Abstract
Restless legs syndrome (RLS) has a high prevalence in the elderly and can impact sleep quality and sleep quantity, reduce quality of life (QoL), and increase the risk of falls during episodes of night-time ambulation. In patients unable to verbalize their sensory symptoms, certain behavioral cues may help with the diagnosis. A state of brain iron deficiency could play a central role in the pathophysiology of RLS and be upstream to a series of dysfunctions that are not limited to the dopaminergic system. Management should initially emphasize lifestyle modifications and reduction of all possible iatrogenic contributors while maintaining a state of normal–high peripheral iron stores. Oral iron, in patients with ferritin levels < 75 μg/dL, appears to be effective, although iron infusions should be considered when more immediate benefit or oral iron have not been effective. When other attempts fail and patients continue to experience chronic RLS symptoms substantially interfering with QoL, pharmacological agents may present a favorable benefit versus risk profile. Such agents may include α-2-δ drugs or dopaminergic agents, after careful consideration of the risk of RLS augmentation with the latter class. In patients with established RLS augmentation from the use of dopaminergic drugs, the addition of α-2-δ agents or low-dose opioids, with subsequent slow tapering of dopaminergic agents, is recommended. With any of these agents, caution should be made with regard to the risk of drug–drug interactions and altered pharmacokinetics in this fragile population. Although showing excellent long-term safety data in non-elderly adults with RLS, studies are needed to ascertain that such treatments are effective and well tolerated in older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Restless legs syndrome (RLS) is a sensorimotor disorder often described as an unpleasant ‘creepy’, ‘crawly’ sensation associated with an urge to move the legs. It is worse with rest, as well as in the evening and at night, and is improved with activity such as ambulation. Age appears to be a strong risk factor, with a consistently reported two- to threefold increase in prevalence between young adults and adults aged 60 years and older [1]. Clinically significant RLS (at least 2 ×/week associated with at least moderate distress) is estimated to affect between 3 and 5% of those over the age of 60 years [2, 3]. In addition to reducing sleep quality and quantity, RLS is associated with an increased risk of falls in older adults due to night-time ambulation [4]. Unfortunately, this condition tends to be chronic, with gradually worsening symptoms, leading most patients to seek treatment in their fifth or sixth decade of life, at which point many patients require daily preventative pharmacotherapy [5]. A number of guidelines exist for the treatment of RLS, however no specific recommendations exist for the management of elderly patients with RLS.
This article will first review the diagnostic criteria of RLS, including in patients with severe cognitive impairment, as well as current evidence on the pathophysiology of this disease. Recent guidelines are then reviewed, and the short- and long-term efficacy and safety for each pharmacological agent in the elderly is discussed.
2 Diagnosis
2.1 Diagnosis of Restless Legs Syndrome (RLS) in the Adult Population
A diagnosis of RLS is based on history alone and does not require ancillary testing. The four cardinal criteria can easily be remembered as the acronym ‘URGED’: Urge to move the legs associated with unpleasant leg sensations; Rest induces symptoms; Gets better with activity; Evening and nighttime worsening; Does not occur due to an RLS mimic, such as leg cramps, etc. In addition, symptoms should not be solely accounted for by another condition [6]. Supportive features include a family history of RLS in a first-degree relative, a positive response to dopaminergic drugs, and the presence of periodic limb movements (PLMs) during wake or sleep. Of note, PLMs, although sensitive in RLS [7], are not specific for this disease but are associated with a wide range of cardiovascular and neurologic conditions, as well as male sex and aging.
Although RLS can affect both legs in a symmetric fashion, a large proportion of patients may experience more symptoms in one leg, as well symptoms in their upper extremities or torso, not just in their lower extremities. Depending on the frequency and intensity of symptoms, RLS can be characterized based on the frequency and intensity of symptoms. It is considered ‘intermittent’ when occurring, on average, no more than 1 day per week, and ‘chronic’ when occurring at least 2 days per week. RLS can be mild, moderate, or severe, based on the widely used International RLS Study Group 40-point scale [8].
