Abstract
Hexaminolevulinate [Cysview® (USA); Hexvix® (EU)], an ester derivative of 5-aminolevulinate, is a valuable option for the diagnosis of bladder cancer when used in conjunction with blue-light (BL) cystoscopy. In clinical trials, the addition of hexaminolevulinate-guided BL cystoscopy to white-light (WL) cystoscopy was generally better at detecting non-muscle-invasive bladder cancer lesions than WL cystoscopy alone, as assessed by a number of endpoints (e.g. increased number and rate of lesion detection), leading to increased rates of complete treatment decisions. Add-on hexaminolevulinate-guided BL cystoscopy reduced tumour recurrence rates relative to WL cystoscopy alone in follow-up studies and, although further studies are needed, may potentially improve survival-related outcomes. Hexaminolevulinate is generally very well tolerated when used to guide BL cystoscopy, with the most common adverse events (i.e. haematuria, dysuria, pain and bladder spasm) being expected as a consequence of the resection procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adis evaluation of hexaminolevulinate–guided blue light cystoscopy as an add-on to white-light cystoscopy in the diagnosis of bladder cancer
Has high sensitivity and specificity rates |
↑ detection of NMIBC lesions relative to WL cystoscopy alone, leading to more complete treatment decisions |
↓ rates of tumour recurrence and may potentially improve some survival-related outcomes |
Very well tolerated with the same most common adverse events as WL cystoscopy alone |
Estimated to be cost saving relative to WL cystoscopy alone, as its addition ↑ quality-adjusted life-years and, due to better lesion detection, ↓ future treatment costs |
BL blue light, NMIBC non-muscle-invasive bladder cancer WL white light, ↑ increases, ↓ decreases |
Why add blue-light cystoscopy to white-light cystoscopy when diagnosing bladder cancer?
Bladder cancer often requires ongoing monitoring, surveillance and/or treatment to reduce recurrence and prevent progression, as non-muscle-invasive bladder cancer (NMIBC) lesions have a tendency to recur [1,2,3,4,5]. Correct diagnosis and removal of all visible lesions using cystoscopy and transurethral resection of bladder tumour (TURBT) are crucial in the diagnosis and treatment of bladder cancer. Management of each patient will vary, depending on the biopsy findings (i.e. histology, grade, depth of invasion) and the estimated likelihood of recurrence and progression. NMIBC lesions include [1, 2]:
-
Flat carcinoma in situ (Cis or Tis) High malignancy potential; generally progressive; require timely and intensive treatment. Lesions may be missed or misinterpreted as an inflammatory lesion during cystoscopy if not biopsied.
-
Noninvasive papillary carcinomas largely confined to the mucosa (Ta) Low-grade lesions have a high risk of recurrence but a low risk of progression; require risk-specific treatment and continued surveillance. High-grade lesions are generally progressive and require intensive treatment in a timely manner.
-
Noninvasive papillary carcinomas largely confined to the submucosa or lamina propria (T1) High malignancy potential; generally progressive; require timely and intensive treatment.
Incomplete identification of lesions leads to incomplete initial TURBT, persistent tumours and early recurrence of NMIBC [1,2,3,4,5]. White light (WL) cystoscopy detects large, protruding lesions (e.g. T1 lesions), but may not detect flat (e.g. Cis) or smaller lesions. When added to WL cystoscopy, hexaminolevulinate-guided blue-light (BL) cystoscopy increases the detection of NMIBC lesions (particularly smaller lesions and Cis) relative to WL cystoscopy alone, and is included in EU [1] and US [2] guidelines for the diagnosis and treatment of NMIBC.
This article provides a narrative review of the use of hexaminolevulinate-guided BL cystoscopy in the diagnosis of bladder cancer.
Why use hexaminolevulinate to guide BL cystoscopy?
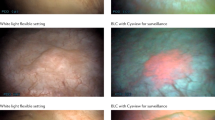
BL cystoscopy uses the photoactive nature of certain compounds, such as 5-aminolevulinic acid (5-ALA) and its derivatives, to enhance the visual differentiation between normal and neoplastic tissue [4,5,6,7]. These compounds accumulate preferentially in neoplastic tissue and are metabolized to form photoactive porphyrins that fluoresce red when illuminated with BL (wavelength 375–440 nm), thereby providing better visualization of tissue that is suspicious for tumours than the use of WL alone [4,5,6,7].
The first agent used in the BL detection of NMIBC lesions was 5-ALA, an endogenous and non-fluorescent compound involved in the biosynthesis pathway of haeme (Fig. 1a) [8]. Exogenous administration of 5-ALA overwhelms the haeme pathway, producing an excess protoporphyrin IX, a fluorescent haeme precursor that preferentially accumulates in tumour cells and is detectable by BL cystoscopy (Fig. 1b) [8]. However, the use of 5-ALA, which is not approved for photodynamic detection, is limited by some of its pharmacological properties [3,4,5].
