestyramine due to intolerance of side effects. These endocrinological interventions resulted in control of his hyperthyroid state. However, he continued to have recurrent non-sustained and sustained VT, so his AT was adjusted. Sotalol was added and titrated to the target dose of 80 mg twice daily, propranolol was changed to a selective β-blocker (bisoprolol 10 mg daily), and IV lidocaine was switched to oral mexiletine 200 mg twice daily. Clinical monitoring over several days confirmed the quiescence of his VAs. Multi-disciplinary discussions deemed an attempt at catheter-based radiofrequency ablation (VT ablation) to have a high risk/benefit ratio and advised against pursuing this treatment modality. Given the strong family history of SCD, advanced HF, and recurrent VAs that required aggressive AT, the multidisciplinary Advanced HF Therapy Committee decided to list him for heart transplantation and/or LVAD placement.
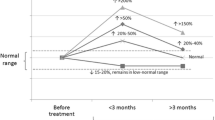
Imaging was performed in order to differentiate Type-I versus Type-II AIT: a TC-99M thyroid uptake scan revealed a uniform uptake, and an ultrasound of the thyroid gland showed low vascularity on Doppler. Subsequently, a corticosteroid tapering regimen was initiated with the aim to complete the course over a few weeks. However, his symptoms worsened, and he presented again to the emergency department with worsening of his TFTs in spite of compliance with high dose carbimazole of 20 mg three times daily. This confirmed a diagnosis of Type-II AIT (destructive amiodarone-induced thyroiditis), for which his corticosteroid dose was increased and his carbimazole dose was decreased as mentioned above. The trend of his TFTs and the main related clinical interventions are represented in Fig. 1.
The patient remains hemodynamically and electrically stable as an outpatient. He has not had any further VAs or ICD shocks. From a thyroid standpoint, he is following regularly in the endocrinology clinic, his last TFTs are within normal range. The patient is well-controlled on Mexiletine 200 mg three times daily, and he is not on any thyroid medications. The patient is INTERMACS profile 6, and he is listed for heart transplantation as UNOS status 2.
Discussion
Mechanisms precipitating ventricular arrhythmias in heart failure
The etiology behind arrhythmogenesis in HF patients is explained by a multifactorial process that results from progressive maladaptive structural remodeling along with altered cardiomyocyte properties [6, 8]. The pathological process involves mechanical and neurohormonal factors, and metabolic as well as ischemic changes. Furthermore, HF medications precipitate many electrolyte abnormalities (mainly related to potassium, sodium, and magnesium) which potentiate the proarrhythmic effect of other medications. Altogether, these mechanisms result in a disturbance of normal contractile performance and (directly or indirectly) precipitate VAs [6, 9].
Approach for ventricular arrhythmia management in heart failure
The management of VAs in HF patients requires a systematic, multifaceted approach by controlling underlying causes, eliminating/minimizing triggers and predisposing factors, managing VT, and preventing SCD. GDMT for HF reduces the incidence and clinical severity of VAs [6, 8, 9]. The aforementioned pathological processes that underlie advanced stages of HF trigger the development of VAs via direct and indirect mechanisms, with the latter being primarily secondary to pump failure [8, 10]. Targeting these pathways can retard and potentially reverse the progression of myocardial remodeling and fibrosis, enhance pump efficiency, and thus reduce VAs and SCD which are important causes of mortality in patients with advanced HF [8, 11, 12]. A suggested algorithm for the treatment of VAs in Advanced HF is displayed in Fig. 2.
The benefits of guideline directed medication therapy/disease modifying therapy in the treatment of ventricular arrhythmias
Since VAs are the main mode of SCD in HF populations, with pump failure being the next most frequent mode, it is essential to manage these VAs appropriately, prophylactically, and secondarily. The early initiation and dosing optimization of GDMT is indicated in all HF patients presenting with VAs [1, 6, 8].
