Abstract
Purpose
Previous research has suggested that prescribers’ knowledge of drug costs in Ireland is deficient. We aimed to update this finding by asking prescribers to estimate drug costs for low-and high-cost drugs, as well as examining their familiarity with a national prescribing initiative.
Methods
We conducted a survey of five groups of prescribers and one group of medical students asking them to estimate the drug costs. Drugs recommended by the Preferred Drugs Initiative in Ireland were included, as were high-cost monoclonal antibody products and nutrition supplements. A 25% margin of error was allowed for a correct estimate. Comparisons were performed across participant groups and between drugs. A number of qualitative questions were included to provide context.
Results
The survey received 122 responses. General practitioners (GPs) had the most accurate estimates, with medical students having the least. The percentage with a correct estimate was lower for higher cost drugs across all participant groups. GPs were also most certain about the estimates and most familiar with the Preferred Drug Initiative, while the students rated worst for both these questions. The cost of most drugs was overestimated. Most prescribers were uncertain about their estimates, which was reflected by the large variation in estimates. Eighty-three percent of prescribers would consider a trade-off of drug efficacy for affordability at least sometimes.
Conclusions
Prescribers’ knowledge of drug costs in Ireland remains poor and may negatively affect patient outcomes and national drug budgets. A national program provides recommendations to improve cost-effective prescribing; however, further alterations to national prescribing practices and policies are required to raise awareness of drug costs and these recommendations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Prescribers’ knowledge of drug costs in Ireland remains poor, with significant uncertainty across all drug types. |
General practitioners are most accurate in their estimates of drug prices, possibly reflecting their greater experience with these frequently prescribed drugs. |
Most prescribers would consider a trade-off of drug efficacy for better affordability. |
Introduction
In Ireland, the state pharmaceutical budget amounts to approximately €2 billion, with 75% of that expenditure in ambulatory care [1]. The financial crisis and subsequent recession beginning in 2008, led to reviews of Irish pharmaceutical spending that was initiated by the International Monetary Fund [2, 3]. Supply side containment policies such as internal reference pricing were introduced, along with demand side reforms such as increases in out-of-pocket payments (OOPs) for community drug schemes [4]. Most healthcare systems use OOPs to control increases in drug expenditure by reducing moral hazard [5]. A frequent OOP is where patients pay for a portion of the drug cost, a fee called a co-payment. The OOP may also take the form of a deductible, with a threshold after which the drug costs are paid for by the healthcare system. In Ireland, OOPs are present as small co-payments (e.g. €1.50 per drug per month) for those eligible for state-funded healthcare, while a deductible (currently €114) caps the monthly cost of drugs for all others [6]. In developing countries, OOPs may equate to the full drug cost causing financial hardship for the sick [7, 8]. OOP and other user charges have been highlighted as an issue of healthcare equity by the World Health Organization (WHO), among others [9, 10]. The negative effect of OOPs on patient adherence to drug regimens is well-described, as is the effect of increasing OOPs on subsequent drug utilization [11,12,13,14]. Reduced drug use is also more evident following comparisons of patients with higher versus lower co-pay insurance schemes [15]. Studies suggest that failed initiation may occur in up to 15–31% of cases for treatments of chronic diseases such as hypertension and diabetes [16,17,18]. The consideration of drug cost should be a key component of any clinical consultation during which a new treatment is commenced. This allows the prescriber to choose the most cost-effective treatment providing best value for the payer, whether this is the patient, the healthcare system, or both. Informing the patient of the drug cost should positively influence their behaviour. It is reasonable to suggest a discussion with the patient about the treatment cost will result in a reduction in failed initiations and improve adherence, which should then improve subsequent health outcomes.
Annual increases in healthcare expenditure are perpetually outstripping inflation. This is an international phenomenon and rising drug costs have been identified as one driver of these increases, particularly in the US where price controls are lacking [19,20,21]. Health systems internationally continue to have difficulty ensuring access to novel therapeutics due to the cost implications and uncertainty regarding the value of some new drugs [22]. Therefore, the optimal use of resources to pay for established and lower-cost treatments has been a health policy focus. European countries have introduced reforms to reduce pharmaceutical spending on drugs with expired patents so that budgets for new drugs are available [23, 24]; however, efforts to control drug costs are limited if prescribers are not aware of and encouraged to use the most cost-effective options. Programs to support prescribers and increase the use of generic medicines and biosimilars have been established in many jurisdictions to address this issue. For example, prescribing initiatives implemented by National Health Service (NHS) Scotland have ensured the majority of statin and proton pump inhibitor prescriptions are for generic options [25, 26]. The Health Services Executive (the body responsible for operating the Irish public health system) established the Medicines Management Program (MMP) in Ireland to provide expertise and advice so that frequently prescribed drugs are used in a cost-effective manner. Among its functions, the MMP reviews options for commonly prescribed conditions to find the most cost-effective drug—this is the Preferred Drug Initiative. A list of preferred drugs is published online, along with the evidence and rational for the choice. Similar approaches to improve prescribing by way of a preferred drugs list have been implemented elsewhere. One example is the Wise List in Stockholm, for which significant effectiveness has been reported in terms of prescribers’ adherence [27, 28]. The MMP also conducts nationwide education sessions for prescribers.