Overview of (a) the haeme biosynthesis pathway under physiological circumstances, and (b) its disruption by the addition of exogenous hexaminolevulinate or 5-aminolevulinate for the purpose of photodynamic detection with fluorescence cystoscopy [8]
Hexaminolevulinate [Hexvix® (EU) [9]; Cysview® (USA) [10]] was developed to have a better pharmacological profile than 5-ALA [4]. It is an ester derivative of 5-ALA, and consists of a lipophilic hexyl moiety bound to 5-ALA [11]. Relative to 5-ALA, hexaminolevulinate has several advantages in BL diagnosis of NMIBC lesions, including increased protoporphyrin IX fluorescence at lower concentrations (e.g. a 2-fold increase in fluorescence at a 45-fold lower concentration [12]), a shorter instillation time (1 vs 4 h) [13, 14], faster and greater penetration of the urothelium tissue in vitro [12], and higher specificity for malignant urothelial carcinoma [14].
What is the approved indication for hexaminolevulinate?
Hexaminolevulinate-guided BL cystoscopy is indicated in the diagnosis of bladder cancer as an adjunct to WL cystoscopy in patients with a known or high suspicion of bladder cancer [9, 10]. It is not a replacement for random bladder biopsies or other procedures used in the detection of bladder cancer [10], and must be performed by healthcare professionals who are trained and proficient in its use. Table 1 provides a summary of the use of hexaminolevulinate in the diagnosis of bladder cancer in the EU [9] and USA [10].
Based on the evidence available at the time of regulatory assessment, hexaminolevulinate-guided BL cystoscopy does not appear to represent a risk when used as follow-up in patients with bladder cancer (European Medicines Agency) [9] or is not indicated for repetitive use (US FDA) [10]. Studies in the clinical-practice setting in 62–80 patients with multiple (2–12) instillations of hexaminolevulinate found no significant differences between the first and subsequent use of hexaminolevulinate-guided BL cystoscopy with regard to its tolerability and safety profiles, with no induction of allergization against the substance being shown [15, 16]. The results of a clinical study evaluating the repeated use of hexaminolevulinate-guided BL flexible cystoscopy in the surveillance office setting [17] are awaited with interest.
What was the diagnostic effectiveness of hexaminolevulinate-guided BL cystoscopy?
The use of hexaminolevulinate-guided BL cystoscopy as an add-on to WL cystoscopy in the diagnosis of bladder cancer has been evaluated in many clinical studies; the discussion in this section focuses on data from clinical trials [18,19,20,21,22,23,24,25,26,27,28,29,30], pooled analyses of data from trials of similar design [31], well-conducted meta-analyses of clinical trial data [32,33,34,35,36], studies in the clinical-practice setting [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] and studies of BL flexible cystoscopy [17, 53,54,55,56,57,58]. Three phase 3 trials (B301, B302 and B303; n = 146–211) [18,19,20,21] and a clinical practice study from the Blue Light Urologic Endoscopy group (the BLUE study; n = 305) [37] primarily assessed tumour detection, whereas other randomized trials and their follow-up periods primarily assessed tumour recurrence (n = 86–402) [22,23,24,25,26,27,28,29,30].
Sensitivity and specificity
At the lesion level, hexaminolevulinate-guided BL cystoscopy as an add-on to WL cystoscopy was associated with numerically higher sensitivity rates in the detection of NMIBC lesions than WL cystoscopy alone (92 vs 68% sensitivity in the phase 3 B302 trial [19, 20] and 93.8 vs 78.2% sensitivity in the BLUE study [37]).
At the patient level in B302 [19], no statistically significant between-group differences (BGDs) in sensitivity (87 vs 83%) or specificity (82 vs 72%) were reported. Across several clinical trials [18,19,20,21, 23, 27, 37], the rate of false-positives was 11–39% with add-on hexaminolevulinate-guided BL cystoscopy and 10–31% with WL cystoscopy alone. However, in another trial in which all patients received TURBT with WL cystoscopy (including those that subsequently received hexaminolevulinate-guided BL cystoscopy) [25], the corresponding rates were 32 and 55%. In the BLUE study [37], the rate of false-negatives was 2.2% with add-on hexaminolevulinate-guided BL cystoscopy and 25.8% with WL cystoscopy alone. The addition of hexaminolevulinate-guided BL cystoscopy also improved sensitivity and/or specificity rates relative to WL cystoscopy alone in clinical-practice studies in 53–90 patients with NMIBC [38,39,40,41,42,43,44].
Tumour detection
Hexaminolevulinate-guided BL cystoscopy was a better detection method for NMIBC than WL cystoscopy alone [18,19,20,21,22,23, 27, 37], in terms of numerically higher detection rates and additional detected lesions (at both the lesion and patient level) in the studies in which tumour detection was the primary outcome [18,19,20,21, 37], as well as in tumour recurrence trials that also assessed tumour detection [22, 23, 27].
The findings in individual trials are supported by tumour detection rates in a pooled analysis [31] of the three B301-3 trials [18,19,20,21], as well as meta-analyses of 9 [32] or 16 [33] trials.
-
All lesion types Add-on hexaminolevulinate-guided BL cystoscopy was associated with a higher overall detection rate than WL cystoscopy alone, with a significant 19.0% (95% CI 15.2–23.6) BGD in the proportion of lesions detected. One or more additional tumours were detected in 15% (95% CI 9.8–21.1) of patients [33].
