Abstract
Purpose
Results of a retrospective single-institution study recently suggested improved prognostic outcomes in patients undergoing photodynamic diagnosis (PDD)-assisted transurethral resection of bladder tumor (TURBT) prior to radical cystectomy (RC). We sought to validate the prognostic influence of PDD-assisted TURBT on survival after RC by relying on a multi-institutional dataset.
Methods
To provide a homogeneous study population, patients with organ metastasis at the time of RC and/or after neoadjuvant chemotherapy were excluded from analysis, which resulted in overall 549 bladder cancer (BC) patients from 18 centers of the Prospective Multicenter Radical Cystectomy Series 2011 (PROMETRICS 2011). To evaluate the influence of PDD conducted during primary or final TURBT on cancer-specific mortality (CSM) and overall mortality (OM) after RC, bootstrap-corrected multivariate Cox proportional-hazards regression models were applied (median follow-up: 25 months; IQR: 19–30). Sensitivity analyses were performed for both patients with pure urothelial carcinoma and patients undergoing one single TURBT only.
Results
In 88 (16.0 %) and 100 (18.2 %) patients, PDD was used in primary and final TURBTs, respectively. In 335 (61.0 %) patients, a single TURBT was performed prior to RC; in 194 patients (35.3 %), TURBT had been performed in a different center. CSM and OM rates at 3 years were 32 and 40 %, respectively. Use of PDD during primary or final TURBT was no independent predictor of CSM or OM. These results were internally valid and were confirmed in sensitivity analyses.
Conclusions
PDD utilization during TURBT prior to RC does not independently impact the prognosis of BC patients after RC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radical cystectomy (RC) is the therapeutic gold standard in patients with non-metastatic muscle-invasive bladder cancer (MIBC) and is recommended also for BCG (Bacille Calmette–Guérin)-refractory high-grade non-MIBC (NMIBC). In addition, RC should be considered in NMIBC patients with high risk of progression and can provide a last-resort palliative option in locally advanced tumor stages (T4b) [1, 2]. Pathological tumor and nodal stage, soft tissue surgical margin (SM) status and the extent of the lymph node dissection (LND) constitute established prognostic factors for the outcome of non-metastatic MIBC after RC [1–4]. Thus, the surgeon’s influence on prognosis is likely to be restricted to providing negative SM during surgery and to performing an extended LND (level of evidence 3) [1]. Additionally, prognosis of RC patients may be improved by complying with some quality of care standards, i.e., the offer of neoadjuvant cisplatin-based chemotherapy to eligible patients (grade of recommendation; GoR A in stages cT2-T4aN0M0), restriction of the time interval between the diagnosis of MIBC and RC to ≤3 months (GoR B) and the consideration of adjuvant cisplatin-based chemotherapy in select patients (GoR C in urothelial carcinoma stages pT3/4 and/or pN+) [1].
In a recent retrospective single-center study by Gakis and colleagues on 224 BC patients undergoing RC between 2002 and 2010, patients who had undergone PDD-assisted TURBT prior to RC had an improved oncological outcome [5]. In 38.8 % of patients, ≥1 TURBT had been performed under PDD-assistance, and in 29.5 %, hexaminolevulinate (HAL) had been used. In patients who had undergone TURBT under HAL-assistance, disease recurrence rates, cancer-specific mortality (CSM) and overall mortality (OM) decreased by 70 % (p < .001), 52 % (p = .044) and 44 % (p = .025), respectively [5]. The authors concluded that HAL-based TURBT results in favorable prognostic long-term outcomes after RC. However, due to the retrospective single-institution study design, the need of an external validation of those results in a larger multi-institutional study group seems obvious in order to confirm any influence of PDD-assisted TURBT prior to RC on patients’ survival [5].
To validate the hypothesis by Gakis et al., we sought to compare CSM and OM in cystectomy patients who had undergone PDD-assisted TURBT versus those who had undergone white-light TURBT prior to RC by analyzing the data from a prospectively collected multi-institutional European dataset (PROspective MulticEnTer RadIcal Cystectomy Series/PROMETRICS 2011).
