Abstract
Background
Topical non-steroidal anti-inflammatory drugs (NSAIDs) have lower risks for cardiovascular disease and gastrointestinal adverse effects compared to oral NSAIDs, but there are little data regarding their kidney risks in chronic kidney disease (CKD). We evaluated the risk of adverse acute kidney outcomes in CKD according to route of NSAID administration.
Methods
Retrospective cohort study of adults with CKD (eGFR less than 60 ml/min/1.73 m2) who received prescriptions between 2015 and 2017 from a major healthcare cluster in Singapore. The adverse acute kidney outcomes were acute kidney injury (AKI) and need for nephrology specialist consult within 30 days.
Results
Among 6298 adults with CKD (mean age 72.1 ± 13.3 years and eGFR 41.9 ± 12.2 ml/min/1.73 m2), systemic and topical NSAIDs were prescribed in 16.7% and 32.0%, respectively. Incident AKI (any severity), KDIGO Stage 2 or 3 AKI, and need for nephrology specialist consult occurred in 16.7%, 2.6%, and 10.6% of the study cohort, respectively. After adjusting for age, diabetes, recent cardiovascular hospitalization, baseline eGFR, RAAS blocker and diuretic, systemic NSAIDs, and topical NSAIDs, compared with the no-NSAID group, were independently associated with incident AKI [adjusted OR 1.77 (95% CI 1.46–2.15) and 1.38 (1.18–1.63), respectively]. Moderate and severe AKI (adjusted OR 1.68, 95% CI 1.09–2.58, p = 0.02) and need for nephrology consults (adjusted OR 1.41, 95% CI 1.09–1.82, p = 0.008) were also increased in systemic NSAIDs.
Conclusion
Among adults with CKD, both systemic and topical NSAIDs were independently associated with acute adverse kidney outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Analgesics are frequently used in chronic kidney disease (CKD), including non-steroidal anti-inflammatory drugs (NSAIDs) in 12–33% [1,2,3]. Notably, the increasing prevalence of NSAIDs use in CKD has been attributed to limited therapeutic options, lack of evidence for pain management in CKD, and opioid avoidance due to the opioid crisis [2]. In the prospective Chronic Renal Insufficiency Cohort study of 39,339 adults aged 21–74 years with estimated glomerular filtration rates between 20 and 70 ml/min/1.73 m2, nearly a quarter (24%) reported oral NSAID use despite the well-established risk of acute kidney injury (AKI) [4, 5], or “acute-on-chronic kidney injury” in this context. Less is known about the prevalence or effect of topical NSAIDs in CKD. Topical NSAIDs are effective analgesics despite lower bioavailability and maximal plasma concentration. As a result of reduced risk for cardiovascular disease and severe gastrointestinal adverse effects compared to oral NSAIDs [6,7,8], topical NSAIDs are favored in other at-risk individuals such as older adults [9]. Yet, a survey of Australasian kidney and rheumatology specialists found that a fifth would not prescribe topical NSAID in moderately severe kidney impairment [10], possibly due to reports of systemic absorption and AKI with topical NSAIDs [11, 12]. Recently, we had shown that topical NSAIDs’ use in older adults with co-morbid conditions was associated with increased risk of acute adverse kidney events [13], but there is a paucity of data regarding the effect of topical NSAIDs in CKD. Hence, we aimed to evaluate the risk of adverse acute kidney outcomes among individuals with CKD according to route of NSAIDs administration.
Methods
This was a retrospective cohort study of all adults 21 years and older with CKD, defined as CKD-EPI estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2, who received prescriptions between March 2015 and December 2017 from the country’s largest cluster of public healthcare institutions that provide primary and specialist care to nearly a third of the country, including the Singapore General Hospital and seven SingHealth Polyclinics (Bukit Merah, Outram, Marine Parade, Bedok, Tampines, Pasir Ris, and Sengkang).
Cohort and risk factors
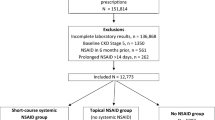
The original dataset was obtained from the Inappropriate nephrotoxic Non-Steroidal Anti-Inflammatory Drug in Diabetes, Elderly and Renal Impairment (INSIDER) study [13], which evaluated potentially inappropriate NSAID prescriptions. NSAID prescriptions [including selective NSAIDs such as cyclooxygenase II (COX II) inhibitors and topical NSAIDs, Supplementary Table S1] were identified from outpatient and discharge electronic pharmacy records. Prescription dates, type, route, dose, and duration of each NSAID were retrieved. We categorized those with oral or parenteral NSAIDs as “systemic” NSAIDs. If there was no systemic NSAID prescription, then individuals were further categorized into topical NSAID and no-NSAID (did not have any systemic or topical NSAID prescription) groups. The cohort entry date for the NSAID groups was the date of first NSAID prescription, while the cohort entry date for the “no NSAID” group was the date of the first prescription during the study period. We excluded individuals with missing serum creatinine within 6 months before cohort entry, since their CKD status could not be ascertained. The study cohort (Fig. 1) excluded those who had (1) prescriptions for NSAID within 60 days prior to cohort entry (n = 1791), so that the NSAID groups comprised incident NSAID users and those in the “no NSAID” group will not have had any recent NSAID prescription, (2) missing serum creatinine and/or potassium values within 30 days after cohort entry (n = 16,701), since the kidney outcomes could not be ascertained, and (3) advanced or severe kidney dysfunction defined as baseline eGFR < 15 ml/min/1.73 m2 (n = 3649), as this group included those on dialysis and serum creatinine fluctuations cannot be interpreted as AKI.
