Abstract
Aim
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used analgesics among older adults. Adverse effects may be avoided by careful patient selection. We aimed to evaluate the incidence of acute kidney injury (AKI) and/or hyperkalemia, risk factors, and the accuracy of an NSAID risk prediction model in a cohort of Asian older adults.
Methods
We conducted a retrospective cohort study of older adults, age 65 years and above, who received prescriptions between March 2015 and December 2017 from Singapore’s largest cluster of public healthcare institutions. Factors associated with 30-day incident acute kidney injury and/or hyperkalemia were evaluated with multivariable regression analysis. Calibration and discrimination of the Nash prediction model were assessed using the Hosmer-Lemeshow goodness-of-fit test and C-statistic, respectively.
Results
The primary outcome occurred in 16.7% of 12,798 older adults. Topical NSAIDs (adjusted OR 1.29, 95% CI 1.15–1.45), systemic NSAIDs of 1–14 days’ duration (adjusted OR 1.43, 95% CI 1.27–1.62), and systemic NSAIDs > 14 days (adjusted OR 1.84, 95% CI 1.37–2.49) were independently associated with the primary outcome, compared with no NSAID. Diabetes mellitus, cardiovascular disease, lower estimated glomerular filtration rate (eGFR), and diuretics were also independently associated with increased incident AKI and/or hyperkalemia. When applied to older adults with systemic NSAIDs > 14 days (n = 305), the Nash risk model had poor calibration (p < 0.001) and poor discrimination with C-statistic 0.527 (0.438, 0.616).
Conclusions
Longer NSAID duration and systemic compared with topical route were associated with incremental odds for acute renal events. Further studies are required to improve the available risk model to guide NSAID prescriptions in older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used analgesics among older adults and careful patient selection may minimize adverse effects. The Nash risk prediction model was developed in a predominantly Caucasian older adult population but external validation in other cohorts is lacking. |
This study evaluated 12,798 Asian older adults aged 65 years and older who received prescriptions from Singapore’s largest cluster of public healthcare institutions and confirmed that systemic NSAIDs > 14 days, systemic NSAIDs prescribed for 1–14 days, and topical NSAIDs increased the risk of acute adverse kidney events. |
The Nash risk model had poor calibration and discrimination among Asian older adults prescribed systemic NSAIDs for > 14 days. Further research to guide NSAID prescriptions in older adults is required. |
1 Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used analgesics but may be associated with adverse cardiovascular, renal, and gastrointestinal events [1, 2]. Acute kidney injury (AKI) can occur when an NSAID inhibits the prostaglandin-mediated dilation of the afferent renal arteriole in conditions where renal glomerular perfusion is reduced. Older adults are particularly susceptible to NSAID-induced AKI [3]. Despite evidence-based clinical guidelines that have repeatedly cautioned against potentially inappropriate NSAID prescription in older adults with heart failure, severe hypertension, and chronic kidney disease (CKD) [1, 2, 4], NSAID prescription remains frequent. The Chronic Renal Insufficiency Cohort reported baseline NSAID use in 13% of 1140 participants aged 65 years and older, while a recent large population-based study in Canada found that individuals 66 years or older formed the majority of those who had received one or more NSAID for > 14 days [5]. Hence, more needs to be done to prevent potentially inappropriate NSAID prescription. The Nash clinical risk model for 30-day risk of AKI and/or hyperkalemia [5], developed in Canadian older adults in the general population and accessible as an online calculator (https://qxmd.com/calculate-by-qxmd), may be a useful decision support tool for clinicians to avoid prescribing NSAIDs to older adults at risk of AKI [6]. However, this tool has yet to be extensively validated in other populations. As most of the evidence of NSAID-induced AKI in the community is obtained from predominantly Caucasian populations [3, 7], it is unclear if the risks of AKI will be different in Asians. We thus aimed to evaluate the factors associated with risk of acute adverse renal events and the accuracy of the Nash risk model in a cohort of multi-ethnic Southeast Asian older adults.