It is clear from past studies that prescribers’ knowledge of drug costs is suboptimal. A survey of prescribers’ knowledge of drug costs in the US reported that prescribers correctly identified the cost of the drugs less than half the time [29]. A Scottish study also identified an awareness of drug costs as a knowledge gap for general practitioners (GPs), although the prescribers believed cost was an important consideration [30]. A systematic review of physicians’ awareness of drug costs reported a similar conclusion [31]. More recently, a study in Nigeria again reported that physicians had very poor knowledge of drug costs and suggested health economic education was necessary to address this deficit [32]; however, there is a paucity of contemporary evidence on the topic. Furthermore, previous studies have rarely included newer groups of prescribers such as nurse prescribers.
This study aimed to (1) assess the accuracy of drug cost estimates for groups of prescribers, using an online survey, i.e. hospital doctors, GPs, GPs in training, nurse prescribers, and medical students; (2) assess this accuracy for low- and high-cost drugs; (3) determine if prescribers are familiar with the Irish national program to facilitate cost-effective prescribing and whether its recommendations influence their prescribing decisions; and (4) identify resources prescribers use to inform themselves of drug costs.
Methods
Setting, population and distribution
To examine prescribers’ knowledge of a selection of drugs commonly prescribed in Ireland, an electronic survey was distributed in April and May 2019 to a relevant population, by email via gatekeepers. The newly implemented European Union General Data Protection Regulation prohibited the attaining of prescribers’ email addresses so that gatekeepers were required for groups of prescribers. The groups included nurse prescribers (via the Office of the Nursing and Midwifery Services Director), doctors working at St James Hospital Dublin (via the William Stokes Postgraduate Centre), qualified GPs, GPs in training (via four Dublin GP training networks) and one class of medical students attending Trinity College Dublin (TCD). The students were in the third year of medical school, undertaking a module in pharmacology that included pharmacoeconomic components, and they had been integrated into clinical teams at TCD university teaching hospitals. For the purpose of the study, interns were regarded as a separate group from other hospital doctors (house officers, registrars, consultants). Gatekeepers were chosen based on their involvement with professional educational groups, for example, the hospital postgraduate education centre and GP training networks. At least one reminder email was sent to each gatekeeper.
Survey development
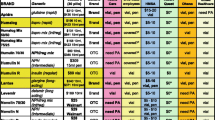
The survey consisted of three main sections. The first section asked basic information about the participants (professional status, age category, and year of graduation), while the second section asked participants about their knowledge of prescribing costs of 23 drugs and nutritional products, based on a 4-week supply, except inhalers (30 days) and denosumab (6 months). The drugs were chosen based on their inclusion in the MMP Preferred Drug Initiative and so that they reflected a range of costs and therapeutic indications. The list of drugs were simvastatin, atorvastatin, esomeprazole, lansoprazole, ramipril, perindopril, bisoprolol, nebivolol, duloxetine, venlafaxine, rivaroxaban, warfarin, apixaban, Complan Shake® (oral nutritional supplement), Ensure Plus Advance® (oral nutritional supplement), Seretide Diskus® (inhaler), Bufomix Easyhaler® (inhaler), mycophenolate mofetil (proprietary and generic products), alendronate, adalimumab (proprietary and biosimilar products), and denosumab. The latter two drugs were considered high-cost drugs. Examples of the typical packaging of the drugs were selected at random, photographed, and added to the questionnaire for illustrative purposes. The survey was piloted on departmental staff prior to implementation.
Where multiple prices existed for the same product, all available prices obtained from the Health Service Executive (HSE) Primary Care Reimbursement Service were gathered, and an average was found. €5 was added to all drugs to represent the average pharmacy fee, and respondents were informed of this at the outset of the survey, along with the source of the costs.