2.2 Differential Diagnosis
RLS should be distinguished from a number of common mimics that share one or more features of the cardinal symptoms of RLS: neuropathy is often worse at night and causes discomfort, however it is not associated with an urge to move the legs and is not relieved by movement; akathisia does not have a circadian evening propensity and the sensation of restlessness is typically diffuse without predilection for the legs; muscle cramps can predominantly or solely occur during sleep but result in painful muscle contractions and are not associated with an urge to move; habitual foot taping is a behavioral phenomenon that is not associated with an urge to move, and can be suppressed.
2.3 Diagnosis of RLS in Patients Unable to Verbalize Their Sensory Symptoms
Specific consideration should be given to the diagnosis of RLS in patients whose cognitive impairment is such that they cannot verbalize sensory symptoms of RLS. In these circumstances, motor manifestations of RLS and some behavioral cues may help with the diagnosis. Expert opinion issued in 2003 suggested ‘signs’ of leg discomfort such as ‘rubbing’ or ‘kneading’ the legs or ‘groaning while holding the lower extremities’. As well as excessive motor activity such as pacing, fidgeting and kicking, tossing and turning in bed are essential criteria in diagnosing RLS in this population [9]. Others have since reported that nocturnal ambulation and mannerisms could also be motor manifestations of RLS in the geriatric population, especially in those treated with selective serotonin reuptake inhibitors (SSRIs) [10, 11]. Supportive features can include low iron status and, although not specific, a high index of PLMs, a finding that can be observed during wake and/or sleep [6]. None of these criteria are sufficient or definitive and represent a first step in the diagnostic criteria of RLS in patients with dementia. Further research is needed to refine and validate the diagnostic criteria for RLS in these patients. Such studies could involve validation of clinical diagnosis by expert clinicians, and longitudinal studies in individuals with a definite diagnosis of RLS made prior to dementia.
3 Pathogenesis
Although the pathophysiology of RLS is not fully understood, genetic factors have been discovered. As many as 20% of patients with RLS have a first-degree relative affected by the disease. The three main known genetic variants involve the BTBD9, MEIS1, and MAP2K5/SKOR1 genes [12, 13].
Research suggests that several neurotransmitter pathways could be involved at different levels, and possibly in a complementary fashion—the dopaminergic, adenosinergic, and glutamatergic pathways, in addition to a state of central (brain) iron deficiency [14, 15]. Of these pathways, the dopaminergic pathway has been the most extensively studied.
RLS is thought to be a ‘hyperdopaminergic state’, which is illustrated by the efficacy of drugs targeting the inhibitory dopamine D3 receptor (D3R). However, long-term use of dopaminergic agonists results in upregulation of the excitatory D1 receptor (D1R), shown to be associated with RLS augmentation, a complication that we discuss below. Interestingly, D1Rs increase with age, which may explain how age alone is a risk factor for RLS. The adenosinergic pathway has also recently been shown to be implicated. Low central nervous system (CNS) iron stores may lead to downregulation of the adenosine 1 receptor (A1R), which in turn contributes to both a hyperdopaminergic and hyperglutamatergic state [17, 18].
The opioid system, via the mu receptor, is also involved in RLS. An autopsy study shows relative deficiency in CNS opioid receptors in patients with RLS compared with controls [19], while another study suggests that the beneficial effect of opioids in RLS is not a mere consequence of mu receptor activation but is related to improved dopaminergic transmission [20].
Several conditions, including arthritis, sensory neuropathy, radiculopathy, neurodegenerative conditions, diabetes, and renal disease, are associated with RLS and are also more prevalent in seniors. With the exception of renal disease, it is not clear whether these are only comorbid conditions or risk factors, or whether they share a common pathophysiology with RLS. In addition, the prevalence of psychiatric disorders such as depression and anxiety is increased 1.5- to 2-fold in patients with RLS, with a proposed bidirectional relationship between these psychiatric disorders and RLS.