GDMT, such as β-blockers, Angiotensin Receptor Blockers (ARBs) and Angiotensin-Converting Enzyme inhibitors (ACEi) have proven morbidity and mortality benefits. For instance, several studies and trials reported that β-blockers use in HF patients decreases all-cause mortality by 35% and SCD by 45% [8, 9] and also decrease the occurrence of VT by acting as antiarrhythmic agents, irrespective of the concomitant use of other GDMT [8, 10, 11]. On the other hand, although ARBs and ACEi, cause substantial reverse remodeling of the ventricle and are associated with remarkable morbidity and mortality benefits, these agents have failed to demonstrate an effect on VAs or SCD [8, 10, 13]. However, Sacubitril-Valsartan, the only approved Angiotensin Receptor-Neprilysin Inhibitor (ARNi), significantly reduced all-cause mortality and hospitalization (~21.8 %), when compared to ACEi on top of background therapy with β-blockers and mineralocorticoid receptor antagonists (MRA) [8, 13]. MRAs are proven to decrease all-cause mortality (~35%) and SCD (~29%) by targeting multiple pathological pathways [13, 14]. Furthermore, Sodium/Glucose Cotransporter-2 (SGLT-2) inhibitors are showing remarkable beneficial outcomes in HF. Recent trials reported that SGLT-2 inhibitors are associated with a 30%–35% lower risk of hospitalization for HF in patients with diabetes [15, 16]. However, the cardiovascular benefits were shown to be independent of renal function and glucose levels [15]. In terms of the effect of SGLT-2 inhibitors on SCD, a recent meta-analysis included the results of 9 randomized controlled trials (RCTs) of patients with HF, diabetes, and chronic kidney disease. All of which compared different SGLT2 inhibitors to placebo and included the incidence of SCD. After pooling the results of these trials there was no significant association between SGLT2 inhibitors and SCD [Risk Ratio (RR) 0.74, 95% Confidence Interval (CI) 0.50-1.08; P = 0.12] [16].
Although GDMT can reduce morbidity and mortality, a small proportion of cases progress to advanced stages of HF (ACC/AHA Stage D) and require advanced HF therapies; durable left ventricular assistance device (LVAD) implantation, and/or heart transplantation [10, 13]. The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) utilizes risk profiles to optimally select patients for advanced HF therapies. Arrhythmia is the "A factor" in the INTERMACS profile and is a modifier for any profile. This registry previously reported that 19% of the overall population and 20% of profile 1 and 2 patients were limited by frequent VAs. Data suggest that patients with arrhythmias will not necessarily have worse outcomes and generally improve following advanced HF therapies [17, 18].
Antiarrhythmic therapy in heart failure
If VAs persist in HF patients despite GDMT optimization, specific AT may be utilized for management. This includes antiarrhythmic drugs (AADs) or non-pharmacological interventions such as ICD implantation, catheter-based VT ablation, and, in resistant cases, cardiac sympathetic denervation (CSD) therapy with bilateral stellate ganglionectomy surgeries [19, 20].
Antiarrhythmic drugs
The choice of AAD is guided by the type of VT (PVC, non-sustained VT, sustained VT, VF), the pharmacological properties of AADs, and, above all, the hemodynamic and clinical status of the patient [9, 19]. According to the available studies, none of the AADs are associated with improved survival or decreased SCD when used for primary or secondary prevention of VAs, except for β-blockers (e.g., metoprolol succinate and carvedilol) [12]. However, their use is beneficial in suppressing VAs and improving symptoms [9, 19]. Importantly, class II AADs (β-blockers) remain the mainstay therapy and the only class that is attributed to a reduction in mortality as well as SCD [8, 9, 11]. These agents provide dual action in a patient with HF and VAs by maintaining a normal heart rate and managing HF [11, 19]. Certain AAD medications, such as flecainide, sotalol, disopyramide, and dronedarone are better avoided in HF patients as they exacerbate the underlying myocardial dysfunction [7, 9, 19]. Generally, class I sodium-channel-blocking drugs carry the highest risk of provoking arrhythmia and causing hemodynamic worsening related to their negative inotropic effect [9]. On that account, class IA and IC are not recommended to be used in HF patients except for quinidine, since it has minimal negative inotropic actions [9, 19].