2 Methods
This was a retrospective cohort study of all older adults aged ≥ 65 years who received incident prescriptions between 1 March 2015 and 30 December 2017 from Singapore General Hospital and seven SingHealth Polyclinics (Bukit Merah, Outram, Marine Parade, Bedok, Tampines, Pasir Ris, and Sengkang). Together, the Singapore Health Services (SingHealth) cluster is Singapore’s largest cluster of public healthcare institutions that provides integrated clinical services spanning primary care and tertiary referral centers.
2.1 Cohort and Risk Factors
Data was obtained from the INSIDER (Inappropriate nephrotoxic Non-Steroidal Anti-Inflammatory Drug in Diabetes, Elderly and Renal Impairment) study, which evaluated potentially inappropriate NSAID prescription in at-risk individuals who received any prescriptions between 2015 and 2017 in Singapore General Hospital and SingHealth Polyclinics [8]. NSAID prescriptions (including selective NSAIDs such as cyclooxygenase II [COX II] inhibitors, Supplementary Table S1, see electronic supplementary material [ESM]) were identified from outpatient and discharge electronic pharmacy records. Prescription dates, type, route, dose, and duration of each NSAID was retrieved. We categorized systemic NSAID prescription by (a) NSAID (oral or parental route) for > 14 days according to the Nash study definition for NSAID users [5], and (b) short course for 1–14 days. Following the Nash study [5], the date of the first NSAID prescription for > 14 days was defined as the cohort entry date for those with multiple NSAID prescriptions. Additionally, if there was no NSAID prescription > 14 days, then the date of the first systemic NSAID prescription for 1–14 days was defined as the cohort entry date. If there was no systemic NSAID prescription, then individuals were further categorized into (c) topical NSAID, and (d) no NSAID (i.e. did not have any systemic or topical NSAID prescription). The cohort entry date for the ‘no-NSAID’ group was the date of the first prescription during the study period. Among individuals prescribed systemic NSAIDs, any topical NSAID in the same prescription was documented as ‘co-prescribed topical NSAID’. We excluded individuals with the following: (i) prescriptions for NSAIDs within 60 days prior to cohort entry so that the NSAID groups are incident NSAID users and those in the ‘non-NSAID’ group will not have had any recent NSAID prescription; (ii) missing serum creatinine and/ or potassium values within 6 months before and 30 days after cohort entry; and (iii) advanced or severe kidney dysfunction defined as baseline estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2.
Variables collected included demographic data, co-morbid conditions, and biochemistry (most recent serum creatinine and potassium values within 6 months preceding cohort entry and peak values within 30 days after cohort entry). Baseline eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD EPI) equation using the most recent serum creatinine value within 6 months preceding cohort entry. CKD was present if eGFR was < 60 mL/min/1.73 m2. Diabetes mellitus was identified from the SingHealth Diabetes Registry, an electronic medical record (EMR)-based registry that defined diabetes based on diagnosis codes, drug prescriptions of glucose-lowering medications and laboratory results (e.g. plasma glucose and HbA1c). Baseline cardiovascular disease (CVD) was present if there was any CVD-related hospitalization for ischemic heart disease or congestive heart failure in the preceding 6 months. Renin-angiotensin-aldosterone system (RAAS) blockers (such as angiotensin-converting enzyme inhibitor, angiotensin-receptor blocker, and mineralocorticoid receptor blocker) or thiazide or loop diuretics prescribed within 6 months before and up to 30 days after cohort entry were also recorded as high-risk medications that may result in adverse renal outcomes when administered concurrently with NSAIDs [5, 9, 10].
2.2 Renal Events
The primary outcome in this study was the incidence of AKI and/or hyperkalemia within 30 days. AKI was defined if serum creatinine increased ≥ 26.5 µmol/L or ≥ 50% from baseline, according to the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 criteria that was used by the Nash study [5, 11]. Incident hyperkalemia was present if baseline serum potassium was < 5.5 mmol/L and subsequently increased to ≥ 5.5 mmol/L. Earlier studies had established that the risk of NSAID-related AKI was highest within 30 days after treatment initiation [12], thus a 30-day follow up was chosen for this study. Data was censored at last follow up or at 30 days, whichever was earlier.
This study was conducted according to the Declaration of Helsinki. Waiver of informed consent for use of de-identified EMR data was approved by the SingHealth Centralized Institutional Review Board (2018/2567).