-
Cis lesions Detection rate was higher with add-on hexaminolevulinate-guided BL cystoscopy than with WL cystoscopy alone in all analyses. One or more Cis lesions were detected in 87 versus 75% of 174 patients (p = 0.006) [31]; odds ratio (OR) 12.37 (95% CI 6.34–24.13; p < 0.001) [32]. Of all patients with Cis lesions, 26.7% had one or more additional Cis lesions detected only by hexaminolevulinate-guided BL cystoscopy [32]; significant BGD in the proportion of Cis lesions detected (15.7%; 95% CI 6.9–24.5) [33].
-
Ta lesions Detection rate was higher with add-on hexaminolevulinate-guided BL cystoscopy than with WL cystoscopy alone (OR 4.898; 95% CI 1.937–12.390; p < 0.001) [32]. Of all patients with Ta or T1 lesions, 24.9% (p < 0.001) had at least one additional Ta or T1 lesion detected only by hexaminolevulinate-guided BL cystoscopy [32]; significant BGD in proportion of Ta lesions detected (5.9%; 95% CI 1.4–10.3) [33].
-
T1 lesions Based on limited data, differences between add-on hexaminolevulinate-guided BL cystoscopy and WL cystoscopy alone were not significant in either meta-analysis (OR 2.25; 95% CI 0.999–5.081 [32]; BGD in proportion −1.2%; 95% CI −3.3 to −5.7) [33].
The addition of hexaminolevulinate-guided BL cystoscopy to WL cystoscopy also improved the detection of bladder cancer lesions, especially Cis lesions, relative to WL cystoscopy alone in studies of patients with NMIBC in the clinical-practice setting (n = 48–403 patients) [38,39,40,41,42,43,44,45,46,47]. For example, in a clinical-practice study in 403 patients [44], add-on hexaminolevulinate-guided BL cystoscopy detected 6.8% more cancer lesions and 25% more Cis lesions (p < 0.0001) than WL cystoscopy alone, as well as detecting ≥1 additional positive lesions in 10% of patients and providing a diagnosis of NMIBC in 2.2% of patients that would otherwise be missed. In this analysis, hexaminolevulinate-guided BL cystoscopy would need to be added to WL cystoscopy in 16 patients to detect one additional patient with a cancerous lesion [44].
Tumour detection with BL flexible cystoscopy
The use of hexaminolevulinate-guided BL flexible cystoscopy in the outpatient or office-based setting is increasing, as improved tumour detection rates may potentially lead to less extensive and more cost effective management of patients with bladder cancer [17]. In a phase 3 study conducted in the clinical office setting in 17 US academic institutions, 234 patients under surveillance because of their high risk of tumour recurrence were randomized to WL flexible cystoscopy alone or in combination with hexaminolevulinate-guided BL flexible cystoscopy [17]. According to preliminary results, almost half (44%) of patients were referred to the operating room within 6 weeks because of suspected tumour recurrence, where WL and hexaminolevulinate-guided BL cystoscopy were repeated [17].
In smaller (n = 20–73) studies, hexaminolevulinate-guided BL flexible cystoscopy was reliable in detecting bladder tumours, with detection rates that were equivalent [53] or almost equivalent [54, 55] to those with hexaminolevulinate-guided BL rigid cystoscopy [54, 55], and/or comparable to standard rigid WL cystoscopy [54] and better than those with standard flexible WL cystoscopy [55,56,57,58].
Complete treatment plans
As a result of better lesion detection, more complete NMIBC treatment plans could be made following tumour detection with add-on hexaminolevulinate-guided BL cystoscopy than after WL cystoscopy alone, as reported in the following trials:
-
B302 trial [19] 14% of patients had their proposed WL cystoscopy-based treatment plan changed after hexaminolevulinate-guided BL cystoscopy, and 81.3% had their proposed treatment plan confirmed. Hexaminolevulinate-guided BL cystoscopy was useful as an add-on and for selecting further management options in 66 and 51% of cases, respectively.
-
B303 trial [21] 17% of patients received a more complete treatment plan based on add-on hexaminolevulinate-guided BL cystoscopy than based on WL cystoscopy alone. Hexaminolevulinate-guided BL cystoscopy was useful as an add-on and for making further treatment decisions in 78 and 42% of cases, respectively.
-
Tumour recurrence trial [27] Add-on hexaminolevulinate-guided BL cystoscopy had significantly (p ≤ 0.001) higher rates of changes in tumour grading and treatment decisions than WL cystoscopy alone (e.g. a decision of no treatment was changed to treatment with bacillus Calmette-Guérin in 7.7 vs 1.4% of cases, no treatment changed to mitomycin-C in 4.2 vs 0.7%, and overall changes in risk grading with a change in treatment plan in 19.0 vs 6.3%).
Tumour recurrence, disease progression and survival
Regardless of the length of follow-up, the overall tumour recurrence rate (primary endpoint) was significantly lower in patients with NMIBC who had received add-on hexaminolevulinate-guided BL cystoscopy than in those who received WL cystoscopy alone in most follow-up studies [22,23,24, 26,27,28], with the exception of some relatively small studies [25, 29, 30] (Table 2).
The addition of hexaminolevulinate-guided BL cystoscopy to WL cystoscopy also showed significant benefits over WL cystoscopy alone with regard to a number of other NMIBC tumour recurrence-related endpoints in several trials (Table 2) [22,23,24,25,26,27,28,29,30]. Moreover, although the trials were not adequately powered for subgroup analysis, findings in various subgroups generally suggest a lower recurrence rate with add-on hexaminolevulinate-guided BL cystoscopy than with WL cystoscopy alone (Table 2) [22,23,24, 26, 28].