A Likert scale was included after the estimation to assess certainty, with 1 indicating complete uncertainty and 10 indicating complete certainty of cost. Data were collected and collated using Google Forms.
Data analysis
It would not have been reasonable to expect participants to know the exact cost of drugs (there are often discrepancies between published sources of cost information); hence, in keeping with other studies of doctors’ perceptions of costs, we accepted as accurate those estimates that were within 25% of the actual cost [30]. Estimates for each group and all prescribers (therefore excluding medical students) were calculated as a ratio of the actual drug cost to allow comparison between drugs and groups of participants. All data were imported into JMP v15 (SAS Institute Inc., Cary, NC, USA). Cost estimates were compared between the participant categories using the Kruskal–Wallis tests for non-normally distributed data. The proportion of correct, underestimates, and overestimates were compared between participant categories using the Chi-square statistic. As standard, the minimum level of statistical significance was 5% (p < 0.05).
Ethics
Ethical permission to conduct the survey was granted by the TCD School of Medicine Ethics Committee. The data were collected anonymously and no personal data were collected, except for basic demographics (professional status, age category, and year of graduation). Completion was voluntary and participants received no incentive for completion of the survey, monetary or otherwise.
Results
A total of 122 responses were included in this study. The breakdown of participant categories is 14.8% GPs, 12.3% GP trainees, 12.3% hospital doctors, 23.8% interns, 15.6% nurses, and 21.3% students. The response rate was 10.4%. The majority of respondents (67%) were aged under 35 years, while 7.3% were over 55 years of age (eight GPs, one nurse prescriber). The cost estimations varied significantly across the participant categories for most drugs included (Table 1). For example (and excluding students), the mean cost estimates varied from €60 (GP trainees) to €109 (nurse prescribers) for rivaroxaban, from €16 (GP trainees) to €30 (nurses) for esomeprazole, and from €28 (GPs) to €122 (interns) for alendronate. The cost estimates of high-cost drugs also varied significantly, with the mean estimates for adalimumab (proprietary) varying from €249 (hospital doctors) to over €2000 (nurses), and from €161 (hospital doctors) to €3958 (nurse prescribers) for denosumab.
As evident from Table 1, the estimates for all prescribers (excluding medical students) were similar for drugs listed on the MMP Preferred Drug Initiative (simvastatin, lansoprazole, ramipril and bisoprolol) compared with their drug class counterparts (atorvastatin, esomeprazole, perindopril, nebivolol). Most drug costs were overestimated, with notable examples being mycophenolate, alendronate, and denosumab, for which the estimates were many multiples of the actual cost. The cost of very few drugs was consistently underestimated across the participant groups, though this was the case for nutritional supplements, Seretide Diskus® inhaler and adalimumab in its proprietary form. Those in a primary care setting (GPs and GP trainees) estimated the cost of the adalimumab biosimilar to be more than that of the proprietary product.
GPs had the greatest proportion of correct cost estimates at 31% (Fig. 1), followed by GP trainees (28%) and nurse prescribers (27%), with students having the least success (12%). All groups were more successful at estimating drug costs when compared with the medical student group (all groups p < 0.05). Of potential interest is the comparison of GPs with hospital doctors, which demonstrated that the latter were more accurate with their estimates (p < 0.001). All groups were more likely to overestimate than underestimate the drug cost.
The proportion of correct cost estimates between the prescriber groups was similar for drugs across a number of different therapeutic groups, for example ramipril, Bufomix Easyhaler®, and mycophenolate; however, the proportion of correct estimates also varied significantly across many other drugs. For example, 72% of GPs correctly estimated the cost of denosumab, compared with just 20% of GP trainees and no medical students. Fifty percent of GPs correctly estimated the cost of Seretide Diskus® inhaler, in contrast to 7% of hospital doctors and 4% of students. All groups had greater difficulty accurately estimating the cost of higher-cost drugs, with the GPs fairing best (Fig. 2).
Participants were also asked to rank their familiarity with the MMP’s Preferred Drugs Initiative. Again, this varied across the participant categories, with GPs being the most familiar with the initiative; 44% of GPs were very familiar with the initiative, 72% were at least somewhat familiar with it, and only 6% were not familiar with it at all. Unsurprisingly, 85% of the medical student group were not familiar with the program; however, there was also poor awareness among hospital doctors, with 60% only slightly familiar with the program or not familiar with it at all (Fig. 3). When increased familiarity (very/moderately familiar) was plotted against the percentage of correct estimates, the GPs and medical students were placed at the opposite ends of the graph, indicative of the level of prescribing experience (Fig. 4a). This familiarity was not a discerning feature for the other prescriber categories, which were clustered in the centre.
