Abstract
Restless legs syndrome (RLS; also known as Willis-Ekbom disease) is a common neurological, sensorimotor disorder. RLS is initially managed with lifestyle modifications, elimination of possible iatrogenic contributors and maintenance of normal-high peripheral iron stores. Moderate-to-severe RLS may be treated with pharmacological therapy, which generally involves the use of α-2-δ ligands (e.g. gabapentin enacarbil, pregabalin, gabapentin) and dopamine agonists (e.g. pramipexole, rotigotine, ropinirole), as well as opioids for treatment-resistant RLS. The chosen drug class/specific drug depends on patient factors (e.g. the most prominent symptoms, comorbidities, age-related issues, preferences) and drug factors (e.g. tolerability profile, augmentation risk).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Common and progressive condition
Restless legs syndrome (RLS; also known as Willis-Ekbom disease) is a common neurological, sensorimotor disorder characterized by an irresistible urge to move the legs, usually in response to unpleasant leg sensations (e.g. creeping, crawling, itching, pulling, throbbing) [1,2,3]. Although the arms and/or other parts of the body may be involved, the legs are usually firstly and most severely affected [4]. RLS symptoms begin or worsen during periods of inactivity or rest, are worse during the evening or night, and are partially or fully relieved by movement [2]. However, symptoms may progress to a point where they are no longer confined to the evening or night and no longer relieved by movement [2].
An estimated 7–10% of the US population have RLS [3]. Although RLS can start at any age, middle-aged or older individuals tend to be more severely affected than younger individuals, and symptoms generally become more frequent and longer lasting with age [3]. It is also more common in Parkinson’s disease (PD) patients than in the general population [5].
RLS can reduce sleep quality and quantity, impair daytime functioning, increase the risk of falls (especially in older adults because of night-time ambulation), and decrease health-related quality of life (HR-QoL) [1, 3]. By the time most patients seek treatment (usually in their fifth or sixth decade of life), daily preventative pharmacotherapy is usually needed [1]. This article summarizes the pharmacological management of RLS [1, 2, 5].
Diagnose based on patient history
RLS is diagnosed based on history alone, without the need for ancillary testing [1]. For a diagnosis of RLS, a patient must meet the five criteria shown in Fig. 1, with these features not able to be solely accounted for as symptoms of another behavioural or medical condition (e.g. arthritis, habitual foot tapping, leg cramps, leg oedema, myalgia, positional discomfort, venous stasis) [2].
Supportive factors should also be considered (Fig. 1) [1, 3, 6]. For example, as many as 20% of RLS patients have a first-degree relative with the disorder [1], and > 80% of experience periodic limb movements during sleep (PLMS), with involuntary jerking or twitching movements in the leg (and sometimes arm) that typically occur every 15–40 s, sometimes throughout the night [3]. As the pathophysiology of RLS involves dopamine dysfunction, patients with RLS should have a positive response to these drugs [3].
RLS has a high prevalence in the geriatric population, and those with cognitive impairment who are unable to verbalize their sensory symptoms (e.g. those with dementia) should be given special consideration (Fig. 1) [1]. Clinicians can look for specific behavioural cues, such as excessive motor activity (e.g. fidgeting and kicking, pacing, tossing and turning in bed), rubbing or kneading the legs, or groaning while holding the lower extremities. Although these ‘signs’ are not definitive or sufficient, they represent a first step in diagnosing RLS in this patient population [1].
The clinical course of RLS is categorized based on the average frequency of symptoms per week in the past year when RLS was left untreated as follows: intermittent when symptoms occur less than twice weekly on average; and chronic-persistent when symptoms occur at least twice weekly on average [2]. Based on the widely used International RLS Study Group 40-point scale, RLS is also categorized as mild, moderate or severe [1].
Start with non-pharmacological approaches
Treatment of RLS begins with non-pharmacological approaches (Fig. 1), which provide satisfactory outcomes in patients with mild RLS [1].
Lifestyle modifications include moderate, consistent exercise (can worsen RLS if excessive), avoidance of alcohol (can worsen RLS if consumed), and alleviating measures, such as stretching before or around symptom onset, and applying warm (or occasionally cold) water on legs before bedtime [1].
The use of drugs that can worsen RLS needs to be minimized or avoided [1]. Such drugs include:
-
All antidopaminergic agents (antiemetics, antipsychotics).
-
Histamine-1 (H1) blockers (anti-allergy drugs, anti-vertigo drugs, over-the-counter sleeping aids)
-
Serotonergic agents (antidepressants, except bupropion).
Of note, H1 blockers and sedative antidepressants are frequently used to treat insomnia, but can worsen RLS symptoms [1].