Meta-analyses also indicate that the risk of NMIBC tumour recurrence with add-on hexaminolevulinate-guided BL cystoscopy is lower than that with WL cystoscopy [34,35,36]. In a network analysis of 15 trials, the risk of NMIBC tumour recurrence with hexaminolevulinate-guided BL cystoscopy was lower than with WL cystoscopy (OR 0.58; 95% CI 0.45–0.74; p < 0.0001) and did not differ significantly from narrow-band imaging (OR 1.11; 95% CI 0.55–2.101) [34]. Likewise, in the subgroup of 9 hexaminolevulinate trials in a meta-analysis of 14 5-ALA/hexaminolevulinate trials, relative to WL cystoscopy alone, the addition of hexaminolevulinate-guided BL cystoscopy significantly reduced the relative risk (RR) of bladder cancer at recurrence at 3–12 months (RR 0.76; 95% CI 0.62–0.93) and >1 year (RR 0.75; 95% CI 0.62–0.92), and the risk of disease progression (RR 0.51; 95% CI 0.28–0.96), but did not reduce the risk of mortality or recurrence at <3 months to a significant extent [36].
A meta-analysis of five studies found that the rate of progression of bladder cancer was significantly lower with hexaminolevulinate-guided BL cystoscopy than with WL cystoscopy (6.8 vs 10.7% of patients; median OR 1.64; 95% CI 1.10–2.4; p = 0.01) [35]. Of note, disease progression results depend on the definition of disease progression in NMIBC. When data from the retrospective study with a median follow-up time of 4.5 years [24] (Table 2) were re-analyzed using a new proposed definition, additional patients in both the hexaminolevulinate-guided BL cystoscopy and WL cystoscopy alone groups progressed (12.2 vs 17.6%) and the time to progression was significantly (p = 0.05) longer with hexaminolevulinate-guided BL cystoscopy [59].
In the clinical-practice setting (n = 190-808), the addition of hexaminolevulinate-guided BL cystoscopy was also effective in reducing tumour recurrence [48,49,50] and improving survival-related outcomes (i.e. overall [49, 51], cancer-specific [51], recurrence-free [49, 51] and/or progression-free [52] survival). For example, in the study in 808 patients with bladder cancer who underwent complete TURBT, rates of early recurrence (i.e. at the first follow-up cystoscopy) were significantly lower with hexaminolevulinate-guided BL cystoscopy than with WL cystoscopy (13.6 vs 30.9% of patients; OR 2.9; 95% CI 1.6–5.0; p < 0.001) [48], as were rates of 3-year recurrence (42.2 vs 48.8%) and overall and risk-group adjusted recurrence-free survival (p ≤ 0.01) [49].
In a retrospective single-centre study in 224 patients who underwent subsequent radical cystectomy for bladder cancer after fluorescence-guided TURBT [51], Kaplan-Meier estimates of recurrence-free survival (77.8 vs 52.4% of patients), cancer-specific survival (83.9 vs 59.7%) and overall survival (74.0 vs 56.5%) at 3 years were significantly (p < 0.05) higher with hexaminolevulinate-guided BL cystoscopy than with WL cystoscopy. Moreover, hexaminolevulinate-guided BL cystoscopy was an independent predictor of improved survival after radical cystectomy [51]. In contrast, in a multicentre study in 549 patients with bladder cancer, the use of hexaminolevulinate-guided BL cystoscopy during primary or final TURBT did not independently affect 3-year rates of cancer-specific (32%) or overall mortality (40%) after radical cystectomy to a statistically significant extent [60].
What is the tolerability profile of hexaminolevulinate?
Hexaminolevulinate is associated with minimal systemic absorption following bladder instillation for ≈1 [61] or 2 h [62], and is very well tolerated. The absolute bioavailability of radiolabelled hexaminolevulinate was 7% in healthy volunteers [61], and the systemic absorption of hexaminolevulinate 4, 8, or 16 mmol/L was ≈5% in patients with bladder cancer [62]; no skin sensitivity or adverse reactions could be attributed to its use [62].
The addition of hexaminolevulinate-guided BL cystoscopy to WL cystoscopy in the diagnosis of bladder cancer was very well tolerated in clinical trials and the post-marketing setting. The tolerability profile and frequency of adverse events reported in the post-marketing period (>400,000 hexaminolevulinate-guided procedures to date) is comparable to that in pivotal trials despite the broader inclusion criteria for its use in the real-world setting.