Participants were also asked to rate the certainty of their estimates on a scale of 0–10, with 10 being the highest level of certainty. There was a low level of certainty for almost all estimates, across all participant groups. The mean certainty across all drugs for each category is as follows: GPs, 3.9; GP trainee, 2.6; hospital doctors, 3.4; interns, 2.9; nurses, 3.8; and students, 1.7. While a clear correlation was not evident on plotting the certainty of estimates against the percentage of correct estimates, the GPs and medical students were again at each extreme (Fig. 4b). GP trainees were less certain than their success at estimation suggested, while the opposite was the case for hospital doctors.
Participants were asked to provide their source of drug cost information in a free-text box. The most commonly cited sources were Irish Medicines Formulary (45%), British National Formulary (35%), the local/hospital pharmacist (34%), Monthly Index of Medical Specialities Ireland (25%), or a combination of the above. Interestingly, 29% of prescribers suggested they would ‘always’ or ‘often’ be willing to trade-off some degree of efficacy for drug affordability, and 54% would do so ‘sometimes’ (Fig. 5).
Discussion
This study found that those in primary care, GPs and GP trainees, had a better knowledge of drug costs than other groups, with the medical student group fairing worst, as expected. The more accurate estimates by GPs may reflect greater experience but GPs are also more familiar with the MMP Preferred Drugs Initiative and may therefore be more cost conscious. Costs were more likely to be overestimated rather than underestimated in general; however, there was significant estimate variation between and within groups for many drugs. This variation reflected the uncertainty apparent from the Likert scale component of the study. This uncertainty correlates with previous research involving Irish hospital doctors, where 88% stated they often felt unaware of actual drug costs [33]; however, relaying the cost of a drug to the patient on initiation is an important component of the therapeutic plan. The cost is particularly important if the drug is expensive and if OOPs are involved.
That GPs and GP trainees were the most successful at estimating drug costs may indicate a greater appreciation and knowledge of drug costs than is the case for hospital-based prescribers. The consideration of cost may be less of a factor for hospital prescribers, who may be more inclined to prescribe novel, expensive, on-patent drugs under the guidance of an institutional Drugs and Therapeutics Committee [34]; however, the role of hospital prescribers in containing drug expenditure is critical. It has been demonstrated in many studies that prescribing decisions in a hospital setting are perpetuated in primary care [35,36,37]. Nurse prescribers in Ireland prescribe from a limited personal formulary as their role is usually limited to a specific specialty or condition. It is therefore not surprising that their estimates were less accurate and more varied, as indicated by estimate standard deviations. Medical students were least accurate, in keeping with previous research on the topic comparing doctors with medical students [38]. Medical students in Ireland do not have prescribing rights and knowledge of drug costs will undoubtably improve with experience as they practice.
Previous research has suggested that prescribers are likely to overestimate the cost of inexpensive drugs and underestimate the cost of more expensive drugs [31]. Furthermore, the accuracy of drug cost estimates improved for more expensive drugs. In this study, all groups were also more likely to overestimate the cost of inexpensive drugs. In terms of the expensive drugs, while the cost of adalimumab was underestimated, there was a large variation in the estimates for denosumab. It was evident that the groups had less success estimating the cost of high-cost drugs compared with low-cost drugs (Fig. 2).
The prescriber’s awareness of the cost effectiveness of the treatments they prescribe is a key component in managing healthcare expenditure as well as the financial impact to the patient. A US study of physicians suggested they prioritize reducing the OOP drug cost for patients over those for a health service or insurer [39]. Previous research has suggested that physicians consider cost effectiveness as an important component of their clinical decision making [40,41,42]. Nearly two-thirds of prescribers were at least somewhat familiar with the MMP Preferred Drug Initiative, which provides guidance for cost-effective prescribing. Interestingly, our study found that 29% of prescribers are always/often willing to concede some degree of efficacy for more affordable drug costs, and 83% will consider this trade-off at least sometimes. The apparent willingness to consider this cost-efficacy trade-off is inconsistent with previous Irish research asking this question of hospital doctors [33]. We considered whether the alternative position evident in this study may be due to the inclusion of primary care and nurse prescribers. Qualitative research has suggested that GPs consider individual patient requirements above the opportunity cost for the population when prescribing and that cost minimization rather than cost effectiveness was considered by GPs, although the success of more recent prescribing initiatives suggest cost effectiveness is a central consideration [27, 42]. However, in this study, 30% of hospital-based prescribers were always/often willing to consider sacrificing some efficacy to make drugs more affordable, a similar figure to that for those in primary care (29%).