3 Statistical Analysis
Statistical analysis for risk factors was performed using IBM SPSS Statistics 25 (IBM Corp., Armonk, NY, USA). Categorical variables were presented as proportions and continuous variables summarized as means with standard deviations. Pearson chi-square test was used to compare categorical variables and student t-test or one-way Anova test for continuous variables, as appropriate. Binary logistic regression analysis (enter method) was used to obtain odds ratios (ORs) and 95% confidence intervals (CIs) for pre-selected factors associated with the outcome based on available literature [3, 5, 9, 13], as well as factors with p < 0.20 in univariate analysis. Hence, the variables included in the multivariable regression model were age, diabetes mellitus (yes vs no), CVD (yes vs no), eGFR, serum potassium, NSAID type and duration (vs no-NSAID), RAAS blocker (yes vs no), and diuretic (yes vs no). Multicollinearity was checked by examining the correlation matrix for coefficient values ≥ 0.80. Cox regression analysis was also performed to generate the survival curves for time to the primary outcome according to NSAID type and duration. Sensitivity analysis was performed for older adults with serum creatinine values at baseline and follow up to evaluate the outcome of incident AKI. All tests were two-tailed and statistical significance was defined as p < 0.05 unless otherwise stated.
External validation analysis of the Nash clinical risk model was performed using R (R Core Team, Vienna, Austria) [14] with ResourceSelection [15] and pROC [16] packages for the Hosmer-Lemeshow goodness-of-fit tests and C-statistic calculations, respectively. C statistic, the equivalent of the area under a receiver operating characteristic (ROC) curve (AUC), was computed as a measure of discrimination for a binary outcome, where a value of 0.5 suggests poor predictive performance (similar to that of a coin toss) while a value of 1.0 suggests perfect ability to differentiate between individuals with and without the outcome. Calibration of the model was evaluated by the Hosmer-Lemeshow goodness-of-fit test and graphically by logistic regression models with loess smoothers [17], where p < 0.05 suggests poor agreement between predicted and observed risks. The sensitivity, specificity, positive predictive value, and negative predictive value were calculated at 1%, 5%, and 10% risk. Since the Nash risk score was developed in a relatively low-risk cohort [5], we performed sensitivity analysis for older adults without CVD. An additional sensitivity analysis included those without follow-up serum creatinine and potassium results by assuming they did not have incident AKI or hyperkalemia.
4 Results
4.1 Population and Renal Events
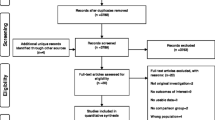
Figure 1 describes the cohort identification and exclusions. We identified 12,798 older adults with incident prescriptions between March 2015 and December 2017. Half were female (50.9%). The multi-ethnic cohort were mostly Chinese (n = 10,369, 81.0%), followed by Malay (7.5%), Indian (6.3%), and other ethnicities (5.2%). Comorbid conditions such as diabetes (36.2%) and CKD (36.4%) were frequent, as was use of RAAS blockers (36.5%) and diuretics (25.1%).
NSAIDs were prescribed in 7210 individuals (56.3%). Systemic NSAIDs were prescribed in 3640 individuals (28.4%), of whom 305 (2.4%) had systemic NSAID prescriptions for > 14 days and 3335 (26.1%) received short-course systemic NSAID prescriptions for 1–14 days. The majority of systemic NSAIDs were COX II inhibitors (61.0%). Co-prescription of topical NSAIDs was present in 19.7% and 13.1% of those with systemic NSAID prescriptions for > 14 days and 1–14 days, respectively. Another 3570 (27.9%) received only topical NSAIDs.
Table 1 compares the baseline characteristics and acute renal outcomes by route and duration of NSAID prescription. The group with systemic NSAID prescriptions > 14 days were significantly younger, more likely to be female but less likely to have CVD, CKD, RAAS blockers, and diuretics, and had higher eGFR than the other groups. In contrast, those with topical NSAID prescriptions were significantly older, more likely to have diabetes, CVD, and CKD, and had lower eGFR and higher baseline serum potassium than the other groups.
The primary outcome of 30-day AKI and/or hyperkalemia occurred in 2137 individuals (16.7%). Table 1 shows that it was most frequent in the group prescribed systemic NSAIDs for > 14 days (20.0%), compared with short-course systemic NSAIDs (17.1%), topical NSAIDs (19.0%), and no NSAIDs (14.8%).