A pooled safety analysis of hexaminolevulinate-guided BL cystoscopy [63] included data from six registration trials [18,19,20,21, 23, 25, 64], in a total of 1324 and 499 hexaminolevulinate-guided BL cystoscopy and WL cystoscopy recipients, respectively (safety set), and 9 years of post-marketing data in >200,000 patients. In the pooled registration trials, 44–58% of patients receiving hexaminolevulinate-guided BL cystoscopy reported at least one treatment-emergent adverse event (TEAE), with almost all (97%) of TEAEs being mild to moderate in severity and not related to hexaminolevulinate. Overall, it appears that the addition of hexaminolevulinate-guided BL cystoscopy for bladder tumour resection adds very little risk to that seen with WL cystoscopy alone (41% of recipients of WL cystoscopy alone reported an adverse event) [63]. In pooled trials that directly compared add-on hexaminolevulinate-guided BL cystoscopy and WL cystoscopy alone (n = 533 and 499), the most common TEAEs were renal and urinary disorders (25.7 and 23.4% of patients), gastrointestinal disorders (6.8 and 6.8%), urinary tract infection (5.3 and 5.0%), and procedural pain (5.3 and 4.0%) [63]. According to the EU summary of product characteristics, the most common TEAEs across clinical studies in hexaminolevulinate-guided BL cystoscopy recipients involved urinary tract-related disorders, including bladder spasm (2.4% of patients), dysuria (1.8%), bladder pain (1.7%) and haematuria (1.7%) [9]. Of the 183 unique serious TEAEs reported in the pooled analysis, none were considered to be probably related to hexaminolevulinate-guided BL cystoscopy and eight events in six patients had an uncertain relationship [63]. Less than 1% of patients discontinued hexaminolevulinate-guided BL cystoscopy because of a TEAE [63].
During ≈9 years of post-marketing in European countries and the USA, hypersensitivity reactions possibly related to hexaminolevulinate-guided BL cystoscopy were reported in four patients (two cases each of anaphylactoid reactions and possible hypersensitivity) [63]. Repeated use of hexaminolevulinate-guided BL cystoscopy has not been associated with anaphylaxis-related TEAEs [15, 16, 63]. Nevertheless, as there is a potential risk of serious anaphylactic/anaphylactoid reactions to hexaminolevulinate instillation, trained personnel and advanced life support facilities should be readily available (Table 1).
None of the rare and generally mild changes in laboratory or physical examination findings or vital signs in the pooled analysis were considered to be related to hexaminolevulinate-guided BL cystoscopy [63].
Is hexaminolevulinate-guided BL cystoscopy cost effective?
The addition of hexaminolevulinate-guided BL cystoscopy to WL cystoscopy appears to be cost saving. In modelled analyses of the cost utility of hexaminolevulinate-guided BL cystoscopy versus WL cystoscopy in the detection of bladder cancer from a national healthcare payer perspective, hexaminolevulinate-guided BL cystoscopy was estimated to be cost-saving relative to WL cystoscopy in the USA [65], Italy [66] and England/Wales [67], as hexaminolevulinate-guided BL cystoscopy reduced recurrence rates and the requirement for repeated treatment procedures, and improved utility [e.g.quality-adjusted life years (QALYs) gained] and effectiveness scores, regardless of the time horizon used (lifetime [66, 67] or 5 years [65]).
In a modelled cost-consequence analysis in Canada, the addition of hexaminolevulinate-guided BL cystoscopy to WL cystoscopy increased healthcare payer costs over the 5-year period, but decreases in disease recurrence and/or progression over the longer-term may reduce the need for hospital care in the future, potentially leading to cost effectiveness and even cost savings [68].
Moreover, in a prospective study from a UK healthcare payer perspective, office-based WL flexible cystoscopy and/or hexaminolevulinate-guided BL flexible cystoscopy was cost saving relative to inpatient WL cystoscopy, as office-based treatment was less costly and provided more QALYs [58].
What conclusions can be made regarding hexaminolevulinate–guided tumour diagnosis?
Hexaminolevulinate-guided BL cystoscopy is a valuable addition to WL cystoscopy in the diagnosis of NMIBC in patients who are suitable candidates for its use. According to current EU guidelines on the diagnosis of NMIBC [1], fluorescence-guided (e.g. hexaminolevulinate-guided) biopsies should be performed when the equipment is available, as WL cystoscopy alone may not identify all lesions and hexaminolevulinate-guided BL cystoscopy is more sensitive than conventional procedures for the detection of malignant tumours (particularly Cis). The US guidelines [2] state that, when available, BL cystoscopy should be offered to the patient at the time of TURBT to increase detection and decrease recurrence of tumours, and should be considered in patients with a history of NMIBC with normal cystoscopy and positive cytology. The use of add-on hexaminolevulinate-guided BL cystoscopy in the detection of NMIBC is based on the following clinical evidence.
-
Selectivity and sensitivity Associated with high sensitivity and specificity rates.
-
Tumour detection Detects more lesions, especially with regard to high-risk Cis lesions, than WL cystoscopy, leading to an increased proportion of patients with a complete treatment plan. Office-based BL flexible cystoscopy provides reliable tumour detection and is increasing in popularity.
-
Impact on long-term outcomes Reduces tumour recurrence relative to WL cystoscopy and, based on limited data, may improve some survival-related outcomes (further studies are needed).
-
Tolerability Very well tolerated, with only a few reports of hypersensitivity reactions including anaphylactic/anaphylactoid reactions.
-
Pharmacoeconomic considerations BL rigid and flexible cystoscopy is predicted to be cost saving relative to WL cystoscopy due to improvements in clinical outcomes and utility and decreases longer-term costs.
References
Babjuk M, Böhle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71(3):447–61.
Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196(4):12021–9.
Daneshmand S, Schuckman AK, Bochner BH, et al. Hexaminolevulinate blue-light cystoscopy in non-muscle-invasive bladder cancer: review of the clinical evidence and consensus statement on appropriate use in the USA. Nat Rev Urol. 2014;11(10):589–96.