Many jurisdictions use multi-tier formularies to encourage the use of cost-effective drugs. This includes using generic versions where possible. These formularies may be coupled with reference pricing so that the provider or patient will bear the excess cost above the best value option if they choose an alternative. This has been an effective method of cost control for traditional small molecule drugs. The application of this approach to more complex biological drugs has been more problematic, although considerable recent process is evident [43, 44]. Despite the access to biosimilars, the medical community has been slow to adapt at times, possibly unaware of the value proposition on offer. In this study, we found that the cost of the most commonly prescribed monoclonal antibody drug was consistently underestimated. This was particularly the case for hospital doctors, who will be initiating the drug in Ireland (it must be initiated in hospital by a specialist), with a significant underestimate evident for both the proprietary and biosimilar adalimumab products. The larger estimates by primary care prescribers for the biosimilar versus the proprietary adalimumab may suggest they were unfamiliar with the available biosimilars at the time of the survey. This is reasonable as prescribers in primary care do not initiate adalimumab, and an MMP project to integrate biosimilars into prescribing practice is more recent than the survey.
Prescribers may consider cost, but evidence suggests it is inconsistently applied to their prescribing decisions [40]. Prescribers have reported time constraints and patient demand as factors when choosing a drug treatment, which may affect the influence of cost on decision making [45]. Resources are required to improve this consistency. One such resource identified in this study is the community pharmacist, whom 34% of prescribers surveyed identified as an information source for drug costs; however, previous research has suggested that while GPs may value the pharmacist to identify prescription errors, they did not consider them as an aid in terms of deciding treatment plans [45]. Currently, most prescribers reported they used a national formulary as the information source for drugs costs. These formularies display the cost in the drug monograph but do not include pharmacy fees. When information is available, such as drug price lists from health insurers, evidence suggests that prescribers do not refer to these regularly [29]. The HSE-MMP has produced, and updates, a list of cost-effective drugs for commonly treated conditions (e.g. hypertension, depression) in its Preferred Drug Initiative. Excluding medical students, 62% of prescribers were very, moderately, or somewhat familiar with the initiative. However, guidance to aid prescribers in choosing cost-effective options is often ignored or awareness of such guidance is poor so that other solutions are also necessary [29].
The most pertinent systematic review on the topic of prescribers’ knowledge of drug costs was performed in 2007 [31]. This was prior to the implementations of many initiatives to increase generic drug prescribing and the availability of biosimilars. However, two areas to address the knowledge gap identified by the authors at that time, and still relevant today, were education and clinical decision support. Passive educational interventions aimed at medical professionals often have no or minimal benefit in terms of improving healthcare quality in the longer term. As with other healthcare quality issues, this is true of prescribers’ knowledge of drug costs [46]; however, it is also recognizing that interventions with multiple aspects are unlikely to succeed without an educational component. Prescribers’ and medical students’ willingness to consider drug costs when prescribing has been previously established, and an understanding of cost effectiveness has been suggested as a key component of pharmaco-education [38]. Irish hospital doctors have previously indicated they had little formal education about drug costs and were not aware of where to find such information [33]. A review of health economics education for medical graduates reported that cost containment was the primary focus rather than cost effectiveness and value [47]. Therefore, curricula components focusing on pharmacoeconomics is required at an undergraduate and postgraduate level for healthcare professionals.
It is uncertain whether the use of clinical decision support systems (CDSS) improves cost control in clinical settings. Alerts to display the cost of laboratory and radiological investigations have shown mixed results [48]. Older evidence for employing CDSS to reduce drug costs is unconvincing [49,50,51,52]. More recent studies have been more favourable. One US study using a web-based solution to provide drug cost information showed a reduction in costs but not in OOPs [53]. Formulary decision support for e-prescribing demonstrated improvements in the use of more cost-effective drug options but did not show an improvement in co-payments and adherence when these outcomes were modelled [6, 54]. An alert system to optimize prescribing of high-cost medicines in a hospital setting was successful, but for a limited number of drugs [55]. The regular use of information technology (IT) did not necessarily lead to improved knowledge of drug prices and no single type of IT resource was identified as being particularly useful [56]. It is also worth noting that one study that found a reduction in drug expenditure with implementation of a CDDS reported that this saving offset the cost of a subscription to the service, resulting in no net saving [57]. The success of CDDS and e-prescribing solutions are likely to be setting- and situation-dependent.