4.2 Factors Associated with Acute Kidney Injury and Hyperkalemia
In univariate analysis compared according to the primary outcome (Supplementary Table S2, see ESM), those with AKI and/or hyperkalemia were significantly older with lower eGFR and more likely to have diabetes, CVD, RAAS blockers, and diuretics than those without. Co-prescription of systemic and topical NSAIDs was not significantly different between the group with the primary outcome and the group without (12.1% vs 13.9%; p = 0.23).
Multivariate analysis by logistic regression for the occurrence of the primary outcome within 30 days in Table 2 shows that NSAIDs, compared with no NSAIDs, were independently associated with increased odds of the primary outcome. The odds ratios were incrementally higher for topical NSAIDs (adjusted OR 1.29, 95% CI 1.15–1.45), systemic NSAIDs 1–14 days (adjusted OR 1.43, 95% CI 1.27–1.62), and systemic NSAIDs >14 days (adjusted OR 1.84, 95% CI 1.37–2.49). Diabetes, CVD, lower eGFR, and diuretics were also independently associated with increased 30-day incident AKI and/or hyperkalemia. None of the covariates were significantly correlated. Cox regression adjusting for age, diabetes, CVD, baseline eGFR and serum potassium, and use of RAAS blockers and diuretics similarly showed that systemic route and longer duration increased the risk for the primary outcome (Fig. 2). CVD, lower eGFR, and diuretics continued to be independently associated with the primary outcome (Supplementary Table S3, see ESM).
Comparison of time to acute kidney injury or hyperkalemia according to prescribed NSAID route and duration, after adjustment for age, diabetes, cardiovascular disease, baseline estimated glomerular filtration rate and serum potassium and use of renin-angiotensin-aldosterone system blockers and diuretics by Cox regression. NSAIDs for > 14 days (adjusted HR 1.50, 95% CI 1.15–1.96), systemic NSAIDs for 1–14 days (adjusted HR 1.26, 95% CI 1.13–1.41), and topical NSAIDs (adjusted HR 1.12, 95% CI 1.01–1.24) were significantly associated with incident AKI and/or hyperkalemia, compared with no-NSAIDs. AKI acute kidney injury, CI confidence interval, HR hazard ratio, NSAIDs non-steroidal anti-inflammatory drugs
Supplementary Table S4 (see ESM) showed the sensitivity analysis performed for the outcome of AKI among 13,221 individuals with serum creatinine at baseline and follow up. Prescribing NSAIDs for > 14 days (adjusted OR 2.13, 95% CI 1.54–2.95), systemic NSAIDs for 1–14 days (adjusted OR 1.72, 95% CI 1.50–1.98), and topical NSAIDs (adjusted OR 1.41, 95% CI 1.24–1.61) were significantly associated with 30-day incident AKI, compared with no NSAIDs.
4.3 External Validation
When applied to our cohort of 305 older adults with systemic NSAID prescriptions > 14 days, the Nash risk model had poor calibration (p < 0.001, Supplementary Fig. S2, see ESM), and poor discrimination with C-statistic 0.527 (0.438, 0.616) (Supplementary Fig. S3, see ESM). The calibration (goodness-of-fit, p < 0.001) and discrimination (C-statistic 0.518 [0.424, 0.611]) remained poor in the subgroup without CVD (n = 294). Supplementary Table S5 demonstrates the clinical utility of the Nash model to identify high-risk individuals in our cohort based on different predicted risk thresholds. The sensitivity values were 77% and 28% and the specificity values were 22% and 82% for predicted risk thresholds of > 5% and > 10%, respectively.
In the sensitivity analysis where those without follow-up serum creatinine and potassium were assumed not to have AKI or hyperkalemia, the Nash risk model was applied to 2786 older adults with systemic NSAID prescriptions > 14 days. Incident AKI and/or hyperkalemia occurred in 62 (2.2%). The calibration (goodness-of-fit p < 0.001) and discrimination (C-statistic 0.529 [0.441, 0.613]) remained poor in this cohort. The sensitivity values were 4.8% and 1.6% and the specificity values were 98.8% and 99.7% for predicted risk thresholds of > 5% and > 10%, respectively.
