Abstract
Obesity in children, often accompanied by comorbidities, is increasingly common. For many frequently used paediatric drugs, information on dosage adjustment in obese children is lacking or absent. Scalars, such as total body weight, are not always helpful as obese children may weigh more than adults, but differ with regard to aspects of their anatomy and physiology, especially hepatic function. Further pharmacokinetic studies in obese children are urgently needed and, in the interim, close monitoring for therapeutic effect and toxicity is recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Drug dosage in obese children a weighty challenge
Obesity in children and adolescents is a big problem, affecting at least 124 million children and carrying significant comorbidity [1]. Childhood obesity is defined by the World Health Organization (WHO) as having a body mass index (BMI, kg/m2) above the 95th percentile of individuals the same age and sex in children aged 5–18 years inclusive, and above the 99th percentile in those aged up to 5 years [1]. Other organizations, such as the American Academy of Pediatrics [2], include “late adolescents” aged 18–21 years in the paediatric population. For simplicity, “children” and “paediatric” in this review refers to all paediatric age groups, including adolescents.
Although paediatric obesity is common, there are few pharmacokinetic or other studies on the best approach to use for drug dosage adjustment in this patient population [3]. Total body weight (TBW), the scalar most commonly used in the overall paediatric population, may be inappropriate for those who are obese. Their bodyweight (BW) may equate to or exceed that of adults, but aspects of their anatomy and physiology may differ [4,5,6,7]. The paucity of data results in inadequate or excessive drug levels in a huge percentage of obese children, with up to two-thirds being susceptible to either toxicity or treatment failure [3]. This article summarises current information on obesity-related dosage adjustment for commonly prescribed paediatric drugs, including those prescribed for obesity-related comorbidities, as reviewed by Kyler et al. [8].
Start with size, but be aware of pharmacokinetics
While paediatric dosage adjustments have traditionally been size-related, an all-round view that also considers patient physiology and drug physiochemical properties may be more clinically relevant [5]. Aside from TBW, proposed allometric or size-based measures include: normal fat mass scaled for volume, BW or body surface area (BSA); ideal BW (IBW); lean BW (LBW); and BMI or BSA alone [5].
Size-based measures are complicated by obese children’s abnormal vertical growth. In childhood and/or puberty, overweight children grow taller than normal-weight peers [9], but by adulthood they are shorter or the same height [8]. Nomographs, which show relationships between ≥ 3 variables [4], or obesity-specific dose curves [8] may eventually provide better dose-adjustment models.
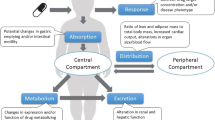
Size aside, obese children may display variations in pharmacokinetics and possibly even disease phenotypes relative to normal-weight children (Table 1) [10]. Drug absorption, distribution, metabolism and excretion all tend to differ between the two groups due to differing anatomy and physiology (Table 1) [7], but specifics are ill-defined, because of the limited physiological data for obese children (Table 1). Although more information is available for obese adults, such data can provide only a broad context regarding pharmacological changes, as physiology, hepatic function and other pharmacokinetic parameters differ between children and adults [8].
Physiochemical drug properties, such as lipophilicity, suggest another possible path to optimal drug dosages [8]. In theory, highly lipophilic drugs have a higher volume of distribution (Vd) in obesity, suggesting that higher dosages, especially initially, are needed. However, while this seems logical, inconsistent relationships between lipophilicity and Vd have been observed, with factors such as unpredictable sequestering in, and release from, adipose tissue potentially affecting the correlation [7, 17, 19]. The pharmacokinetic profiles of hydrophilic drugs in obese individulals also require clarification [19].
Adjust dosages cautiously then monitor carefully
Clear guidelines on dosage adjustments in obese children are lacking, but pharmacokinetic studies and analyses in this patient population provide pointers to dosage adjustment for some commonly used drugs (Table 2). Studies to date focus on the in-hospital acute or intensive care setting, as well as on drugs with a narrow therapeutic index and serious potential toxicity [21]. As no paediatric data are yet available for many commonly used drugs, some adult information is included in Table 2; such data should be considered with caution and not just simply extrapolated to children. As information is sparse or uncertain, clinicians need to carefully monitor obese children’s responses to treatment, and watch for signs of toxicity [8].