Limited published data exists about the safety and efficacy of the remaining AADs in HF population, and the decision regarding the most suitable choice remains challenging. Out of all AADs, as previously mentioned selective β-blockers are considered the first-line therapy due to their excellent safety profile, proven survival benefits from RCT, and significant relative reduction in SCD (~45%) [6, 8, 11]. But since β-blockers are part of the GDMT, most HF patients will already be on this class of medications, which places amiodarone as the best next choice in management of VA after GDMT optimization. Amiodarone, a class IIIa AAD that blocks the voltage-dependent potassium channels, is a reasonable and effective option. However, the wide range of possible end-organ toxicities (thyroid, hepatic, ocular, dermatological) make it a second-line therapy; it is mainly reserved for refractory cases or as a bridge therapy for cardiac ablation [11, 19]. In case of treatment failure, despite the initial approach of β-blockers initiation/optimization, the addition of amiodarone or sotalol (class III AADs) is indicated [6, 19]. Generally, it is not preferred to start with sotalol which could cause worsening of HF due to its proarrhythmic action, albeit this effect is not significant in patients with ICD [6, 11]. Sotalol could be an alternative therapy if the patient is not a candidate for amiodarone due to contraindications or adverse drug reactions or in case of amiodarone treatment failure [6, 19] Overall, amiodarone is superior to sotalol in terms of SCD and mortality reduction, and it is frequently used to suppress VAs [1, 12].
Intravenous lidocaine, and its oral derivative mexiletine, are Class IB AADs that cause rapid dissociation at the voltage-gated sodium channels and are first-line therapies for the short and medium-term management of recurrent VT given the proven efficacy and highly-tolerable side effects profile [11, 12, 19]. Yet, mexiletine should be used with caution in patients with severely impaired left ventricular function because it increases systemic vascular resistance and decreases the cardiac and stroke volume indexes resulting in myocardial depression and hemodynamic deterioration [9, 21]. Lidocaine (Class 1B) is considered an option for acute recurrent/refractory cases of VT or PVC owing to its short half-life and good safety profile; in the setting of drug plasma concentrations within the desired therapeutic range [9, 12]. These agents could be acceptable options as monotherapy or in combination with other class III AADs to suppress VAs in HF patients, despite the lack of strong evidence supporting their role [9, 11].
Non-pharmacological interventions
ICDs are effective as adjuvant therapy for recurrent and/or life-threatening VAs in HF patients, whether used for primary or secondary prevention of SCD [9, 19]. Cardiac resynchronization therapy with defibrillator therapy (CRT-D) is also associated with a relative reduction in SCD (~56%) [9, 22]. Catheter-based VT ablation is indicated in patients with sustained monomorphic VT who are nonresponsive to or cannot tolerate AAD, and in select polymorphic VT cases with identifiable PVC triggers [11, 19, 20]. Noteworthy, success rates vary between 75% to 95% of patients, with a recurrence rate of up to 35% [23]. As a last resort, albeit usually in non-HF-associated cases of resistant VT, certain quaternary referral centers of expertise perform cardiac sympathetic denervation (CSD) therapy with bilateral stellate ganglionectomy, with some retrospective data to show long-lasting efficacy [20].
For HF patients with ICD and persistent VAs despite HF therapy optimization and device re-programming, initiating amiodarone is recommended [19]. Also, it may be a reasonable option if patients suffer from incessant VAs and are not a candidate for ICD [9]. Generally, amiodarone, sotalol, and/or other β-blockers are acceptable adjunctive therapies to ICD since the pro-arrhythmia risk may be less of a consideration. Also, they could be used to manage symptomatic VT in patients who are unsuitable to have ICD, though amiodarone could be preferred in HF patients because it is less pro-arrhythmic [1, 6, 9]. The main disadvantage of amiodarone is the high risk of toxicity with chronic use, therefore the utilization of the lowest effective dose with close monitoring is recommended [6, 19].