5 Discussion
This study comprising 12,798 older adults found that topical NSAIDs (adjusted OR 1.29), systemic NSAIDs for 1–14 days (adjusted OR 1.43) and systemic NSAIDs for >14 days (adjusted OR 1.84) were independently associated with 30-day incident AKI and/or hyperkalemia. While prolonged systemic NSAIDs use is associated with AKI [5], there was little data on the nephrotoxicity of short-term NSAIDs or topical NSAIDs. We had recently demonstrated that short-course systemic NSAIDs and topical NSAIDs were associated with increased AKI and/or hyperkalemia [8]. The findings from this study further showed that there were incremental odds of an acute adverse renal outcome with longer NSAID duration and systemic route, compared with topical. This is consistent with the greater nephrotoxicity expected with greater cumulative exposure, since topical NSAIDs have lower bioavailability and plasma concentrations compared with oral NSAIDs. While recent studies on NSAID-associated AKI in older adults did not study the impact of NSAID duration or route [18,19,20], NSAIDs were associated with increased risk of acute myocardial infarction in a dose-dependent manner in a meta-analysis that included 446,763 individuals [21]. Thus, our findings relating NSAID prescription duration and acute adverse renal outcomes complement those in acute cardiovascular events.
The other risk factors of diabetes [7, 12], lower eGFR, and diuretics identified by this study are consistent with known literature on NSAID-associated AKI [5, 9, 10]. In addition, we found that recent CVD-associated hospitalizations for ischemic heart disease or congestive heart failure were strongly associated with AKI and/or hyperkalemia, even after accounting for use of RAAS blockers and diuretics. CVD may adversely affect renal hemodynamics and reduce glomerular flow, thereby potentiating NSAID-induced AKI mediated by reduced renal prostaglandin synthesis and reduced renal blood flow [22]. However, CVD was not included as a risk factor in the Nash risk model [5]. While age, diabetes, and diuretic use were similar between the groups, our cohort of older adults who received systemic NSAIDs > 14 days were more likely to have had CVD-related hospitalization within 6 months preceding cohort entry than the Nash NSAID users who had a mean of 0.2 ± 0.5 hospitalizations in the preceding year [5]. Thus, the poor calibration and discrimination of the Nash model may be due to differences in case mix in the two studies. However, the performance of the Nash model did not improve in the subgroup without CVD, nor in the sensitivity analysis.
At a predicted risk threshold of > 5%, the Nash model had high sensitivity but low specificity, while at a predicted risk threshold of > 10%, the Nash model had low sensitivity but high specificity. The positive predictive values were low and negative predictive values were high for both levels of predicted risk threshold. When applied to clinical practice, the Nash model can potentially aid physicians in risk stratifying older adults before prescribing potentially nephrotoxic systemic NSAIDs. However, the acceptable risk threshold remains debatable and may need to be individualized according to the individual’s comorbid conditions and tolerance for AKI or hyperkalemia. It can be argued that the adverse renal outcomes identified by the study definitions may include mild cases of AKI or hyperkalemia. These may be trade-offs for adequate pain relief and an improved quality of life, especially when alternative effective analgesic options such as opioids, anti-epileptics, and anti-depressants are limited due to comorbid conditions that reduce drug clearance or predispose to drug interactions [23, 24]. Hence, the choice of risk threshold should reflect the harms of false positives (e.g. not prescribing an NSAID resulting in inadequate pain relief or use of other inappropriate analgesic) and false negatives (e.g. prescribing prolonged systemic NSAIDS to high-risk individual and causing AKI and/or hyperkalemia); and the benefits of identifying true positives (e.g. instituting close monitoring after NSAID prescription to identify adverse events early or even avoid prescription of prolonged systemic NSAIDs). These are likely to change according to the clinical context [25], so shared decision making to tailor therapy to the needs of the individual is required. The results from this study support the recommendations from the American Geriatrics Society 2019 updated AGS Beers Criteria® that systemic NSAIDs be prescribed at the lowest effective dose for shortest possible duration [24]. Topical NSAIDs had been frequently recommended for better gastrointestinal and cardiovascular tolerability [26, 27]. Considering the limitations of the Nash model when applied to a higher-risk cohort, and that topical NSAIDs were also associated with acute renal events [8], albeit with a lower risk magnitude than prolonged systemic NSAIDs, future studies may want to consider evaluating short-course systemic NSAIDs and topical NSAIDs in predictive risk models to guide NSAIDs prescriptions to older adults. These newer models will need to be externally validated in cohorts of varying risks before they can be widely implemented in appropriately risk-stratified older adults [8].