Use available data in common comorbidities
Obese children and adolescents are prone to the metabolic and cardiovascular disorders frequently seen in obese adults and, therefore, may require medications usually prescribed to adults. Information on appropriate dosage adjustment for these drugs is also scant [8]. Available information regarding some of the drugs commonly used to treat comorbid conditions in obese children suggests the following:
-
Metformin Consider increasing dosages in line with increased TBW and LBW to compensate for increased clearance and perhaps Vd [51, 52]. Of note, metformin acts as a substrate for OCT1, a hepatic uptake transporter that increases in obese patients, potentially leading to interactions between metformin and other OCT1 drug substrates (e.g. cimetidine, tramadol).
-
Calcium channel blockers May need to increase dosages if clinically required, possibly due to secondary increases in Vd [53, 54].
-
ACE inhibitors and ARBs Do not adjust dosage unless clinically required, as both classes of antihypertensives appeared equally effective at standard dosages in a study in three obese and three non-obese paediatric patients with renal disease [53].
-
β-blockers Do not adjust dosage to allow for an increase in Vd on the basis of lipophilia unless clinically appropriate, as these antihypertensives may preferentially target lean body tissue [8].
-
Statins Systemic exposure to statins is not simply a function of BMI, as two- to fivefold increases in exposure to pravastatin were observed due to genetic variations in some obese children’s hepatic uptake [55, 56].
-
Proton pump inhibitors (PPIs) The treatment of GORD is a particular concern, as GORD is six times more common in obese children than in non-obese children, and the use of PPIs is associated with infections, osteopenia and other adverse drug events in children [57]. Base the dosage of pantoprazole on LBW to avoid systemic overexposure, as total CYP2C19-mediated clearance of the PPI from plasma may be reduced [58,59,60].
Take home messages
-
Be aware that obese children often require pharmacological treatment for comorbid conditions, such as T2D and hypertension.
-
Realize that adjusting the dosage based on treatment effect may be the best option for many commonly prescribed drugs, as adjustment based on TBW, as for normal-weight children, is hampered by a lack of reliable data for most drugs.
-
Recognize the urgent need for pharmacokinetic studies in obese children to provide clearer dosage adjustment guidelines.
References
Commission on Ending Childhood Obesity (ECHO). Report of the commission on ending childhood obesity. Geneva: World Health Organzation; 2016.
American Academy of Pediatrics. Council on child and adolescent health: age limit of pediatrics. Pediatrics. 1988;81(5):736.
Harskamp-van Ginkel MW, Hill KD, Becker KC, et al. Drug dosing and pharmacokinetics in children with obesity: a systematic review. JAMA Pediatr. 2015;169(7):678–85.
Callaghan LC. Prescribing in paediatric obesity: methods to improve dosing safety in weight-based dose calculations. Arch Dis Child Educ Pract. 2018;103(5):274–7.
Anderson BJ, Holford NH. What is the best size predictor for dose in the obese child? Paediatr Anaesth. 2017;27(12):1176–84.
Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67.
Kendrick JG, Carr RR, Ensom MHH. Pediatric obesity: pharmacokinetics and implications for drug dosing. Clin Ther. 2015;37(9):1897–923.
Kyler KE, Wagner J, Hosey-Cojocari C, et al. Drug dose selection in pediatric obesity: available information for the most commonly prescribed drugs to children. Pediatric Drugs. 2019;21(5):357–69.
He Q, Karlberg J. BMI in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res. 2001;49(2):244–51.
McGarry ME, Castellanos E, Thakur N, et al. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest. 2015;147(6):1591–8.
Knibbe CAJ, Brill MJE, van Rongen A, et al. Drug disposition in obesity: toward evidence-based dosing. Annu Rev Pharmacol Toxicol. 2015;55:149–67.
Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71–877.
Benedek IH, Blouin RA, McNamara PJ. Serum protein binding and the role of increased alpha 1-acid glycoprotein in moderately obese male subjects. Br J Clin Pharmacol. 1984;18(6):941–6.
Brill MJE, Diepstraten J, van Rongen A, et al. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304.
Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–233.
Bauer LA, Black DJ, Lill JS. Vancomycin dosing in morbidly obese patients. Eur J Clin Pharmacol. 1998;54(8):621–5.
Rowe S, Siegel D, Benjamin DK. Gaps in drug dosing for obese children: a systematic review of commonly prescribed emergency care medications. Clin Ther. 2015;37(9):1923–32.
Small BG, Wendt B, Jamei M, et al. Prediction of liver volume: a population-based approach to meta-analysis of paediatric, adult and geriatric populations—an update. Biopharm Drug Dispos. 2017;38(4):290–300.
Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215–31.
Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–31.
Pediatric Trials Network (PTN). Available frome https://pediatrictrials.org/. Accessed 31 Mar 2020.
Zheng Y, Liu SP, Xu BP. Population pharmacokinetics and dosing optimization of azithromycin in children with community-acquired pneumonia. Antimicrob Agents Chemother. 2018;62:e00686–718.
Natale S, Bradley J, Nguyen WH, et al. Pediatric obesity: pharmacokinetic alterations and effects on antimicrobial dosing. Pharmacotherapy. 2017;37(3):361–78.
Koshida R, Nakashima E, Taniguchi N, et al. Prediction of the distribution volumes of cefazolin and tobramycin in obese children based on physiological pharmacokinetic concepts. Pharm Res. 1989;6(6):486–91.
Schmitz ML, Blumer JL, Cetnarowski W, et al. Determination of appropriate weight-based cutoffs for empiric cefazolin dosing using data from a phase 1 pharmacokinetics and safety study of cefazolin administered for surgical prophylaxis in pediatric patients aged 10 to 12 years. Antimicrob Agents Chemother. 2015;59(7):4173–80.
Smith MJ, Gonzalez D, Goldman JL, et al. Pharmacokinetics of clindamycin in obese and nonobese children. Antimicrob Agents Chemother. 2017;61(4):e02014–e2016.
Madigan T, Sieve RM, Graner KK, et al. The effect of age and weight on vancomycin serum trough concentrations in pediatric patients. Pharmacotherapy. 2013;33(12):1264–72.
Nassar L, Hadad S, Gefen A, et al. Prospective evaluation of the dosing regimen of vancomycin in children of different weight categories. Curr Drug Saf. 2012;7(5):375–81.
Le J, Capparelli EV, Wahid U. Bayesian estimation of vancomycin pharmacokinetics in obese children: matched case–control study. Clin Ther. 2015;37(6):1340–51.
Eiland LS, Sonawane KB. Vancomycin dosing in healthy-weight, overweight, and obese pediatric patients. J Pediatr Pharmacol Ther. 2014;19(3):182–8.
Moffett BS, Kim S, Edwards MS. Vancomycin dosing in obese pediatric patients. Clin Pediatr (Phila). 2011;50(5):442–6.
Heble DE, McPherson C, Nelson MP, et al. Vancomycin trough concentrations in overweight or obese pediatric patients. Pharmacotherapy. 2013;33(12):1273–7.
Barshop NJ, Capparelli EV, Sirlin CB, et al. Acetaminophen pharmacokinetics in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2011;52(2):198–202.
Goday Arno A, Farré M, Rodríguez-Morató J, et al. Pharmacokinetics in morbid obesity: influence of two bariatric surgery techniques on paracetamol and caffeine metabolism. Obes Surg. 2017;27(12):3194–201.
van Rongen A, Välitalo PAJ, Peeters MYM, et al. Morbidly obese patients exhibit increased CYP2E1-mediated oxidation of acetaminophen. Clin Pharmacokinet. 2016;55(7):833–47.
Abernethy DR, Divoll M, Greenblatt DJ, et al. Obesity, sex, and acetaminophen disposition. Clin Pharmacol Ther. 1982;31(6):783–90.
Vaughns JD, Ziesenitz VC, Williams EF, et al. Use of fentanyl in adolescents with clinically severe obesity undergoing bariatric surgery: a pilot study. Paediatr Drugs. 2017;19(3):251–7.
Samuels PJ, Sjoblom MD. Anesthetic considerations for pediatric obesity and adolescent bariatric surgery. Curr Opin Anaesthesiol. 2016;29(3):327–36.
Shibutani K, Inchiosa MA, Sawada K, et al. Pharmacokinetic mass of fentanyl for postoperative analgesia in lean and obese patients. Br J Anaesth. 2005;95(3):377–83.
Vaughns JD, Ziesenitz VC, van den Anker JN. Clinical pharmacology of frequently used intravenous drugs during bariatric surgery in adolescents. Curr Pharm Des. 2015;21(39):5650–9.
Diepstraten J, Chidambaran V, Sadhasivam S, et al. An integrated population pharmacokinetic meta-analysis of propofol in morbidly obese and nonobese adults, adolescents, and children. CPT Pharmacometrics Syst Pharmacol. 2013;2(8):543–51.