4 Evidence-Based Treatment of RLS
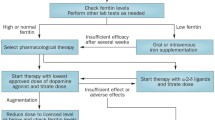
The treatment of RLS can be divided into non-pharmacological and pharmacological approaches. A list of the steps that should be checked for every patient with RLS is provided in Fig. 1; however, to date, no studies have evaluated the effects of these interventions specifically in the elderly.
4.1 Non-Pharmacological Treatment
4.1.1 Lifestyle Modifications
Non-pharmacological approaches are the foundation of RLS treatment. When implemented in a systematic fashion, they can yield satisfactory outcomes in milder cases. Such approaches will include an evaluation of lifestyle and iatrogenic factors, as well as serum iron stores.
Lifestyle factors that contribute to RLS include physical deconditioning, although excessive exercise can worsen RLS. As a result, patients should be encouraged to exercise moderately but consistently. In addition, alcohol can worsen RLS. Alleviating measures such as stretching before or around symptom onset, and warm (or occasionally cold) water, are at least temporarily effective for a number of patients.
4.1.2 Iatrogenic Factors
Many drugs can exacerbate RLS and should thus be minimized or avoided. These broadly include all antidopaminergic agents (antipsychotics, antiemetics), histamine-1 (H1) blockers (anti-vertigo and anti-allergy drugs, over-the-counter sleeping aids), and serotonergic agents (most antidepressants, except for bupropion). This is of particular importance as H1 blockers and sedative antidepressants are often used to treat insomnia and could make symptoms of RLS worse.
4.1.3 Complementary Interventions and Alternative Therapies
Several non-pharmacological interventions for RLS have recently been reviewed [21, 22]. Although the evidence remains limited, based on one double-blind sham-controlled trial, a pneumatic leg compression device may be considered in some patients [23]. Acupuncture may also provide some relief, although its efficacy needs to be supported by more rigorous clinical trials [21]. Additional possibly effective interventions for RLS include near-infrared spectroscopy or transcranial magnetic stimulation [22]. In addition, vibrating pads can improve subjective sleep in patients with RLS, but not RLS symptoms per se.
4.1.4 Iron Therapy
Although RLS is associated with a state of brain iron deficiency, only ‘peripheral’ iron, i.e. serum iron levels, can be routinely measured. Rather than iron concentration, which is subject to circadian variation and is rather insensitive (falsely normal or elevated, particularly by ingestion of iron in the hours before testing), assessment of iron stores requires ferritin and transferrin saturation (iron/TIBC, also called iron saturation in some laboratories), which should ideally be collected in a fasting state. Recent guidelines suggest that oral iron supplementation could be beneficial if serum ferritin is < 75 μg/dL or the transferrin saturation index is < 20% [24]. In these cases, oral ferrous sulfate 325 mg (65 mg elemental iron) taken once daily with vitamin C on an empty stomach is the most effective regimen. More aggressive (two or three times daily) oral repletion is unlikely to succeed, not only due to poor gastrointestinal tolerance (nausea and constipation) but also due to the homeostatic action of hepcidin, whose synthesis is stimulated by oral iron loads, resulting in net inhibition of iron absorption at higher iron doses.
Recent guidelines suggest the use of intravenous iron therapy when rapid response is needed, or when oral repletion fails or is contraindicated [24]. In such cases, a higher ferritin threshold can be considered appropriate for treatment: ferritin levels < 100 μg/dL can justify intravenous repletion, provided that the risk of iron overload is limited (transferrin saturation should be < 45%). Among all intravenous formulations, ferric carboxymaltose and low molecular weight (LMW) iron dextran have the best clinical evidence for RLS. Following a 1 g infusion of either formulation, response rates range between 50 and 60%, with a clinical benefit often delayed by 2–8 weeks. Intravenous treatment can be repeated in case of clinical relapse, especially if iron stores appear to be depleted again, or in patients who have responded to prior infusion(s). Serious hypersensitivity reactions are very rare with current intravenous iron formulations.