Methods
Study population and data assessment
The PROMETRICS 2011 is an institutional review board-approved study with all participating sites providing the necessary data-sharing agreements. A total of 18 European centers (15 German, 2 Austrian, and 1 Italian) prospectively contributed their data resulting in a database consisting of 679 consecutive patients who underwent RC for MIBC or high-risk NMIBC from January 1, 2011 to December 31, 2011. A computerized data collection sheet including a comprehensive definition of all variables was generated for data transfer. After combining the datasets, preliminary reports were generated to identify data inconsistencies or integrity problems. Regular communication among all participating centers ensured that all identified discrepancies were resolved before final analysis. Furthermore, patients with incomplete oncological follow-up or missing information on PDD utilization during primary or final TURBT were excluded from analysis. Aiming at a homogenous study population, organ metastasis and conduction of neoadjuvant chemotherapy were further exclusion criteria. Overall, 549 patients with RC and bilateral LND were included for final analysis. Besides several clinical and pathological parameters, it was also assessed whether primary and/or final TURBT was performed under PDD-assistance or in white-light mode. For the PDD-assisted final TURBTs, HAL was used in all cases. With the exception of only one case of 5-aminolevulinic acid (ALA), HAL was applied used in all primary TURBTs. Hence, PDD-assisted TURBT in this study represents the utilization of HAL. Apart from the total numbers of TURBT performed in each patient, it was also recorded whether TURBT was performed in the same center where the RC took place. Histopathological stages of TURBT and RC were recorded according to the 2009 TNM classification [1].
Follow-up and study endpoints
The extent of preoperative staging diagnostic workup, histopathological review of the in-house TURBT and RC specimens and the modality and frequency of follow-up examinations were defined by standardized protocols within the PROMETRICS study group and were based on current European Association of Urology guidelines [1]. Prior to the initiation of the study, August 2014, December 2016 and June 2019 had been determined as effective dates for a centralized merging of oncological follow-up data. Median follow-up was 25 months (interquartile range (IQR), 19–30). Study endpoints were defined as CSM and OM.
Statistical analyses
Patient and clinical characteristics were assessed by using medians and IQR for continuous and non-normally distributed variables. To evaluate the association of PDD utilization during TURBT with the probability of pT0 stage at RC, a multivariate logistic regression model was created. The latter was adjusted for tumor stage at final TURBT (≥T2 vs. ≤T1), time interval between final TURBT and RC (continuously coded in months), presence of solitary or concomitant carcinoma in situ (CIS) in the final TURBT specimen, number of TURBTs performed per patient (≥1 vs. 1), age at RC (continuously coded in years) and gender.
Cancer-specific survival (CSS) and overall survival (OS) were estimated by the Kaplan–Meier method and equality of survival curves evaluated by the log-rank test. Using bootstrap-corrected multivariable Cox proportional hazard regression models (stepwise analyses of 200 bootstrap samples), the impact of several covariates on the endpoints CSM and OM was assessed. Cox models were adjusted for age at RC, gender, American Society of Anesthesiologists physical status (ASA 3–4 vs. 1–2), histological subtype (pure urothelial vs. non-pure urothelial), pT stage (pT3–4 vs. ≤pT2), pN stage (pN+ vs. pN0), soft tissue SM status (positive vs. negative) in RC specimens, lymphovascular invasion (LVI) in RC specimens, number of lymph nodes removed (continuous) and adjuvant chemotherapy (present vs. absent). Two separate models were established regarding PDD utilization during primary or final TURBT (yes vs. no). In addition, sensitivity analyses were performed in both patients with pure urothelial histology and patients who underwent one single TURBT only prior to RC.
Statistical analyses were performed using SPSS (version 23.0, IBM Corp., Armonk, NY, USA). Two-sided statistical significance was defined as a p value <.05.
Results
Patient and clinical characteristics
80.1 % of the study patients were male. In 16.0 and 18.2 % of cases, PDD had been used during primary and final TURBTs, respectively. One single TURBT had been performed in 61.0 % of patients prior to RC (range: 1–25 TURBT), and 35.3 % of patients had undergone TURBT in a hospital different to where the RC was performed. Invasion of muscularis propria was detected in 53.3 and 66.9 % at primary and final TURBTs, respectively. In 22.3 %, solitary or concomitant CIS was diagnosed. Median time from final TURBT to RC was 1 month (IQR: 0–1) with a time interval >3 months in 7.4 % of patients. Median patient age at RC was 70 years (IQR: 62–76); 48.7 % had an ASA status 3–4. In RC specimens, pure urothelial histology was diagnosed in 87.1 %. The fraction of RC specimens with a stage ≥pT3 and pN+ was 47.0 and 26.3 %, respectively. Positive soft tissue SM and LVI were described in 8.2 and 34.2 %, respectively. Median number of lymph nodes removed was 16 (IQR: 9–23), and adjuvant chemotherapy was administered in 17.7 %.
Predictors of pT0 stage at RC
At RC, pT0 stage was diagnosed in 59 patients (10.7 %). However, multivariate logistic regression analysis showed that PDD-assistance during final TURBT did not translate into increased presence of pT0 stage at RC (odds ratio (OR) = 1.34, 95 % confidence interval (CI) = [.66–2.69], p = .417). The only independent predictor of pT0 stage was non-muscle-invasive tumor stage in the final TURBT specimen (OR = .49, 95 % CI = [.28–.85], p = .012) (Table 1).