Falling costs in data storage and increased computing power is issuing in a golden age in bioinformatics. Machine learning using datasets of patient records has been shown to be effective at preventing prescribing errors with the potential for considerable cost savings [58]. The application of similar artificial intelligence (AI) capabilities must be considered as the next logical step in cost-effective prescribing. Data analysis features based on machine learning of prescriber trends would allow for the creation of a medicine management program that provides real-time feedback to prescribers on their prescribing habits, including cost-effective prescribing. With the correct implementation strategy, these tools will be an essential component of any solution to improve cost-effective prescribing.
It is important to clarify that knowing the cost of a drug will not necessarily lead to improved prescribing, adherence, and allocative efficiency. Rather, the value of the drug is the crucial information required, of which cost is an inherent component. The patient may not be able to judge or perceive the value of the treatment for themselves, particularly for preventive medicines, therefore conveying the actual cost is also necessary. However, when comparing a number of options for treatment, it is cost effectiveness that the prescriber should consider as the unit of cost for a beneficial health outcome. Given the magnitude of this task, doing so is only possible with guidance from an independent source that assesses the value of available drug treatments.
Strengths and limitations
This study has a number of strengths. It involved a comprehensive survey that included high-cost as well as low-cost drugs. Its format allowed comparisons of nationally recommended options against others, and it included a qualitative aspect to assess prescribers’ use of national recommendations and whether they were amenable to trade-offs of effectiveness for cost. The survey involved a number of different prescriber groups and included medical students to create what was effectively a control group. Limitations of the study include the relatively low response rate, which may be due to the use of gatekeepers to disseminate email requests in line with the General Data Protection Regulation requirements. The survey included hospital doctors from one site, although the GPs and nurse prescribers were from a broader area. Another limitation was that the hospital doctors were at different career stages and experience of prescribing these frequently used drugs (e.g. dermatologists). Furthermore, the study was performed in Ireland, where prescribing is not focused by national formularies or guidance, therefore it may not be applicable to jurisdictions with stringent enforcement of prescribing guidance.
Conclusion
Prescribers’ knowledge of drug costs remains poor and may negatively affect patient outcomes and national drug budgets. While resources are provided by a national program to improve cost-effective prescribing, further work is required to successfully implement these prescribing recommendations in practice. Solutions are multifactorial, while a focus on value rather than cost is essential.
References
Connors J. Health budget oversight and management: alignment of health budget and national service plan. Department of Public Expenditure and Reform. 2018. http://www.budget.gov.ie/. Accessed 24 Dec 2020
Leopold C, Mantel-Teeuwisse AK, Vogler S, et al. Effect of the economic recession on pharmaceutical policy and medicine sales in eight European countries. Bull World Health Organ. 2014;92:630–40.
Vogler S, Zimmermann N, Leopold C, et al. Pharmaceutical policies in European countries in response to the global financial crisis. South Med Rev. 2011;4:69.
Kenneally M, Walshe V. Pharmaceutical cost-containment policies and sustainability: recent Irish experience. Value Health. 2012;15:389–93.
Gemmill MC, Thomson S, Mossialos E. What impact do prescription drug charges have on efficiency and equity? Evidence from high-income countries. Int J Equity Health. 2008;7:12.
Fischer MA, Vogeli C, Stedman M, et al. Effect of electronic prescribing with formulary decision support on medication use and cost. Arch Intern Med. 2008;168:2433–9.
Cameron A, Ewen M, Ross-Degnan D, et al. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373:240–9.
Kastor A, Mohanty SK. Disease-specific out-of-pocket and catastrophic health expenditure on hospitalization in India: do Indian households face distress health financing? PLoS ONE. 2018;13:e0196106.
Thomson S, Cylus J, Evetovits T. Can people afford to pay for health care? New evidence on financial protection in Europe. Geneva: World Health Organization; 2019.
Lexchin J, Grootendorst P. Effects of prescription drug user fees on drug and health services use and on health status in vulnerable populations: a systematic review of the evidence. Int J Health Serv. 2004;34:101–22.
Sinnott S-J, Buckley C, O’Riordan D, et al. The effect of copayments for prescriptions on adherence to prescription medicines in publicly insured populations; a systematic review and meta-analysis. PLoS One. 2013;8:64914.
Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing associations with medication and medical utilization and spending and health. JAMA. 2007;298:61–9.
Sinnott S-J, Whelton H, Franklin JM, et al. The international generalisability of evidence for health policy: a cross country comparison of medication adherence following policy change. Health Policy. 2017;121:27–34.