4.2 Pharmacological Treatment
The pharmacotherapy of chronic RLS broadly encompasses three classes of drugs: α-2-δ ligands, dopaminergic drugs, and opioids.
4.2.1 Dopaminergic Agonists
Oral pramipexole, ropinirole, and transdermal rotigotine are all US FDA-approved for the treatment of moderate–severe primary RLS. The efficacy of dopaminergic drugs is thought to be mediated by the inhibitory D3 receptor. In addition to rapid and often complete symptom relief, dopaminergic drugs usually substantially reduce PLMs. In the elderly, pramipexole (12 h) has a longer half-life than ropinirole (6 h) and is primarily excreted by the kidneys, as opposed to ropinirole, which is primarily metabolized by the liver. This makes ropinirole the dopaminergic drug of choice in patients with advanced renal disease. Doses used in RLS are 50–90% lower than those used to treat parkinsonism (Table 1).
Due to their efficacy for RLS symptoms, dopaminergic agonists have been widely prescribed in such patients. The efficacy of pramipexole and ropinirole is supported by multiple studies showing their superiority over placebo in terms of RLS symptoms, subjective sleep parameters, PLMs, and possibly other objective sleep parameters, as well as QoL and potentially depression [22]. Rotigotine patch has also been shown to reduce RLS symptoms, PLMs, subjective sleep measures, and possibly QoL.
Ergot derivative dopaminergic drugs such as cabergoline and pergolide should be avoided due to the increased risk of valvulopathy and augmentation. Despite their well-supported efficacy, dopamine agonists have a number of adverse effects, including sedation and occasionally sleep attacks during daytime, nausea, orthostatic hypotension, disturbed sleep and insomnia, and, more concerning, augmentation and impulse control disorders. Impulse control disorders occur in approximately 10–15% of patients, resulting in compulsive spending, gambling, sexual activity (often viewing pornography), or binge eating, with a mean time of occurrence of 9 months.
Augmentation of RLS symptoms with chronic daily use is the most common complication of RLS treatment with dopaminergic agents, and the most common reason for their discontinuation. Augmentation is a disastrous consequence of chronic oral dopaminergic drugs, and is most commonly characterized by RLS symptoms starting earlier during the day. In addition, the extension of symptoms to the upper extremities, increased severity of symptoms, shorter latency to symptom onset, and shorter duration of medication benefit are commonly observed. As a result of worsening RLS symptoms, patients and their providers will often gradually increase doses of these agents, leading to a vicious cycle of worsening symptoms and dose escalation. The estimated yearly rate of augmentation with pramipexole is 8%, and as high as 50% after 5 years of continuous use [25]. This rate is even higher with levodopa–carbidopa, and is therefore not recommended for daily use. Almost invariably, the dopaminergic drug will need to be discontinued, which often results in severe RLS exacerbation. As discussed earlier, augmentation may be due to upregulation of excitatory D1Rs [16]. Rotigotine patch may present a lower risk of augmentation than short half-life oral treatments, although it is unclear whether there is truly reduced augmentation or just a temporary masking of earlier symptoms by continuous dopaminergic stimulation. Due to this important and disastrous risk, current guidelines suggest that α-2-δ ligands could be considered first-line when daily treatment is indicated [26]. In those patients taking long-term dopaminergic agonists, frequent assessment for symptoms of augmentation, as well as maintaining moderate doses of these agents (pramipexole < 0.75 mg, ropinirole < 4.0 mg, rotigotine < 3.0 mg) is strongly recommended.
4.2.2 α-2-δ Ligands
This class of drugs includes pregabalin, gabapentin, and gabapentin enacarbil, a controlled-release prodrug of gabapentin. These drugs act via binding at the α-2-δ subunit of the voltage-gated calcium channel, which reduces the release of excitatory neurotransmitters, including glutamate, and which in turn may explain their efficacy in RLS. These drugs are renally excreted, and as nearly 50% of those subjects over 70 years of age will have reductions in estimated glomerular filtration rate, dose should be adjusted in this age group, and certainly in patients with renal failure or insufficiency. In addition, the starting dose should be lower in the elderly than in younger adults (Table 1). α-2-δ ligands have several other benefits, including a reduction in neuropathic pain and improvement in sleep quality, via reduction of arousals and increased slow-wave sleep.