This study has several limitations. Since we sought to evaluate the Nash risk model, we similarly defined NSAID exposure by prescription data. However, the authors acknowledge that NSAID prescriptions may not equate to NSAID use and data on over-the-counter NSAID use was not available. By necessarily including only those with both baseline and follow-up serum creatinine and potassium levels to accurately classify the outcome, we may have selected those who had their biochemistry performed because they were deemed by their physicians to be at risk for renal dysfunction. This contributed to the higher incidence of acute renal events observed, compared with that of the Nash cohort [5]. On the other hand, it is possible that we excluded very sick patients who died before reaching the emergency department where the kidney function would be routinely assessed, whereas their inclusion would have led to an even higher acute adverse event rate. A sensitivity analysis for the outcome of AKI was conducted to include those who were initially excluded for missing serum potassium levels and found that the factors identified remained consistent with the main analysis. While we attempted to address immortal time bias by defining the cohort entry according to first prescription and the subsequent person-time as exposed, excluding those with NSAID prescription prior to cohort entry, and using a Cox regression model to assess time to event [28], we did not censor for death or loss to follow up since the number of affected patients is presumed to be low for the short follow up. We were also unable to account for confounding by indication, where individuals with certain conditions predisposing them to use of systemic and topical NSAIDs may be at increased risk for acute adverse events, such as hyperuricemic gout and rheumatoid arthritis. The authors acknowledge that the comorbidities and co-prescriptions were not exhaustive, and associations elicited in this observational study do not necessarily indicate causality. The external validation was performed in a relatively small cohort of older adults with systemic NSAIDs > 14 days. While this study focused on acute adverse renal events, there are increasing data that NSAIDs may be associated with progressive CKD [23, 29]. Such information will further add to the risk–benefit discussion when physicians consider prescribing NSAIDs to older adults. This deserves greater study but is beyond the scope of this work. However, this study attempted to address the current knowledge gap by quantifying the risk of NSAID-associated acute nephrotoxicity by NSAID route and duration in multi-ethnic Asians, after considering well established risk factors. We found that systemic NSAIDs > 14 days, systemic NSAIDs prescribed for 1–14 days, and topical NSAIDs increased the risk of acute adverse kidney events among older adults. This study also provided insight into potential limitations of the Nash risk model when it is applied in this population to assess their acute adverse renal events after systemic NSAIDs; hence, further studies are required to improve the available risk model to guide NSAID prescriptions in older adults.
References
Panel AGSBCUE, Fick DM, Semla TP, et al. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–94.
Kuhn-Thiel AM, Weiß C, Wehling M. Consensus validation of the FORTA (Fit fOR The Aged) list: a clinical tool for increasing the appropriateness of pharmacotherapy in the elderly. Drugs Aging. 2014;31(2):131–40.
Zhang X, Donnan PT, Bell S, Guthrie B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol. 2017;18(1):256.
O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–8.
Nash DM, Markle-Reid M, Brimble KS, et al. Nonsteroidal anti-inflammatory drug use and risk of acute kidney injury and hyperkalemia in older adults: a population-based study. Nephrol Dial Transplant. 2019;34(7):1145–54.
Lim CC, Tan NC, Ang A, Quek N, Choo J. To give or not to give: no dearth of explicit guidelines on potentially inappropriate prescribing of non-steroidal anti-inflammatory drugs to older adults. Intern Med J. 2019;49(11):1461–2.
Chou C-I, Shih C-J, Chen Y-T, et al. Adverse effects of oral nonselective and cyclooxygenase-2-selective NSAIDs on hospitalization for acute kidney injury: a nested case–control cohort study. Medicine. 2016;95(9):e2645.