Unadjusted Kaplan–Meier estimates
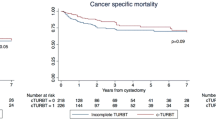
In our study population, CSS and OS at 3 years were 68 and 60 %, respectively. In unadjusted Kaplan–Meier analyses, log-rank tests also showed that PDD-assistance during primary or final TURBT did not significantly impact on CSS (p = .513 and p = .802, respectively). Similarly, no effect on OS was found for PDD-assistance during primary (p = .577) or final TURBT (p = .524).
Adjusted multivariate Cox regression analyses
In adjusted multivariable Cox regression analyses, PDD-assistance during primary or final TURBT did not independently impact on CSM and OM (Tables 2, 3). The results were internally valid (bootstrap-corrected p values) and corroborated by sensitivity analyses in both patients with pure urothelial histology and patients with one single TURBT prior to RC (data not shown).
Discussion
In this analysis of a recent, multi-institutional, prospectively collected dataset, we could not provide any evidence for a favorable impact of PDD utilization during TURBT prior to RC on CSM or OM. These findings were robust on sensitivity analyses limited to patients with pure urothelial histology and patients who had undergone one single TURBT prior to RC only. Hence, the present study does not confirm but contradicts the results of a retrospective single-center series recently published by Gakis et al. [5] who indicated a decline in CSM (−52 %; p = .044) and OM (−44 %; p = .025) in patients who had undergone PDD-assisted TURBT prior to RC (n = 66/224). Comparing the results of our study with those of Gakis and colleagues, we need to take methodological differences into account. In the single-institution study by Gakis, patients who underwent RC between 2002 and 2010 were split into three subgroups according to the modality of TURBT (HAL, 29.5 %; ALA, 10.3 %; white-light, 60.2 %). In addition, Gakis et al. [5] did not differentiate between PDD-assistance during primary or final TURBT but rather compared HAL- versus ALA- versus white-light-TURBT. Furthermore, as Gakis et al. did not show a prognostic advantage for their ALA-subgroup, they collapsed this subgroup with the white-light subgroup for further comparative multivariable mortality analysis in comparison to HAL-assistance during TURBT. Thus, the benefit reported by Gakis et al. [5] regarding disease recurrence, CSM and OM is restricted to those 29.5 % of patients who had undergone HAL-assisted TURBT only. In our study, only one single patient had undergone ALA-assisted PDD with primary TURBT; in this patient, HAL was then also used for the final TURBT. Therefore, this patient was not excluded from analysis. In addition, it must be considered that overall, HAL-assisted TURBT was less frequently used in our prospective study (primary TURBT: 15.8 %; final TURBT: 18.2 %) compared to the retrospective study by Gakis et al. [5] (overall: 29.5 %), which is most likely related to the separate analysis of primary versus final TURBT in our study. Although Gakis et al. [5] provided the aforementioned hypothesis, no rationale for the observed prognostic benefit in the HAL-group was generated.
However, several explanatory approaches might disclose the underlying causes leading to favorable outcomes when HAL-assistance is used. First, the feasibility of a radical resection during TURBT is likely to be improved under HAL-assistance compared to white-light mode. Thus, the probability of pT0 stage at RC would increase, which might translate into a significant decrease in CSM, as shown in a previous PROMETRICS analysis [6]. Nevertheless, Gakis et al. failed to show significant differences in pT0 stages at RC after TURBT when the use of HAL (4.6 %), ALA (4.3 %) or white-light (5.9 %) was compared. In our study, HAL-assistance during final TURBT did not have an independent impact on achieving pT0 stage at RC (OR = 1.34; p = .417), which was seen in 59 patients (10.7 %). Secondly, PDD utilization during TURBT might ease the detection of a high-risk profile in patients with NMIBC and thus facilitate a timely indication of BCG therapy or RC. Retrospective studies often fail to distinguish such effects, which emphasizes the need for long-term observations from prospective randomized trials comparing HAL- to white-light-assisted TURBT. In our study, patients undergoing PDD-assisted (primary or final) TURBT did not harbor solitary or concomitant CIS more frequently nor did they have a tumor-free muscularis propria more often in the cases of NMIBC (data not shown). However, we observed a significant difference between the groups regarding tumor multi-focality, which was more often detected in patients who underwent PDD-assisted final TURBT compared to white-light TURBT (69 vs. 49 %; p = .001). Remarkably, there was no significant difference in the time interval <3 months between final TURBT and RC among the groups (p = .674). Finally, another issue should be addressed when hypothesizing on reasons for a possible survival benefit related to HAL-assistance as postulated by Gakis et al. In their single-institution study, the proportions of oncological favorable pTa/pT1 tumors at RC did not significantly differ between the HAL- and ALA-/white-light-group, but proved to be clinically important (24.2 vs. 17.7 %). Gakis and colleagues dichotomized tumor stage (≥pT3 vs. ≤pT2) in the prognostic outcome models. Hence, a proper adjustment of this descriptive difference in the distribution of more favorable tumor stages might have been lacking [5]. To mirror the exact outcome models by Gakis et al., we dichotomized pathological tumor stages for mortality analyses in the same manner; nonetheless, we could not confirm any prognostic effect of PDD-assistance during TURBT on CSM and OM.