In this drug class, gabapentin enacarbil is supported by the highest quality evidence, with four double-blind, randomized controlled trials showing superiority over placebo for RLS symptoms, and a likely improvement in sleep measures other than PLMs, in addition to QoL and mood [22]. At doses of at least 150 mg/day, pregabalin has also shown to likely be effective in RLS symptoms, sleep measures, and QoL. Head-to-head studies comparing pregabalin and pramipexole revealed no differences in terms of RLS symptoms, but possible superiority of pregabalin regarding sleep parameters other than PLMs, and QoL [27, 28]. Importantly, this 1-year study demonstrated a substantially higher rate of augmentation with pramipexole compared with pregabalin.
Although gabapentin is one of the recommended first-line agents [26], and likely the most widely prescribed drug in this class, no long-term prospective studies have been conducted demonstrating its efficacy in RLS. On the other hand, even though it is approved by the FDA for the treatment of RLS, gabapentin enacarbil has not shown superior efficacy compared with pregabalin [29].
The most common adverse effects of the α-2-δ ligands include sedation (most notable at treatment introduction), dizziness, and weight gain at higher doses. Early concerns regarding suicide were later contradicted by a large retrospective study in over 130,000 patients, suggesting that gabapentin is not associated with an increased risk of suicide attempts [30].
The main disadvantage of gabapentin resides in its erratic and non-linear pharmacokinetics due to limits in gastrointestinal absorption, which at least partially explains why some patients require significantly higher doses than others; some patients respond to doses of 300 mg or less, while others require up to 2000 mg/day to reach a therapeutic dose. For this reason, split-dosing of gabapentin is recommended to circumvent saturation of gastrointestinal receptors if daily doses greater than 600–900 mg are desirable. Gabapentin enacarbil, a prodrug of gabapentin, has linear pharmacokinetics, and thus predictable serum levels. It is also the only drug of this class approved by the FDA for the treatment of RLS. Gabapentin enacarbil is best absorbed with food and has a slow onset of action, which can exceed 2 h, but a longer duration of action compared with other drugs in this class, achieving steady-state within a few days. Pregabalin has a shorter (usually 60 min) onset of action compared with other drugs in this class.
In summary, α-2-δ ligands remain the drug of choice in many chronic RLS cases due to their efficacy and short- and long-term safety profile without the risk of augmentation. When choosing any of these three drugs, for the best benefit–risk ratio, considerations should be given to their respective profile in terms of rate of absorption and effective elimination half-life. However, guidelines from the American Geriatric Society (AGS) recommend cautious use of gabapentinoid drugs in elderly patients. The AGS specifically recommend a dose adjustment in those with creatinine clearance < 60 mL/min, and warn against the risk of overdose when combined with opioids [31].
4.2.3 Opioids
These agents are most commonly used in patients with augmented RLS symptoms from dopaminergic drugs when adequate symptom control is not obtained with α-2-δ agents. In the US, the most commonly used opioids for the treatment of RLS are oxycodone and methadone, although it appears that all opioids can provide symptom relief in RLS. Their efficacy is likely related to their action on the mu receptor. In addition to this class effect, some opioids have other pharmacological effects that may contribute to their efficacy in RLS, such as the NMDA glutamate receptor antagonists methadone and tramadol.
Therapeutic doses of opioids in RLS are usually much lower than in the treatment of chronic pain, and are typically below 50 mg morphine equivalent daily (Table 1). Other than the starting dose, particular consideration should be given to the drug half-life; immediate-release oxycodone has a short half-life of 3–4 h, while extended-release formulations have an effective half-life of approximately 5–6 h. By comparison, the half-life of methadone is much longer, ranging from 8 to 60 h (although generally approximately 24 h) with substantial interindividual variability.