Amiodarone induced thyroid toxicity
Thyroid toxicity is one of the most concerning side effects associated with long-term amiodarone therapy. Amiodarone can induce either hypothyroidism (especially in iodine-sufficient areas) or hyperthyroidism (more common in iodine-deficient regions) [12, 24]. However, amiodarone-induced hyperthyroidism is usually more symptomatic and clinically significant in patients with underlying cardiovascular disease [25]. The main cause of amiodarone effects on the thyroid gland are the structural similarities between the drug and the thyroid hormone thyroxine (T4), the high iodine content of amiodarone (39% iodine by weight), and the direct cytotoxic effect of amiodarone on the gland [24, 26]. Noteworthy, these effects of amiodarone can persist for longer than six months, even after drug discontinuation. This is because of the very long half-life resulted from amiodarone storage in adipose tissue [25]. AIT can occur regardless of pre-existing thyroid gland functionality; both apparently-normal and abnormal thyroid glands are susceptible [24]. Usually, it is clinically challenging to distinguish between the types of AIT, which further complicates treatment. However, imaging is helpful to differentiate between these types [24, 26]. The expected changes to thyroidal function with amiodarone and the resulting various diagnostic phenotypes are represented in Table 1.
Thyroid storm and thyrotoxicosis management
Thyroid storm (TS) is a life-threatening complication of thyrotoxicosis, with the classical presentation being tachycardia, arrhythmias, and death from cardiac arrest. The prevalence of TS amongst cases of thyrotoxicosis is felt to be 1-2% [27]. Cardiovascular complications are one of the most common life-threatening clinical manifestations of thyrotoxicosis. Nearly half of thyrotoxicosis patients are admitted to the hospital due to cardiovascular complications [28]. At the same time, development of thyrotoxicosis in HF patients could be fatal and difficult to manage. Immediate diagnosis and initiation of therapy are imperative, with therapy being comprised of high doses of non-selective β-blockers alongside antithyroid drugs such as Propylthiouracil and/or Carbimazole [29]. The antithyroid drugs will achieve a euthyroid state by blocking hormone synthesis, whereas the β-blockers will control the other systemic manifestations of thyrotoxicosis [29]. Propranolol, the most studied non-selective β-blocker that is used in thyrotoxicosis, contributes to the gradual reduction in thyroid levels by as much as 30%. This happens via blocking the enzyme that converts T4 to the more biologically potent form of T3 hormone: monodeiodinase, and causing T4 to be converted to rT3 which is inactive and removed quickly from the body [30]. Metoprolol and atenolol, both selective β-blockers, comparably reduce FT3 levels, however, other selective β-blockers like sotalol and nadolol do not have the same impact [31].
What makes propranolol the preferred choice in TS is its high lipid solubility, with resultant rapid tissue absorption and diffuse inhibition of monodeiodinase. Moreover, propranolol is associated with a significant increase in rT3 levels [32]. Despite the lack of confirmatory evidence of the concomitant advent of adrenal insufficiency in TS, oral glucocorticoids are strongly recommended (strong recommendation, low-quality evidence) within the latest European and American societal guidelines [29, 33]. The reason behind that is the provision of prophylaxis against relative adrenal insufficiency, along with the reduction in conversion of T4 to T3. The usual recommended dose is hydrocortisone 100 mg every 6–8 h (300–400 mg/day) or dexamethasone 2 mg IV every 6 h (8 mg/day), to be continued until TS resolution [34]. Other supportive measures, such as acetaminophen administration and electrolyte and fluid replacement can be considered. Finally, it is essential to eliminate all triggers which could aggravate the thyroid storm [29, 30].
Typically, the treatment of AIT is clinically challenging because of the severity of underlying arrhythmia and cardiac disease, for which the drug was started initially, which renders stopping amiodarone almost impossible. The role of amiodarone discontinuation in thyrotoxicosis is debatable because of several factors, including its role in the cardiac inhibition of T4 to T3 conversion. This inhibition is beneficial in reducing the cardiac symptoms (arrhythmia, HF, and cardiac arrest) of AIT. Therefore, the decision should be individualized based on patient factors, as recommended by the American Thyroid Association [29].
There is emerging data on the potential role of bile acid sequestrants in cases of severe TS [29]. Cholestyramine acts as an ion exchange resin that interferes with thyroid hormone absorption by binding to these particles during the enterohepatic re-circulation, therapy decreasing serum thyroid hormone levels during hyperthyroidism [35]. In this instance, an RCT showed that the addition of cholestyramine to standard therapy (methimazole and propranolol) was well-tolerated and caused a more rapid decline in thyroid levels compared to standard therapy [36]. Accordingly, adjunct use of cholestyramine with standard therapy could be helpful in normalizing thyroid levels, especially in severe and/or resistant cases.