Pietzak EJ. The impact of blue light cystoscopy on the diagnosis and treatment of bladder cancer. Curr Urol Rep. 2017;18(5):39.
Oude Elferink P, Witjes JA. Blue-light cystoscopy in the evaluation of non-muscle-invasive bladder cancer. Ther Adv Urol. 2014;6(1):25–33.
Liu JJ, Droller MJ, Liao JC. New optical imaging technologies for bladder cancer: considerations and perspectives. J Urol. 2012;188(2):361–8.
Lerner SP, Goh A. Novel endoscopic diagnosis for bladder cancer. Cancer. 2015;121(2):169–78.
Wachowska M, Muchowicz A, Firczuk M, et al. Aminolevulinic acid (ALA) as a prodrug in photodynamic therapy of cancer. Molecules. 2011;16(5):4140–64.
Hexvix 85 mg: UK summary of product characteristics. Slough: Ipsen Ltd.; 2016.
Cysview® (hexaminolevulinate HCl): US prescribing information. Princeton: Photocure ASA; 2011. Accessed.
van den Berg NS, van Leeuwen FW, van der Poel HG. Fluorescence guidance in urologic surgery. Curr Opin Urol. 2012;22(2):109–20.
Marti A, Lange N, van den Bergh H, et al. Optimisation of the formation and distribution of protoporphyrin IX in the urothelium: an in vitro approach. J Urol. 1999;162(2):546–52.
Lange N, Jichlinski P, Zellweger M, et al. Photodetection of early human bladder cancer based on the fluorescence of 5-aminolaevulinic acid hexylester-induced protoporphyrin IX: a pilot study. Br J Cancer. 1999;80(1–2):185–93.
Marti A, Jichlinski P, Lange N, et al. Comparison of aminolevulinic acid and hexylester aminolevulinate induced protoporphyrin IX distribution in human bladder cancer. J Urol. 2003;170(2 Pt 1):428–32.
Lane GI, Downs TM, Soubra A, et al. Tolerability of repeat use of blue light cystoscopy with hexaminolevulinate for patients with urothelial cell carcinoma. J Urol. 2017;197(3 Pt 1):596–601.
Apfelback M, Grimm T, Kretschmer, et al. Follow-up of high-risk bladder cancer: is it safe to perform bluorescence endoscopy multiple times in the same patient? Urol Oncol. In Press. Doi: http://dx.doi.org/10.1016/j.urolonc.2017.06.002.
Daneshmand S, Patel S, Lotan Y, et al. Blue light flexible cystoscopy (BLFC) with hexaminolevulinate (HAL) and white light flexible cystoscopy: a prospective, comparative, within-patient controlled multicenter phase 3 study in the detection of bladder cancer during surveillance [abstract no. PPTLBA-02 ]. J Urol. 2017;197(4 Suppl):e608.
Schmidbauer J, Witjes F, Schmeller N, et al. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol. 2004;171(1):135–8.
Fradet Y, Grossman HB, Gomella L, et al. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter study. J Urol. 2007;178(1):68–73.
Grossman HB, Gomella L, Fradet Y, et al. A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J Urol. 2007;178(1):62–7.
Jocham D, Witjes F, Wagner S, et al. Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: a prospective, phase III multicenter study. J Urol. 2005;174(3):862–6.
Geavlete B, Jecu M, Multescu R, et al. HAL blue-light cystoscopy in high-risk nonmuscle-invasive bladder cancer: re-TURBT recurrence rates in a prospective, randomized study. Urology. 2010;76(3):664–9.
Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol. 2010;184(5):1907–13.
Grossman HB, Stenzl A, Fradet Y, et al. Long-term decrease in bladder cancer recurrence with hexaminolevulinate enabled fluorescence cystoscopy. J Urol. 2012;188(1):58–62.
Hermann GG, Mogensen K, Carlsson S, et al. Fluorescence-guided transurethral resection of bladder tumours reduces bladder tumour recurrence due to less residual tumour tissue in Ta/T1 patients: a randomized two-centre study. BJU Int. 2011;108(8 Pt 2):E297–303.
Karaolides T, Skolarikos A, Bourdoumis A, et al. Hexaminolevulinate-induced fluorescence versus white light during transurethral resection of noninvasive bladder tumor: does it reduce recurrences? Urology. 2012;80(2):354–9.
Geavlete B, Multescu R, Georgescu D, et al. Treatment changes and long-term recurrence rates after hexaminolevulinate (HAL) fluorescence cystoscopy: does it really make a difference in patients with non-muscle-invasive bladder cancer (NMIBC)? BJU Int. 2012;109(4):549–56.
Geavlete B, Multescu R, Georgescu D, et al. Four-year non-muscle invasive bladder cancer recurrence rates-a prospective, randomized comparison between hexaminolevulinate blue light and standard white light cystoscopy [abstract no. 1279]. J Urol. 2013;189(4 Suppl):e523.
Gan C, Chatterton K, Amery S, et al. Long term follow up of a prospective randomised trial of hexylaminolevulinate (Hexvix) photodynamic diagnosis (PDD) assisted versus conventional white-light transurethral resection (TURBT) in newly presenting non-muscle invasive bladder cancer (NMIBC) [abstract no. MP22-08]. J Urol. 2014;191(4 Suppl):e237.