Particular caution should be used with regard to the specific risks or complications with some of these opioids. Due to its serotonergic effect, tramadol may be associated with a risk of augmentation. Methadone presents a risk of cardiac complications due to prolonged QT, and patients should have an electrocardiogram before treatment is initiated and once a stable dose is reached (Table 1).
Despite their long use in RLS, studies evaluating the efficacy of opioids over placebo are scarce. A small randomized controlled crossover trial in 11 patients with moderate to severe RLS receiving oxycodone (mean dose of 16 mg/day) or placebo showed superior efficacy of oxycodone in terms of sensory and motor symptoms [32]. The largest study using opioids in RLS evaluated the efficacy of oxycodone combined with naloxone (a drug available in Europe for treatment-refractory RLS but not available in the US) in over 300 patients, showing superior benefit using a mean dose of oxycodone 22 mg over the 12-week trial [33]. The largest open-label study using methadone in 76 consecutive patients showed long-term efficacy, with minimal dose increase over time and excellent tolerability [25].
Although considered to be effective and relatively well tolerated in the adult population, no studies have specifically evaluated the safety profile of the long-term use of opioids for RLS in older adults. Specifically, there is no evidence of substantial tolerance or abuse in the vast majority of patients with RLS [24, 25, 34], although case series are generally small and from tertiary care centers. Similarly, there is no suggestion of augmentation with the opioids.
As a class, all opioids present the risk of sedation, nausea, and constipation, which warrant even further caution in older adults. Due to their respiratory depressant effects, opioids can aggravate underlying obstructive sleep apnea, resulting in more severe hypoxias, as well as precipitate treatment-emergent central sleep apnea. When not prescribed appropriately, drug–drug interactions between opioids and gabapentinoids or benzodiazepines can lead to overdose. This has lead the AGS to issue a strong recommendation against such drug combinations [31]. Before initiating an opioid for RLS, patients should be questioned about their past history of alcohol or drug abuse as this poses a higher risk of opioid misuse or abuse. In addition, an opioid contract listing risks and certain obligations regarding prescription and refills should be signed by the patient. Assessment of state online prescription drug monitoring programs at each visit is mandated by many states.
4.2.4 Other Drugs in the Treatment of RLS
There is no evidence that benzodiazepines and non-benzodiazepine receptor agonists improve RLS symptoms per se, although they often improve sleep disturbance and/or anxiety symptoms, both of which can exacerbate RLS. We recommend caution in their use in seniors with RLS due to cognitive and fall risk associated with these drugs. Treatment guidelines do not recommend their use in RLS [24, 35]. When pharmacotherapy for insomnia is deemed necessary, the use of short to intermediate half-life benzodiazepine receptor agonists for a limited course of < 90 days is recommended. However, such drugs may trigger parasomnias in patients with RLS [36, 37]. Such risk is particularly concerning in the elderly due to the additional risk of falls, and we therefore advise extreme caution in the use of these agents in elderly patients with RLS.
5 Conclusions
RLS is particularly prevalent in the elderly. In these patients, RLS not only affects sleep quality and quantity but can also increase the risk of falls due to the need to ambulate at night. Low CNS iron could contribute to a series of dysfunctions across different neurotransmitter pathways, including but not limited to the dopaminergic system. The mainstay of management includes lifestyle modifications, reduction of all possible iatrogenic contributors, and maintenance of a state of normal–high peripheral iron stores. In patients with ferritin levels < 75 μg/dL, oral supplementation appears to be effective, although when more immediate benefit or oral iron has not been effective, intravenous iron infusions should be considered. Despite this approach, if patients continue to experience chronic and debilitating RLS symptoms, certain pharmacological agents can be considered, with consideration of their benefit versus risk ratio. These include α-2-δ drugs or dopaminergic agents, after careful consideration of the risk of RLS augmentation with the latter class. In patients with established RLS symptom augmentation related to dopaminergic drugs, the addition of α-2-δ agents or low-dose opioids, with subsequent slow tapering of dopaminergic agents, is recommended. With any of these drugs, in this fragile population at risk of polypharmacy, there is a higher risk of drug–drug interactions and altered pharmacokinetics. Although the evidence shows excellent long-term safety data in non-elderly adults with RLS, studies are needed to ascertain that such treatments are effective and well tolerated in older adults.