Conclusion
VAs are common and can modify the risk profile in patients with advanced HF. GDMT as well as arrhythmia control are warranted to optimize outcomes and prevent SCD. AT may result in undesired toxicities which can further complicate management.
References
Pedersen CT, Kay GN, Kalman J, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Europace. 2014;16(9):1257–83.
Pérez-Serra A, Toro R, Campuzano O, et al. A novel mutation in lamin A/C causing familial dilated cardiomyopathy associated with sudden cardiac death. J Card Fail (Elsevier). 2015;21(3):217–25.
Shah SR, Park K, Alweis R. Long QT syndrome: a comprehensive review of literature and current evidence. Curr Probl Cardiol. 2019;44(3):92–106.
McKenna WJ, Maron BJ, Thiene G. Classification, epidemiology, and global burden of cardiomyopathies. Circ Res (American Heart Association). 2017;121(7):722–30.
Corrado D, Basso C, Judge DP. Arrhythmogenic cardiomyopathy. Circ Res. 2017;121(7):784–802.
Lip GYH, Heinzel FR, Gaita F, et al. EHRA /Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Eur J Heart Fail. 2015;17(9):848–74.
Heidenreich PA, BozkurtChair B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Card Fail. 2022;S1071–9164(22):00075–6.
Alvarez CK, Cronin E, Baker WL, et al. Heart failure as a substrate and trigger for ventricular tachycardia. J Interv Card Electrophysiol Int J Arrhythm Pacing. 2019;56(3):229–47.
Santangeli P, Rame JE, Birati EY, et al. Management of ventricular arrhythmias in patients with advanced heart failure. J Am Coll Cardiol. 2017;69(14):1842–60.
Writing Committee, Maddox TM, Januzzi JL, et al. Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772–810.
Masarone D, Limongelli G, Rubino M, et al. Management of arrhythmias in heart failure. J Cardiovasc Dev Dis. 2017;4(1):3.
Dan GA, Martinez-Rubio A, Agewall S, et al. Antiarrhythmic drugs-clinical use and clinical decision making: a consensus document from EHRA and ESC Working Group on Cardiovascular Pharmacology, endorsed by the HRS, APHRS and ISCP. Europace. 2018;20(5):731–2.
Shah A, Gandhi D, Srivastava S, et al. Heart failure: a class review of pharmacotherapy. Pharm Ther. 2017;42(7):464–72.
Vizzardi E, Regazzoni V, Caretta G, et al. Mineralocorticoid receptor antagonist in heart failure: past, present, and future perspectives. Int J Cardiol Heart Vessels. 2014;3:6–14.
Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Lancet Lond Engl. 2020;396(10254):819–29.
Sfairopoulos D, Zhang N, Wang Y, et al. Association between sodium–glucose cotransporter-2 inhibitors and risk of sudden cardiac death or ventricular arrhythmias: a meta-analysis of randomized controlled trials. EP Eur. 2022;24(1):20–30.
Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant (Elsevier). 2009;28(6):535–41.
Cowger J, Shah P, Stulak J, et al. INTERMACS profiles and modifiers: heterogeneity of patient classification and the impact of modifiers on predicting patient outcome. J Heart Lung Transplant. 2016;35(4):440–8.
Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15(10):e190–252.
Elliott IA, DeJesus M, Dobaria V, et al. Minimally invasive bilateral stellate ganglionectomy for refractory ventricular tachycardia. JACC Clin Electrophysiol. 2021;7(4):533–5.
Gottlieb SS, Weinberg M. Cardiodepressant effects of mexiletine in patients with severe left ventricular dysfunction. Eur Heart J. 1992;13(1):22–7.
Saxon LA, Bristow MR, Boehmer J, et al. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Trial. Circulation. 2006;114(25):2766–72.
Ponti RD. Role of catheter ablation of ventricular tachycardia associated with structural heart disease. World J Cardiol. 2011;3(11):339–50.