Variables collected included demographic data, co-morbid conditions, and biochemistry (most recent serum creatinine and potassium values within 6 months preceding cohort entry and peak values within 30 days after cohort entry) obtained from electronic medical records from the ambulatory clinics and hospitalizations. Baseline eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation using the most recent serum creatinine value within 6 months preceding cohort entry [14]. CKD was present if eGFR was less than 60 ml/min/1.73 m2. Individuals with diabetes mellitus (DM) were identified from the SingHealth Diabetes Registry, an electronic medical record-based registry which defined DM based on diagnosis codes, drug prescriptions of glucose lowering medications, and laboratory results (e.g., plasma glucose and HbA1c). Recent cardiovascular (CV) hospitalization was defined as hospitalizations for ischemic heart disease or congestive cardiac failure in the preceding 6 months. Renin–angiotensin–aldosterone system (RAAS) blockers (such as angiotensin converting enzyme inhibitors, angiotensin-receptor blockers, and mineralococorticoid receptor antagonists) or thiazide or loop diuretics prescribed within 3 months before and up to 30 days after cohort entry were also recorded as high-risk medications that may result in adverse kidney outcomes when administered concurrently with NSAID [15]. Hospitalization episodes and discharge diagnoses from 6 months before until 30 days after cohort entry were retrieved from electronic medical records.
Outcomes: kidney events
The primary outcome was the incidence of AKI within 30 days. AKI was defined if serum creatinine increased ≥ 26.5 µmol/L or ≥ 50% from baseline, according to the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 criteria [16]. Earlier studies had established that the risk of NSAID-related AKI was highest within 30 days after treatment initiation [17]; thus, a 30-day follow-up was chosen for this study. The other commonly used timeframe of 48 h to define AKI was not feasible in this study based largely on outpatient prescriptions and ambulatory clinic follow-up data, since kidney function tests are less likely to be performed so soon after prescription. The secondary outcomes were (1) KDIGO Stage 2 or 3 AKI, (2) incident hyperkalemia if baseline serum potassium was < 5.5 mmol/L and subsequently increased to ≥ 5.5 mmol/L, and (3) need for either inpatient or outpatient nephrology specialist consult, all within 30 days of cohort entry. We hypothesized that patients with acute adverse kidney events, including those assessed by this study (AKI and hyperkalemia) and those not assessed directly by this study (NSAID-related hypertension or fluid overload), may be referred to the nephrologist, and hence, “need for nephrology consult” would be a surrogate measure of adverse kidney outcome and a pertinent measure of healthcare utilization.
This study was conducted according to the Declaration of Helsinki. Waiver of informed consent for use of de-identified electronic medical record data was approved by the SingHealth Centralized Institutional Review Board (2018/2567).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 25 (IBM Corp., Armonk, New York). Baseline characteristics and outcomes were compared by route of NSAID administration. Categorical variables were presented as proportions and compared using Pearson chi-square test. Continuous variables were summarized as means with standard deviations and compared using one-way ANOVA with Bonferroni correction as appropriate. Binary logistic regression analysis (enter method) was used to obtain odds ratio (OR) and 95% confidence interval (CI) for pre-selected factors associated with the kidney outcome based on available literature [4, 18, 19]. Sensitivity analyses were performed by excluding those with subsequent NSAID prescriptions within 30 days of cohort entry. All tests were two-tailed and statistical significance defined as p < 0.05 unless otherwise specified.
Results
Population
We identified 6298 adults with CKD whose mean age was 72.1 ± 13.3 years and baseline eGFR was 41.9 ± 12.2 ml/min/1.73 m2, respectively. Systemic NSAIDs were prescribed in 1054 individuals (16.7%), among whom 56 (0.9%) had prescriptions for > 14 days and 998 (15.8%) had prescriptions for 1–14 days. Among the systemic NSAID group, 420 patients received another NSAID prescription in the following 30 days: 215 and 175 patients received additional systemic and topical NSAID prescriptions, respectively, while 30 individuals received both. In contrast, 2014 individuals (32.0%) received only topical NSAIDs. Among the topical NSAID group, ketoprofen plaster was most frequently prescribed (71.1%) and 1274 received another topical NSAID prescription within 30 days. Table 1 compares the baseline characteristics by route of first NSAID prescription. The groups prescribed systemic and topical NSAIDs were significantly older, female and more had diabetes compared to no NSAIDs. Recent CV hospitalization, more severe kidney disease with CKD Stage G4, RAAS blockers, and diuretics were significantly less frequent in those prescribed systemic NSAID compared to topical or no NSAIDs.