References
Garcia-Borreguero D, Egatz R, Winkelmann J, Berger K. Epidemiology of restless legs syndrome: the current status. Sleep Med Rev. 2006;10(3):153–67.
Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16(4):283–95.
Allen RP, Walters AS, Montplaisir J, Hening W, Myers A, Bell TJ, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165(11):1286–92.
Kuzniar TJ, Silber MH. Multiple skeletal injuries resulting from uncontrolled restless legs syndrome. J Clin Sleep Med. 2007;3(1):60–1.
Walters AS, Hickey K, Maltzman J, Verrico T, Joseph D, Hening W, et al. A questionnaire study of 138 patients with restless legs syndrome: the “night-walkers” survey. Neurology. 1996;46(1):92–5.
Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria—history, rationale, description, and significance. Sleep Med. 2014;15(8):860–73.
Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lespérance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12(1):61–5.
Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–32.
Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–19.
Bliwise DL. Periodic leg movements in sleep and restless legs syndrome: considerations in geriatrics. Sleep Med Clin. 2006;1(2):263–71.
Richards K, Shue VM, Beck CK, Lambert CW, Bliwise DL. Restless legs syndrome risk factors, behaviors, and diagnoses in persons with early to moderate dementia and sleep disturbance. Behav Sleep Med. 2010;8(1):48–61.
Yang Q, Li L, Chen Q, Foldvary-Schaefer N, Ondo WG, Wang QK. Association studies of variants in MEIS1, BTBD9, and MAP2K5/SKOR1 with restless legs syndrome in a US population. Sleep Med. 2011;12(8):800–4.
Schormair B, Zhao C, Bell S, Tilch E, Salminen AV, Pütz B, et al. Identification of novel risk loci for restless legs syndrome in genome-wide association studies in individuals of European ancestry: a meta-analysis. Lancet Neurol. 2017;16(11):898–907.
Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54(8):1698–700.
Li X, Allen RP, Earley CJ, Liu H, Cruz TE, Edden RAE, et al. Brain iron deficiency in idiopathic restless legs syndrome measured by quantitative magnetic susceptibility at 7 tesla. Sleep Med. 2016;22:75–82.
Dinkins ML, Lallemand P, Clemens S. Long-term treatment with dopamine D3 receptor agonists induces a behavioral switch that can be rescued by blocking the dopamine D1 receptor. Sleep Med. 2017;40:47–52.
Ferré S, Quiroz C, Guitart X, Rea W, Seyedian A, Moreno E, et al. Pivotal role of adenosine neurotransmission in Restless Legs Syndrome. Front Neurosci. 2018;11:722.
Rivera-Oliver M, Moreno E, Álvarez-Bagnarol Y, Ayala-Santiago C, Cruz-Reyes N, Molina-Castro GC, et al. Adenosine A1-dopamine D1 receptor heteromers control the excitability of the spinal motoneuron. Mol Neurobiol. 2019;56(2):797–811.
Walters AS, Ondo WG, Zhu W, Le W. Does the endogenous opiate system play a role in the Restless Legs Syndrome? A pilot post-mortem study. J Neurol Sci. 2009;279(1–2):62–5.
Silber MH, Becker PM, Buchfuhrer MJ, Earley CJ, Ondo WG, Walters AS, et al. The appropriate use of opioids in the treatment of refractory restless legs syndrome. Mayo Clin Proc. 2018;93(1):59–67.