Lim CC, Ang ATW, Kadir HBA, et al. Short-course systemic and topical non-steroidal anti-inflammatory drugs: impact on adverse renal events in older adults with co-morbid disease. Drugs Aging. 2021;38(2):147–56.
Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ (Clinical research ed). 2013;346. https://doi.org/10.1136/bmj.e8525.
Dreischulte T, Morales DR, Bell S, Guthrie B. Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin–angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int. 2015;88(2):396–403.
Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138.
Schneider V, Lévesque LE, Zhang B, Hutchinson T, Brophy JM. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006;164(9):881–9.
Griffin MR, Yared A, Ray WA. Nonsteroidal antiinflammatory drugs and acute renal failure in elderly persons. Am J Epidemiol. 2000;151(5):488–96.
R: A Language and Environment for Statistical Computing [Internet] [computer program]. Vienna: R Foundation for Statistical Computing; 2020.
Lele SR KJ, Solymos P. ResourceSelection: Resource selection (probability) functions for use-availability data [Internet]. 2019.
Robin XTN, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;12(1):1–8.
Austin PC, Steyerberg EW. Graphical assessment of internal and external calibration of logistic regression models by using loess smoothers. Stat Med. 2014;33(3):517–35.
Nishtala PS, Chyou T. Identifying drug combinations associated with acute kidney injury using association rules method. Pharmacoepidemiol Drug Saf. 2020;29(4):467–73.
Solomon DH, Husni ME, Wolski KE, et al. Differences in safety of nonsteroidal antiinflammatory drugs in patients with osteoarthritis and patients with rheumatoid arthritis: a randomized clinical trial. Arthritis Rheumatol (Hoboken, NJ). 2018;70(4):537–46.
Kate RJ, Perez RM, Mazumdar D, Pasupathy KS, Nilakantan V. Prediction and detection models for acute kidney injury in hospitalized older adults. BMC Med Inform Decis Mak. 2016;16(1):39–39.
Bally M, Dendukuri N, Rich B, et al. Risk of acute myocardial infarction with NSAIDs in real world use: bayesian meta-analysis of individual patient data. BMJ (Clinical research ed). 2017;357:j1909.
Horl WH. Nonsteroidal anti-inflammatory drugs and the kidney. Pharmaceuticals (Basel, Switzerland). 2010;3(7):2291–321.
Zhan M, Doerfler RM, Xie D, et al. Association of opioids and nonsteroidal anti-inflammatory drugs with outcomes in CKD: findings from the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2020;76(2):184–93.
Panel BtAGSBCUE. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–94.
Wynants L, van Smeden M, McLernon DJ, et al. Three myths about risk thresholds for prediction models. BMC Med. 2019;17(1):192–192.
Altman RD. New guidelines for topical NSAIDs in the osteoarthritis treatment paradigm. Curr Med Res Opin. 2010;26(12):2871–6.
Zeng C, Wei J, Persson MS, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52(10):642–50.
Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol. 2008;167(4):492–9.
Katsuno T, Togo K, Ebata N, et al. Burden of renal events associated with nonsteroidal anti-inflammatory drugs in patients with osteoarthritis and chronic low back pain: a retrospective database study. Pain Ther. 2021;10(1):443–55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by the SHF-Foundation Research Grant (SHF/HSRHO014/2017).
Conflict of interest
All authors declare no relevant conflict of interest.
Ethics approval
This study was conducted according to the Declaration of Helsinki.
Consent
Waiver of informed consent for the use of de-identified electronic medical record data was approved by the SingHealth Centralized Institutional Board (2018/2567).
Consent for publication
Not applicable.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because data sharing will be subject to institutional approval.
Code availability
Not applicable.
Author contributions
CCL, TNC, and JC conceptualized and designed the study; CL, TNC, HAK, and AA obtained data; CL and ET analyzed and interpreted the data; CL wrote the first draft; all authors contributed to and approved the final version of this manuscript for submission.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, C.C., Tan, N.C., Teo, E.P.S. et al. Non-Steroidal Anti-Inflammatory Drugs and Risk of Acute Kidney Injury and Hyperkalemia in Older Adults: A Retrospective Cohort Study and External Validation of a Clinical Risk Model. Drugs Aging 39, 75–82 (2022). https://doi.org/10.1007/s40266-021-00907-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-021-00907-w