Gkritsios P, Hatzimouratidis K, Kazantzidis S, et al. Hexaminolevulinate-guided transurethral resection of non-muscle-invasive bladder cancer does not reduce the recurrence rates after a 2-year follow-up: a prospective randomized trial. Int Urol Nephrol. 2014;46(5):927–33.
Lerner SP, Liu H, Wu M-F, et al. Fluorescence and white light cystoscopy for detection of carcinoma in situ of the urinary bladder. Urol Oncol. 2012;30(3):285–9.
Burger M, Grossman HB, Droller M, et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: a meta-analysis of detection and recurrence based on raw data. Eur Urol. 2013;64(5):846–54.
Di Stasi SM, De Carlo F, Pagliarulo V, et al. Hexaminolevulinate hydrochloride in the detection of nonmuscle invasive cancer of the bladder. Ther Adv Urol. 2015;7(6):339–50.
Lee JY, Cho KS, Kang DH, et al. A network meta-analysis of therapeutic outcomes after new image technology-assisted transurethral resection for non-muscle invasive bladder cancer: 5-aminolaevulinic acid fluorescence vs hexylaminolevulinate fluorescence vs narrow band imaging. BMC Cancer. 2015;15:506. doi:10.1186/s12885-015-1571-8.
Gakis G, Fahmy O. Systematic review and meta-analysis on the impact of hexaminolevulinate- versus white-light guided transurethral bladder tumor resection on progression in non-muscle invasive bladder cancer. Bladder Cancer. 2016;2(3):293–300.
Chou R, Selph S, Buckley DI, et al. Comparative effectiveness of fluorescent versus white light cystoscopy for initial diagnosis or surveillance of bladder cancer on clinical outcomes: systematic review and meta-analysis. J Urol. 2017;197(3):548–58.
Burgués JP, Conde G, Oliva J, et al. Hexaminolevulinate photodynamic diagnosis in non-muscle invasive bladder cancer: experience of the BLUE group. Actas Urol Esp. 2011;35(8):439–45.
Lapini A, Minervini A, Masala A, et al. A comparison of hexaminolaevulinate (Hexvix®) fluorescence cystoscopy and white light cystoscopy for the detection of bladder cancer: results of the HeRo observational study. Surg Endosc. 2012;26(12):3634–41.
Sfetsas K, Mitropoulos D. Reducing understaging of bladder cancer with the aid of photodynamic cystoscopy. J Egypt Natl Canc Inst. 2016;28(2):89–94.
Pagliarulo V, Alba S, Gallone MF, et al. Diagnostic accuracy of hexaminolevulinate in a cohort of patients undergoing radical cystectomy. J Endourol. 2017;31(4):405–11.
Napodano G, Campitelli A, Realfonso T, et al. Photodynamic diagnosis of nonmuscle invasive bladder cancer: preliminary experience [abstract no. 181]. Anticancer Res. 2015;35(6):3736–7.
Porpiglia F, Poggio M, Cossu M, et al. Fluorescence cystoscopy with hexaminolevulinate in diagnosis and follow up of bladder cancer: five-years experience [abstract no. 116]. Anticancer Res. 2013;33(5):2327.
Palou J, Hernandez C, Solsona E, et al. Effectiveness of hexaminolevulinate fluorescence cystoscopy for the diagnosis of non-muscle-invasive bladder cancer in daily clinical practice: a Spanish multicentre observational study. BJU Int. 2015;116(1):37–43.
Bach T, Bastian PJ, Blana A, et al. Optimised photodynamic diagnosis for transurethral resection of the bladder (TURB) in German clinical practice: results of the noninterventional study OPTIC III. World J Urol. 2017;35(5):737–44.
Bazargani ST, Clifford TG, Djaladat H, et al. Blue light cystoscopy for diagnosis of urothelial bladder cancer: results from a prospective multicenter registry [abstract no. MP23-20]. J Urol. 2016;195(4 Suppl. 1):e269–e70.
Osaghae SO, Turner DTL. Photodynamic diagnosis of bladder cancer: Initial experience of a single UK centre. Afr J Urol. 2014;20(3):123–9.
Knoll T, Holten M, Weiss C, et al. Routine half-fluoroscence cystoscopy improves detection rate of carcinoma in situ in transurethral bladder tumor resection [abstract no. MP23-17]. J Endourol. 2014;28(Suppl 1):A216–7.
Mariappan P, Rai B, El-Mokadem I, et al. Real-life experience: early recurrence with Hexvix photodynamic diagnosis-assisted transurethral resection of bladder tumour vs good-quality white light TURBT in new non-muscle-invasive bladder cancer. Urology. 2015;86(2):327–31.
Gallagher K, Gray KL, Lee H, et al. Real life experience: recurrence free survival at 3 years is significantly better with Hexvix PDD-TURBT when compared with Good Quality White Light TURBT (GQ-WLTURBT) in new non muscle invasive bladder cancer (NMIBC)-a prospective controlled study [abstract no. 9]. BJU Int. 2015;115(Suppl 7):12.
Risager M, Nielsen T, Ebbensgaard N, et al. Reduction of recurrence in non-muscle invasive bladder cancer using photodynamic diagnosis and immediate post-TUR-B chemoprophylaxis [abstract no. POD-06.08]. Urology. 2013;82(3 Suppl 1):S20–S1.