Xu XM, Liu Y, Jia SY, Dong MX, Cao D, Wei YD. Complementary and alternative therapies for restless legs syndrome: an evidence-based systematic review. Sleep Med Rev. 2018;38:158–67.
Winkelman JW, Armstrong MJ, Allen RP, Chaudhuri KR, Ondo W, Trenkwalder C, et al. Practice guideline summary: treatment of restless legs syndrome in adults. Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2016;87(24):2585–93.
Lettieri CJ, Eliasson AH. Pneumatic compression devices are an effective therapy for restless legs syndrome: a prospective, randomized, double-blinded, sham-controlled trial. Chest. 2009;135(1):74–80.
Allen RP, Picchietti DL, Auerbach M, Cho YW, Connor JR, Earley CJ, et al. Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: an IRLSSG task force report. Sleep Med. 2018;41:27–44.
Silver N, Allen RP, Senerth J, Earley CJ. A 10-year, longitudinal assessment of dopamine agonists and methadone in the treatment of restless legs syndrome. Sleep Med. 2011;12(5):440–4.
Garcia-Borreguero D, Silber MH, Winkelman JW, Högl B, Bainbridge J, Buchfuhrer M, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. 2016;21:1–11.
Allen R, Chen C, Garcia-Borreguero D, Polo O, DuBrava S, Miceli J, et al. Comparison of pregabalin with pramipexole for restless legs syndrome. N Engl J Med. 2014;370(7):621–31.
Garcia-Borreguero D, Patrick J, DuBrava S, Becker P, Lankford A, Chen C, et al. Pregabalin versus pramipexole: effects on sleep disturbance in restless legs syndrome. Sleep. 2014;37(4):635–43.
Iftikhar IH, Alghothani L, Trotti LM. Gabapentin enacarbil, pregabalin and rotigotine are equally effective in restless legs syndrome: a comparative meta-analysis. Eur J Neurol. 2017;24(12):1446–56.
Gibbons RD, Hur K, Brown CH, Mann JJ. Gabapentin and suicide attempts. Pharmacoepidemiol Drug Saf. 2010;19(12):1241–7.
American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–94.
Walters AS, Wagner ML, Hening WA, Grasing K, Mills R, Chokroverty S, et al. Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16(4):327–32.
Trenkwalder C, Beneš H, Grote L, García-Borreguero D, Högl B, Hopp M, et al. Prolonged release oxycodone-naloxone for treatment of severe restless legs syndrome after failure of previous treatment: a double-blind, randomised, placebo-controlled trial with an open-label extension. Lancet Neurol. 2013;12(12):1141–50.
Walters AS, Winkelmann J, Trenkwalder C, Fry JM, Kataria V, Wagner M, et al. Long-term follow-up on restless legs syndrome patients treated with opioids. Mov Disord. 2001;16(6):1105–9.
Aurora RN, Rosenberg RS, Kristo DA, Bista SR, Casey KR, Rowley JA, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults—an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses. Sleep. 2012;35(8):1039–62.
Howell MJ, Schenck CH. Restless nocturnal eating: a common feature of Willis-Ekbom Syndrome (RLS). J Clin Sleep Med. 2012;8(4):413–9.
Provini F, Antelmi E, Vignatelli L, Zaniboni A, Naldi G, Calandra-Buonaura G, et al. Association of restless legs syndrome with nocturnal eating: a case-control study. Mov Disord. 2009;24(6):871–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflicts of interest
John W. Winkelman reports research support from Merck, National Institute of Mental Health, the Restless Legs Syndrome Foundation, and American Regent, and was a consultant for, or received honoraria from, Merck, Advance Medical, and UpToDate. Emmanuel H. During reports no conflicts of interest in relation to this article.
Rights and permissions
About this article
Cite this article
During, E.H., Winkelman, J.W. Drug Treatment of Restless Legs Syndrome in Older Adults. Drugs Aging 36, 939–946 (2019). https://doi.org/10.1007/s40266-019-00698-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-019-00698-1