Aziz H, Hatah E, Makmor Bakry M, et al. How payment scheme affects patients’ adherence to medications? A systematic review. Patient Prefer Adherence. 2016;10:837–50.
Kemp A, Roughead E, Preen D, et al. Determinants of self-reported medicine underuse due to cost: a comparison of seven countries. J Health Serv Res Policy. 2010;15:106–14.
Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25:284–90.
Shah NR, Hirsch AG, Zacker C, et al. Predictors of first-fill adherence for patients with hypertension. Am J Hypertens. 2009;22:392–6.
Shah NR, Hirsch AG, Zacker C, et al. Factors associated with first-fill adherence rates for diabetic medications: a cohort study. J Gen Intern Med. 2009;24:233–7.
Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024–39.
Vokinger KN, Hwang TJ, Grischott T, et al. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost–benefit analysis. Lancet Oncol. 2020;21:664–70.
Emanuel EJ, Zhang C, Glickman A, et al. Drug reimbursement regulation in 6 Peer countries. JAMA Int Med. 2020;180:1510–7.
Cohen D. Cancer drugs: high price, uncertain value. BMJ. 2017;359:j4543.
Godman B, Malmström R, Bennie M, et al. Prescribing restrictions–a necessary strategy among some European countries to enhance future prescribing efficiency? Rev Health Care. 2012;3:5–16.
Godman B, Shrank W, Andersen M, et al. Comparing policies to enhance prescribing efficiency in Europe through increasing generic utilization: changes seen and global implications. Expert Rev Pharmacoecon Outcomes Res. 2010;10:707–22.
Leporowski A, Godman B, Kurdi A, et al. Ongoing activities to optimize the quality and efficiency of lipid-lowering agents in the Scottish National Health Service: influence and implications. Expert Rev Pharmacoecon Outcomes Res. 2018;18:655–66.
Godman B, Kurdi A, McCabe H, et al. Ongoing activities to influence the prescribing of proton pump inhibitors within the Scottish National Health Service: their effect and implications. GaBI J. 2018;7:142–51.
Gustafsson LL, Wettermark B, Godman B, et al. The ‘Wise List’: a comprehensive concept to select, communicate and achieve adherence to recommendations of essential drugs in ambulatory care in Stockholm. Basic Clin Pharmacol Toxicol. 2011;108:224–33.
Eriksen J, Gustafsson LL, Ateva K, et al. High adherence to the ‘Wise List’ treatment recommendations in Stockholm: a 15-year retrospective review of a multifaceted approach promoting rational use of medicines. BMJ Open. 2017;7:e014345.
Cogdill B, Nappi JM. Assessment of prescribers’ knowledge of the cost of medications. Ann Pharmacother. 2012;46:200–7.
Ryan M, Yule B, Bond C, et al. Do physicians’ perceptions of drug costs influence their prescribing? Pharmacoeconomics. 1996;9:321–31.
Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4.
Fadare JO, Enwere OO, Adeoti AO, et al. Knowledge and attitude of physicians towards the cost of commonly prescribed medicines: a case study in three Nigerian healthcare facilities. Value Health Reg Issues. 2020;22:68–74.
McGuire C, King S, Roche-Nagle G, et al. Doctors’ attitudes about prescribing and knowledge of the costs of common medications. Ir J Med Sci. 2009;178:277.
Grimmsmann T, Schwabe U, Himmel W. The influence of hospitalisation on drug prescription in primary care—a large-scale follow-up study. Eur J Clin Pharmacol. 2007;63:783–90.
Feely J, Chan R, McManus J, et al. The influence of hospital-based prescribers on prescribing in general practice. Pharmacoeconomics. 1999;16:175–81.
Harder S, Fischer P, Krause-Schäfer M, et al. Structure and markers of appropriateness, quality and performance of drug treatment over a 1-year period after hospital discharge in a cohort of elderly patients with cardiovascular diseases from Germany. Eur J Clin Pharmacol. 2005;60:797–805.
Gallini A, Legal R, Taboulet F. The influence of drug use in university hospitals on the pharmaceutical consumption in their surrounding communities. Br J Clin Pharmacol. 2013;75:1142–8.
Schutte T, Tichelaar J, Nanayakkara P, et al. Students and doctors are unaware of the cost of drugs they frequently prescribe. Basic Clin Pharmacol Toxicol. 2017;120:278–83.
Shrank WH, Joseph G, Choudhry NK, et al. Physicians’ perceptions of relevant prescription drug costs: do costs to the individual patient or to the population matter most? Am J Manag Care. 2006;12:545.