Olutoye OA, Yu X, Govindan K, et al. The effect of obesity on the ED95 of propofol for loss of consciousness in children and adolescents. Anesth Analg. 2012;115(1):147–53.
Meyhoff CS, Lund J, Jenstrup MT, et al. Should dosing of rocuronium in obese patients be based on ideal or corrected body weight? Anesth Analg. 2009;109(3):787–92.
Leykin Y, Pellis T, Lucca M, et al. The pharmacodynamic effects of rocuronium when dosed according to real body weight or ideal body weight in morbidly obese patients. Anesth Analg. 2004;99(4):1086–9.
van Rongen A, Brill MJE, Vaughns JD, et al. Higher midazolam clearance in obese adolescents compared with morbidly obese adults. Clin Pharmacokinet. 2018;57(5):601–11.
van Rongen A, Vaughns JD, Moorthy GS, et al. Population pharmacokinetics of midazolam and its metabolites in overweight and obese adolescents. Br J Clin Pharmacol. 2015;80(5):1185–96.
Goleva E, Covar R, Martin RJ, et al. Corticosteroid pharmacokinetic abnormalities in overweight and obese corticosteroid resistant asthmatics. J Allergy Clin Immunol Pract. 2016;4(2):357.e2–60.e2.
Pelaia G, Vatrella A, Busceti MT, et al. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediators Inflamm. 2015;2015:879873.
Anderson WJ, Lipworth BJ. Does body mass index influence responsiveness to inhaled corticosteroids in persistent asthma? Ann Allergy Asthma Immunol. 2012;108(4):237–42.
Farzan S, Khan S, Elera C, et al. Effectiveness of montelukast in overweight and obese atopic asthmatics. Ann Allergy Asthma Immunol. 2017;119(2):189–90.
van Rongen A, van der Aa MP, Matic M, et al. Increased metformin clearance in overweight and obese adolescents: a pharmacokinetic substudy of a randomized controlled trial. Paediatr Drugs. 2018;20(4):365–74.
Bardin C, Nobecourt E, Larger E, et al. Population pharmacokinetics of metformin in obese and non-obese patients with type 2 diabetes mellitus. Eur J Clin Pharmacol. 2012;68(6):961–8.
Hanafy S, Pinsk M, Jamali F. Effect of obesity on response to cardiovascular drugs in pediatric patients with renal disease. Pediatr Nephrol. 2009;24(4):815–21.
Sankaralingam S, Kim RB, Padwal RS. The impact of obesity on the pharmacology of medications used for cardiovascular risk factor control. Can J Cardiol. 2015;31(2):167–76.
Wagner JB, Abdel-Rahman S, Haandel L, et al. Impact of SLCO1B1 genotype on pediatric simvastatin acid pharmacokinetics. J Clin Pharmacol. 2018;58(6):823–33.
Wagner JB, Abdel-Rahman S, Gaedigk R, et al. Impact of genetic variation on pravastatin systemic exposure in pediatric hypercholesterolemia. Clin Pharmacol Ther. 2018;105(6):1501–12.
Stark CM, Nylund CM. Side effects and complications of proton pump inhibitors: a pediatric perspective. J Pediatr. 2016;168:16–22.
Shakhnovich V, Abdel-Rahman S, Friesen CA, et al. Lean body weight dosing avoids excessive systemic exposure to proton pump inhibitors for children with obesity. Pediatr Obes. 2019;14(1):e12459.
Shakhnovich V, Smith PB, Guptill JT, et al. Obese children require lower doses of pantoprazole than nonobese peers to achieve equal systemic drug exposures. J Pediatr. 2018;193:102–28.
Shakhnovich V, Smith PB, Guptill JT, et al. A population-based pharmacokinetic model approach to pantoprazole dosing for obese children and adolescents. Paediatr Drugs. 2018;20(5):4853–95.
Author information
Authors and Affiliations
Contributions
The article was written by employees of Adis International Ltd./Springer Nature and was adapted, in part, from Pediatric Drugs 2019;21(5):357–69 [8].
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Funding
The preparation of this review was not supported by any external funding.
Rights and permissions
About this article
Cite this article
Writers, A.M. Dosage adjustment in obese children, even for common drugs, is largely unclear and a treat-to-effect approach may work best. Drugs Ther Perspect 36, 341–346 (2020). https://doi.org/10.1007/s40267-020-00734-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-020-00734-w