Treat iron deficiency if necessary
Substantial evidence supports iron deficiency in the brain as part of RLS pathophysiology, with iron therapy aiming to correct this deficiency [6]. Of note, severe iron deficiency to the point of anaemia is associated with a sixfold increase in RLS prevalence [6]. Only peripheral (i.e. serum) iron levels can be measured routinely, and results are rather insensitive and subject to circadian variation [1]. Therefore, transferrin saturation and serum ferritin (ideally collected in the morning after an overnight fast) should be used to assess iron stores [1, 6].
Performing a complete iron panel (serum ferritin, transferrin saturation, iron, total iron binding capacity) is recommended when a patient is initially evaluated for RLS, as well as every time the patient has an unexplained worsening of RLS symptoms [6]. If peripheral iron levels are low (serum ferritin < 100 μg/L and/or transferrin saturation < 20%), the patient may benefit from oral or intravenous (IV) iron therapy (Fig. 1) [1]. Not supplementing iron when iron levels are low but somewhat higher (i.e. transferrin saturation > 45% and/or serum ferritin > 300 μg/L) minimizes the risk of peripheral iron overload [6].
For oral iron treatment, ferrous sulfate 325 mg (65 mg elemental iron) + vitamin C 100 mg twice daily (or the total dose once daily) is recommended for 12 weeks if tolerated, with a repeat iron panel done after 12 weeks [6]. For IV iron treatment, ferric carboxymaltose 1000 mg over 15 min, or 500 mg over 7.5 min twice (5–7 days apart) is recommended. Optionally, low-molecular-weight iron dextran 975 mg can be given over 1–4 h after a 25 mg test dose. The iron panel should be repeated at 8 and 16 weeks following the infusion [6].
Iron deficiency should probably not be compensated for in PD patients with RLS [5]. PD is associated with increased iron in dopaminergic areas, so iron supplementation in PD could possibly contribute to neuronal death.
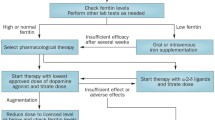
Introduce drugs for moderate-to-severe RLS
Pharmacological treatment is usually started when RLS symptom frequency and severity interfere with HR-QoL [2, 7]. RLS is generally treated with α-2-δ ligands (e.g. gabapentin, pregabalin, gabapentin enacarbil), dopamine agonists (e.g. pramipexole, ropinirole, rotigotine) and opioids (e.g. oxycodone, tramadol, methadone) (Table 1) [1].
Initial treatment should be individualized and targeted based on patient (e.g. most prominent symptoms, comorbidities, age-related issues, preferences) and drug (e.g. tolerability profile, augmentation risk) factors (Table 2) [7, 8]. More specifically, PLMS with can be treated with ropinirole (strong evidence), or pramipexole, rotigotine, cabergoline or pregabalin (moderate evidence) [8]. Other objective sleep measures (e.g. sleep efficiency, sleep latency) can be treated with ropinirole, gabapentin enacarbil or pregabalin (moderate evidence), and subjective sleep measures with gabapentin enacarbil or cabergoline (strong evidence), or ropinirole, pramipexole or pregabalin (moderate evidence).
In patients with concomitant psychiatric symptoms, ropinirole can be considered for anxiety and depression. In individuals with moderate-to-severe RLS-related mood disturbance, pramipexole can be used for depression and anxiety, and gabapentin enacarbil for overall mood. To improve HR-QoL, gabapentin enacarbil, ropinirole, pramipexole and cabergoline, as well as pregabalin, rotigotine and IV iron (ferric carboxymaltose), can be used.
Start with an α-2-δ ligand
An α-2-δ ligand (Table 1) can be considered for initial RLS treatment to prevent augmentation, an iatrogenic worsening of RLS symptoms that is commonly associated with the long-term use of dopamine agonist [7]. The α-2-δ ligands have no significant risk of augmentation [7]. There is strong evidence to support the use of gabapentin enacarbil, moderate evidence to support pregabalin, and insufficient evidence to support or refute gabapentin in the treatment of RLS [8].
Good initial response to dopamine agonists
Most patients have a good initial response to dopamine agonists, but treatment efficacy diminishes and/or augmentation develops over time even at low dosages [7]. In a US community-based study of patients treated with dopamine agonists, only 25% of patients had no indication of augmentation [14]. The risk of developing augmentation with dopamine agonists is strongly correlated with treatment dose and duration, and likely related to the specific action of the dopaminergic system. Therefore, avoiding the use of dopamine agonists is the most effective strategy to prevent augmentation [14].
If dopamine agonists are selected for initial RLS treatment, the lowest daily dosage should be used, with the dosage not exceeding that recommended for RLS (Table 1) [7]. If augmentation is mild, the dose of the dopamine agonist can be divided or taken before the onset of RLS symptom. If breakthrough night-time symptoms occurs, the dose can be increased with caution. However, when augmentation is severe, treatment can be switched to the rotigotine patch or an α-2-δ ligand. Of note, long-term use of rotigotine at a high dose can also cause augmentation. In very severe cases, consider switching to an opioid [7].