Ginsburg ME, Kravitz RL, Sandberg WA. A survey of physician attitudes and practices concerning cost-effectiveness in patient care. West J Med. 2000;173:390.
Polinski JM, Maclure M, Marshall B, et al. Does knowledge of medication prices predict physicians’ support for cost effective prescribing policies. Can J Clin Pharmacol. 2008;15:e286–94.
Prosser H, Walley T. A qualitative study of GPs’ and PCO stakeholders’ views on the importance and influence of cost on prescribing. Soc Sci Med. 2005;60:1335–46.
Moorkens E, Vulto AG, Huys I, et al. Policies for biosimilar uptake in Europe: an overview. PLoS ONE. 2017;12:e0190147.
Moorkens E, Godman B, Huys I, et al. The expiry of Humira® market exclusivity and the entry of adalimumab biosimilars in Europe: an overview of pricing and national policy measures. Front Pharmacol. 2021;11:1993.
Carthy P, Harvey I, Brawn R, et al. A study of factors associated with cost and variation in prescribing among GPs. Fam Pract. 2000;17:36–41.
Korn LM, Reichert S, Simon T, et al. Improving physicians’ knowledge of the costs of common medications and willingness to consider costs when prescribing. J Gen Intern Med. 2003;18:31–7.
Varkey P, Murad MH, Braun C, et al. A review of cost-effectiveness, cost-containment and economics curricula in graduate medical education. J Eval Clin Pract. 2010;16:1055–62.
Bates DW, Kuperman GJ, Jha A, et al. Does the computerized display of charges affect inpatient ancillary test utilization? Arch Intern Med. 1997;157:2501–8.
Ornstein SM, MacFarlane LL, Jenkins RG, et al. Medication cost information in a computer-based patient record system: impact on prescribing in a family medicine clinical practice. Arch Fam Med. 1999;8:118.
Vedsted P, Nielsen JN, Olesen F. Does a computerized price comparison module reduce prescribing costs in general practice? Fam Pract. 1997;14:199–203.
Durand DJ, Feldman LS, Lewin JS, et al. Provider cost transparency alone has no impact on inpatient imaging utilization. J Am Coll Radiol. 2013;10:108–13.
Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Int Med. 2013;173:903–8.
Tseng C-W, Lin GA, Davis J, et al. Giving formulary and drug cost information to providers and impact on medication cost and use: a longitudinal non-randomized study. BMC Health Serv Res. 2016;16:499.
Pevnick JM, Li N, Asch SM, et al. Effect of electronic prescribing with formulary decision support on medication tier, copayments, and adherence. BMC Med Inform Decis Mak. 2014;14:79.
Gipson G, Kelly JL, McKinney CM, et al. Optimizing prescribing practices of high-cost medications with computerized alerts in the inpatient setting. Am J Med Qual. 2016;32:278–84.
Tseng C-W, Brook RH, Alexander GC, et al. Health information technology and physicians’ knowledge of drug costs. Am J Manag Care. 2010;16:E105–10.
McMullin ST, Lonergan TP, Rynearson CS. Twelve-month drug cost savings related to use of an electronic prescribing system with integrated decision support in primary care. J Manag Care Pharm. 2005;11:322–32.
Rozenblum R, Rodriguez-Monguio R, Volk LA, et al. Using a machine learning system to identify and prevent medication prescribing errors: a clinical and cost analysis evaluation. Jt Comm J Qual Patient Saf. 2020;46:3–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Cormac Kennedy, Amelia Smith, Eoin O’Brien, Jamie Rice, and Michael Barry declare they have no conflicts of interest.
Author contributions
Concept and Design: CK, EOB, JR; Acquisition of Data: CK, EOB, JR; Analysis and interpretation of data: CK, AS; Drafting of the manuscript: CK, AS, EOB, JR, MB; Critical revision of the paper for important intellectual content: AS, MB; Statistical analysis: CK, AS; Provision of study materials or patients: Not applicable. Obtaining funding: Not applicable. Administrative, technical, or logistical support: AS; Supervision: MB.
Funding
No funding was requested or received for this study.
Ethics approval
The study was approved by the School of Medicine Ethics Committee, TCD.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent to publish
Not applicable.
Coding availability
Not applicable.
Rights and permissions
About this article
Cite this article
Kennedy, C., Smith, A., O’Brien, E. et al. Prescribers’ knowledge of drug costs: a contemporary Irish study. Drugs Ther Perspect 37, 272–281 (2021). https://doi.org/10.1007/s40267-021-00830-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-021-00830-5