Gakis G, Ngamsri T, Rausch S, et al. Fluorescence-guided bladder tumour resection: impact on survival after radical cystectomy. World J Urol. 2015;33(10):1429–37.
Amend B, Lorch A, Kuehs U, et al. Influence of hexaminolevulinate on progression and tumor specific survival in patients with non-muscle invasive bladder cancer and carcinoma in situ [abstract no. MP-01.14]. Urology. 2013;82(3 Suppl 1):S42.
Karl A, Weidlich P, Adam C, et al. Flexible photodynamic diagnosis of the bladder: ready for the outpatient setting? [abstract MP22-09]. J Urol. 2014;191(4S):e237–8.
Witjes JA, Moonen PM, van der Heijden AG. Comparison of hexaminolevulinate based flexible and rigid fluorescence cystoscopy with rigid white light cystoscopy in bladder cancer: results of a prospective phase II study. Eur Urol. 2005;47(3):319–22.
Loidl W, Schmidbauer J, Susani M, et al. Flexible cystoscopy assisted by hexaminolevulinate induced fluorescence: a new approach for bladder cancer detection and surveillance? Eur Urol. 2005;47(3):323–6.
Hermann GG, Mogensen K, Toft BG, et al. Outpatient diagnostic of bladder tumours in flexible cystoscopes: evaluation of fluorescence-guided flexible cystoscopy and bladder biopsies. Scand J Urol Nephrol. 2012;46(1):31–6.
Bertrand J, Soustelle L, Grès P, et al. Interest of flexible videocystoscopy in blue light (+Hexvix®) in consultation for the diagnosis of vesical tumor [in French]. Prog Urol. 2012;22(3):172–7.
Wong KA, Zisenwe G, Athansious T, et al. Outpatient laser ablation of non-muscle-invasive bladder cancer: is it safe, tolerable and cost-effective? BJU Int. 2013;112(5):561–7.
Kamat AM, Cookson M, Witjes JA, et al. The impact of blue light cystoscopy with hexaminolevulinate (HAL) on progression of bladder cancer: a new analysis. Bladder Cancer. 2016;2(2):273–8.
May M, Fritsche HM, Vetterlein MW, et al. Impact of photodynamic diagnosis-assisted transurethral resection of bladder tumors on the prognostic outcome after radical cystectomy: results from PROMETRICS 2011. World J Urol. 2017;35(2):245–50.
Klem B, Lappin G, Nicholson S, et al. Determination of the bioavailability of [14C]-hexaminolevulinate using accelerator mass spectrometry after intravesical administration to human volunteers. J Clin Pharmacol. 2006;46(4):456–60.
Collaud S, Jichlinski P, Marti A, et al. An open pharmacokinetic study of hexylaminolevulinate-induced photodiagnosis after intravesical administration. Drugs R D. 2006;7(3):173–86.
Witjes JA, Gomella LG, Stenzl A, et al. Safety of hexaminolevulinate for blue light cystoscopy in bladder cancer: a combined analysis of the trials used for registration and postmarketing data. Urology. 2014;84(1):122–6.
Jichlinski P, Guillou L, Karlsen SJ, et al. Hexyl aminolevulinate fluorescence cystoscopy: new diagnostic tool for photodiagnosis of superficial bladder cancer: a multicenter study. J Urol. 2003;170(1):226–9.
Garfield SS, Gavaghan MB, Armstrong SO, et al. The cost-effectiveness of blue light cystoscopy in bladder cancer detection: United States projections based on clinical data showing 4.5 years of follow up after a single hexaminolevulinate hydrochloride instillation. Can J Urol. 2013;20(2):6682–9.
Bennison C, Tempest F, Marteau A, et al. Benefit of hexaminolevulinate (HAL) technology for patients and healthcare systems in non-muscle invasive bladder cancer (NMIBC) in Italy. In: 10th Health Technology Assessment International. 2013.
Marteau F, Kornowski A, Bennison C, et al. Cost-effectiveness of the optical imaging agent hexaminolevulinate for patients with non-muscle invasive bladder cancer [abstract]. Value Health. 2013;16(7):A408–9.
Klaassen Z, Li K, Kassouf W, et al. Contemporary cost-consequence analysis of blue light cystoscopy with hexaminolevulinate in non-muscle-invasive bladder cancer. Can Urol Assoc J. 2017;11(6):173–81.
Acknowledgements
The manuscript was reviewed by: S. Brookman-May, Department of Urology, Ludwig-Maximilians University of Munich Grosshadern, Munich, Germany; P. Mariappan, Edinburgh Urological Cancer Group, Department of Urology, Western General Hospital, Edinburgh, UK. During the peer review process, the marketing authorization holder of hexaminolevulinate was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
K.A. Lyseng-Williamson is an employee of Adis/Springer, are responsible for the article content and declare no conflicts of interest.
Additional information about this Adis Drug Q&A can be found at http://www.medengine.com/Redeem/7ED8F060410C6ED7
Rights and permissions
About this article
Cite this article
Lyseng-Williamson, K.A. Hexaminolevulinate: a profile of its use with blue-light cystoscopy in the diagnosis of bladder cancer. Drugs Ther Perspect 33, 463–472 (2017). https://doi.org/10.1007/s40267-017-0436-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-017-0436-4