Trohman RG, Sharma PS, McAninch EA, et al. Amiodarone and thyroid physiology, pathophysiology, diagnosis, and management. Trends Cardiovasc Med. 2019;29(5):285–95.
Hudzik B, Zubelewicz-Szkodzinska B. Amiodarone-related thyroid dysfunction. Intern Emerg Med. 2014;9(8):829–39.
Loh KC. Amiodarone-induced thyroid disorders: a clinical review. Postgrad Med J. 2000;76(893):133–40.
Pokhrel B, Aiman W, Bhusal K. Thyroid storm. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK448095/. Accessed 5 May 2022.
Ono Y, Ono S, Yasunaga H, et al. Factors associated with mortality of thyroid storm: analysis using a national inpatient database in Japan. Medicine (Baltimore). 2016;95(7):e2848.
Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid Off J Am Thyroid Assoc. 2016;26(10):1343–421.
Klubo-Gwiezdzinska J, Wartofsky L. Thyroid emergencies. Med Clin N Am. 2012;96(2):385–403.
Perrild H, Hansen JM, Skovsted L, et al. Different effects of propranolol, alprenolol, sotalol, atenolol and metoprolol on serum T3 and serum rT3 in hyperthyroidism. Clin Endocrinol (Oxf). 1983;18(2):139–42.
Murchison LE, How J, Bewsher PD. Comparison of propranolol and metoprolol in the management of hyperthyroidism. Br J Clin Pharmacol. 1979;8(6):581–7.
Kahaly GJ, Bartalena L, Hegedüs L, et al. 2018 European thyroid association guideline for the management of Graves’ hyperthyroidism. Eur Thyroid J. 2018;7(4):167–86.
Senda A, Endo A, Tachimori H, et al. Early administration of glucocorticoid for thyroid storm: analysis of a national administrative database. Crit Care. 2020;24:470.
Ha J, Jo K, Kang B, et al. Cholestyramine use for rapid reversion to euthyroid states in patients with thyrotoxicosis. Endocrinol Metab. 2016;31(3):476–9.
Mercado M, Mendoza-Zubieta V, Bautista-Osorio R, et al. Treatment of hyperthyroidism with a combination of methimazole and cholestyramine. J Clin Endocrinol Metab. 1996;81(9):3191–3.
Eaton SEM, Euinton HA, Newman CM, et al. Clinical experience of amiodarone-induced thyrotoxicosis over a 3-year period: role of colour-flow Doppler sonography*. Clin Endocrinol (Oxf). 2002;56(1):33–8.
Bogazzi F, Tomisti L, Rossi G, et al. Glucocorticoids are preferable to thionamides as first-line treatment for amiodarone-induced thyrotoxicosis due to destructive thyroiditis: a matched retrospective cohort study. J Clin Endocrinol Metab. 2009;94(10):3757–62.
Batcher EL, Tang XC, Singh BN, et al. Thyroid function abnormalities during amiodarone therapy for persistent atrial fibrillation. Am J Med. 2007;120(10):880–5.
Bartalena L, Grasso L, Brogioni S, et al. Serum interleukin-6 in amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab. 1994;78(2):423–7.
Wang J, Zhang R. Evaluation of 99mTc-MIBI in thyroid gland imaging for the diagnosis of amiodarone-induced thyrotoxicosis. Br J Radiol. 2016;90(1071):20160836.
Bartalena L, Brogioni S, Grasso L, et al. Treatment of amiodarone-induced thyrotoxicosis, a difficult challenge: results of a prospective study. J Clin Endocrinol Metab. 1996;81(8):2930–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflicts of interest/Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Availability of data and material
Not Applicable.
Code availability
Not Applicable.
Authors’ contributions
All authors contributed to the writing and review of the manuscript.
Consent to participate
Informed consent was obtained from the patient to participate in this case report.
Consent for publication
The patient has consented to the submission of this case report to the Drugs & Therapy Perspectives journal.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alsalama, F., Alzaabi, S., Salloum, C. et al. Ventricular arrhythmias, antiarrhythmic therapy and thyroidal illness in advanced heart failure: a case report and review of the literature. Drugs Ther Perspect 39, 147–155 (2023). https://doi.org/10.1007/s40267-023-00985-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-023-00985-3