Strong evidence supports the use of pramipexole, rotigotine and cabergoline, but cabergoline is rarely used because of the risk of fibrotic complications and cardiac valvulopathy at high dosages [8]. Moderate evidence supports the use of ropinirole, and weak evidence supports levodopa [8]. Levodopa was the first dopaminergic drug that was used to treat RLS, but it has a shorter half-life and a shorter time to augmentation than ropinirole, pramipexole and rotigotine [7]. Although it is not frequently used to treat RLS, levodopa is recommended PD patients with RLS who have contraindications to dopamine agonists (e.g. age > 70 years, cognitive dysfunction/hallucinations, impulse control disorder, severe sleepiness) [5].
Consider opioid therapy when other treatments fail
Opioid therapy can be considered for severe, treatment-resistant RLS, and in patients who develop augmentation with other treatments [2]. There is insufficient evidence to support or refute the use of oxycodone in the treatment of RLS [8]. However, oxycodone is likely efficacious on its own in those with daily RLS symptoms [2]. Oxycodone and methadone are commonly used opioids in the treatment of RLS [1]. Tramadol is used as well, but is associated with a risk of augmentation [1]. Prolonged-release oxycodone/naloxone can be considered where available [2, 8]. Prior to initiating opioid therapy, patients should be screened for a history of alcohol or drug abuse because they pose a higher risk of opioid abuse or misuse [1].
Advance nonpharmacological options may help
Patients and clinicians desiring nonpharmacological approaches to treat RLS should consider the use of pneumatic compression before usual symptom onset [8], and may consider near-infrared spectroscopy (NIRS) or repetitive transcranial magnetic stimulation (rTMS). Pneumatic leg compression is likely effective, and NIRS and rTMS are possibly effective, whereas vibrating pads are possibly ineffective and transcranial direct current stimulation is probably ineffective in treating RLS symptoms. Vibrating pads may work for subjective sleep concerns, but not for RLS symptoms. Evidence is insufficient to either support or refute the use of acupuncture to treat RLS [8].
Take-home messages
-
Initially manage RLS by modifying lifestyle, eliminating possible contributors and maintaining normal-to-high iron stores.
-
Consider pharmacological therapy for moderate-to-severe RLS if symptom frequency and severity interfere with a patient’s HR-QoL.
-
Generally treat with α-2-δ ligands, dopamine agonists or opioids, with the drug class specific drug chosen based on patient and drug factors.
References
During EH, Winkelman JW. Drug treatment of restless legs syndrome in older adults. Drugs Aging. 2019;36(10):939–46.
Gonzalez-Latapi P, Malkani R. Update on restless legs syndrome: from mechanisms to treatment. Curr Neurol Neurosci Rep. 2019;19(8):54.
National Institute of Neurological Disorders and Stroke. Restless legs syndrome fact sheet: NIH publication no. 17-4847. Bethesda (MD): National Institute of Health; 2017.
Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014;15(8):860–73.
Cochen De Cock V. Therapies for restless legs in Parkinson’s disease. Curr Treat Options Neurol. 2019;21(11):56.
Allen RP, Picchietti DL, Auerbach M, et al. Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: an IRLSSG task force report. Sleep Med. 2018;41:27–44.
Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. 2016;21:1–11.
Winkelman JW, Armstrong MJ, Allen RP, et al. Practice guideline summary: treatment of restless legs syndrome in adults: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2016;87(24):2585–93.
Horizant® (gabapentin enacarbil) extended-release tablets: US prescribing information. Atlanta: Arbor Pharmaceuticals, LLC; 2020.
Buchfuhrer MJ. Strategies for the treatment of restless legs syndrome. Neurotherapeutics. 2012;9(4):776–90.
Mirapex® (pramipexole dihydrochloride) tablets: US prescribing information. Ridgefield: Boehringer Ingelheim Pharmaceuticals, Inc.; 2020.
Requip® (ropinirole) tablets: US prescribing information. Research Triangle Park: GlaxoSmithKline; 2020.
Neupro® (rotigotine transdermal system): US prescribing information. Smyrna: UCB, Inc.; 2020.
Allen RP, Ondo WG, Ball E, et al. Restless legs syndrome (RLS) augmentation associated with dopamine agonist and levodopa usage in a community sample. Sleep Med. 2011;12(5):431–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
E.S. Kim is a contracted employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. K.A. Lyseng-Williamson is a salaried employee of Adis International/Springer Nature, is an editor of Drugs & Therapy Perspectives, was not involved in any publishing decision for the manuscript, and has no other conflicts of interest to declare. Both authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Rights and permissions
About this article
Cite this article
Kim, E.S., Lyseng-Williamson, K.A. Choose treatment for restless legs syndrome based on patient and drug characteristics. Drugs Ther Perspect 36, 435–439 (2020). https://doi.org/10.1007/s40267-020-00773-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-020-00773-3