Several limitations have to be taken into account when interpreting the findings of our study. Although PROMETRICS 2011 is a contemporary, multi-institutional and prospectively collected RC series, data on the preceding TURBTs were collected retrospectively. Additionally, we could not ascertain the indication of PDD-assisted TURBT or whether radicalness was aimed for each procedure, which might have led to skewed results. Further prospective TURBT studies are absolutely necessary to establish whether PDD is associated with improved bladder cancer outcomes, specifically in the treatment of NMIBC [7]. With 25 months, the median follow-up of our study is relatively short; however, it is close to that of the study by Gakis et al. [5] (29 months). The upcoming next merging of oncological FU will be in December 2016, indicating the need for future confirmation of these results with longer follow-up (see methods). Furthermore, it needs to be considered that 16 and 18.2 % only of the study patients did receive primary and final PDD-assisted TURBTs, respectively. It should be taken into account that a potential subsequent selection bias might impact the study results as well. Finally, there was a slightly different methodological approach with the separation of primary and final TURBTs in our study which had not been done by Gakis et al. (overall HAL-assisted TURBT).
Conclusions
Based on the results of this multi-institutional series, there is no evidence for an independent impact of HAL-assistance in primary or final TURBT on the oncological outcomes of BC patients after RC. Prospective studies with predefined indications and aimed at radicalness of resection with HAL-assisted TURBT are required in order to reliably investigate whether there is an independent impact on survival after RC or alternatively whether HAL-assisted TURBT might serve as a surrogate for improved BC management.
References
Witjes JA, Comperat E, Cowan NC, De Santis M, Gakis G, Lebret T et al (2014) EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 65:778–792
Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E et al (2013) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 64:639–653
Simone G, Bianchi M, Giannarelli D, Daneshmand S, Papalia R, Ferriero M et al (2015) Development and external validation of nomograms predicting disease-free and cancer-specific survival after radical cystectomy. World J Urol 33:1419–1428
Abdi H, Pourmalek F, Gleave ME, So AI, Black PC (2016) Balancing risk and benefit of extended pelvic lymph node dissection in patients undergoing radical cystectomy. World J Urol 34:41–48
Gakis G, Ngamsri T, Rausch S, Mischinger J, Todenhofer T, Schwentner C et al (2015) Fluorescence-guided bladder tumour resection: impact on survival after radical cystectomy. World J Urol 33:1429–1437
May M, Bastian PJ, Burger M, Bolenz C, Trojan L, Herrmann E et al (2011) Multicenter evaluation of the prognostic value of pT0 stage after radical cystectomy due to urothelial carcinoma of the bladder. BJU Int 108:E278–E283
Faba OR, Palou J, Breda A, Villavicencio H (2012) High-risk non-muscle-invasive bladder cancer: update for a better identification and treatment. World J Urol 30:833–840
Authors’ contributions
Matthias May was involved in project development, data analysis and drafting of the manuscript; Hans-Martin Fritsche was involved in project development, data collection and manuscript editing; Malte W. Vetterlein critically revised the manuscript; Patrick J. Bastian was involved in manuscript editing and supervision; Michael Gierth collected the data and edited the manuscript; Philipp Nuhn collected the data and edited the manuscript; Atiqullah Aziz was involved in project development, data collection and manuscript editing; Margit Fisch collected the data and edited the manuscript; Christian G. Stief was involved in data collection and manuscript editing; Markus Hohenfellner collected the data and edited the manuscript; Manfred P. Wirth was involved in data collection and manuscript editing; Vladimir Novotny collected the data and edited the manuscript; Oliver W. Hakenberg was involved in data collection and manuscript editing; Joachim Noldus collected the data and edited the manuscript; Christian Gilfrich edited the manuscript; Christian Bolenz was involved in data collection and manuscript editing; Maximilian Burger was involved in project development, data collection and manuscript editing; Sabine Brookman-May was involved in project development and critical revision of the manuscript; Collaborators were involved in acquisition of data; MS manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
We confirm that there are no competing financial interests in relation to the work described.
Ethical approval
The study was approved by the local ethics board, and all subjects provided written informed consent.
Additional information
Matthias May and Hans-Martin Fritsche have contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
May, M., Fritsche, HM., Vetterlein, M.W. et al. Impact of photodynamic diagnosis-assisted transurethral resection of bladder tumors on the prognostic outcome after radical cystectomy: results from PROMETRICS 2011. World J Urol 35, 245–250 (2017). https://doi.org/10.1007/s00345-016-1877-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-016-1877-4