Kidney events
Table 1 shows that the primary outcome of incident AKI of any severity occurred in 1050 individuals (16.7%) and was significantly less frequent in those with no-NSAID (14.5%) compared to systemic NSAIDs and topical NSAIDs (19.2% and 18.9%, respectively). More severe AKI (KDIGO Stage 2 or 3) was most frequent in the systemic NSAID group, although the differences were not statistically significant. Nephrology consults were significantly more frequent in the topical and systemic NSAID groups.
Uni-variate analysis found that those with incident AKI were more likely to have diabetes, recent CV hospitalization, lower baseline eGFR, NSAID, and diuretic use (Table 2). In the multi-variable model adjusted for age, gender, diabetes, recent CV hospitalization, baseline eGFR, RAAS blocker, and diuretic (Table 2), systemic NSAID and topical NSAID, compared with the no-NSAID group, were independently associated with incident AKI [adjusted OR 1.77 (95% CI 1.46–2.15) and 1.38 (95% CI 1.18–1.63), respectively].
Table 3 shows that systemic NSAIDs were independently associated with moderate–severe AKI (adjusted OR 1.68, 95% CI 1.09–2.58, p = 0.02) and need for nephrology consults (adjusted OR 1.41, 95% CI 1.09–1.82, p = 0.008), whereas topical NSAIDs were independently associated with need for nephrology consults (adjusted OR 1.69, 95% CI 1.39–2.07, p < 0.001). Incident hyperkalemia was not increased by NSAID use. In sensitivity analyses after excluding those with a second NSAID prescription (Supplementary Table S2), both systemic and topical NSAID prescriptions were independently associated with increased risk for AKI and more severe AKI.
Discussion
This study investigated 6298 adults with CKD, among whom 16.7% were prescribed systemic NSAIDs, while 32.0% were prescribed topical NSAIDs. Both systemic NSAIDs and topical NSAIDs were significantly associated with AKI within 30 days after prescription with adjusted ORs of 1.77 and 1.38, respectively. In comparison, a systematic review that included 5 general population studies with 106,681 individuals with CKD noted that current NSAID use was associated with 63% greater risk of AKI (pooled OR 1.63, CI 1.22–2.19) [4]. However, prior studies focused on oral NSAIDs with little data regarding the risk of acute adverse kidney events after topical NSAIDs. This study showed that the topical NSAIDs in CKD, compared to no-NSAID, were independently associated with increased AKI and the need for nephrology consults. Although this study did not evaluate the duration or intensity of topical NSAID use, we had previously proposed that prolonged or high-dose topical NSAIDs may lead to significant systemic absorption and accumulation [11, 13]. NSAIDs reduce kidney prostaglandin synthesis and blood flow [20], with more pronounced adverse effects in the presence of microvascular disease and impaired kidney hemodynamics which characterize CKD. However, the AKI event rate was largely driven by mild AKI as the risk of moderate and severe AKI was low (2–3%) in all the NSAID groups. This may suggest physician discretion in prescribing NSAIDs, as those who received topical NSAIDs were older and more had comorbidities than those prescribed systemic NSAIDs.
The American Society of Nephrology had identified NSAID avoidance to be an area in patient care most amenable to improvement [21]. It is concerning that despite prescribing guidelines from internationally renowned societies [21, 22], NSAID use remained prevalent among susceptible individuals with established CKD [1,2,3, 9]. There is mounting evidence that NSAIDs also increase the risk of progressive kidney disease [23, 24]. In a prospective cohort study of 3939 adults with estimated glomerular filtration rates of 20–70 ml/min/1.73 m2 and followed up for a median of 6.8 years [24], oral NSAIDs were associated with increased risk of the composite outcome of 50% reduction in the estimated glomerular filtration rate and/or kidney failure requiring kidney replacement therapy (hazard ratio 1.2, 95% confidence interval 1.0–1.5). More recently, a retrospective cohort of 180,371 Japanese patients with osteoarthritis or chronic low back pain noted that the risks of incident progressive CKD were similar in topical (patch) and oral NSAIDs [25]. In addition, a global assessment for risk should consider other risk factors, such as concurrent RAAS blockers, diuretics, and cardiovascular disease [15, 18, 26]. Special attention should also be accorded to the latter, since NSAIDs are also associated with CV disease [27], and CV disease is the leading cause of death in CKD [28]. While the additive CV risk incurred by topical and oral NSAID use in individuals with CKD has not yet been evaluated, the composite of CV events (myocardial infarct, unstable angina, heart failure, stroke, and revascularisation) was 1.87 per 100 person-years for topical NSAID compared to 2.14 per 100 person-years for oral NSAID in rheumatoid arthritis [7], such that topical NSAID had 36% lower risk for CV disease compared with oral NSAID (hazard ratio 0.64, 95% confidence interval 0.43–0.95). Since the presence of multiple risk factors may lead to incremental risks, clinical decision support tools such as integrating risk prediction models into electronic medication alerts or having systematic pharmacist intervention programs can guide prescribing for patients with CKD to improve dose and frequency choices [29, 30], while electronic alerts for AKI in the context of nephrotoxin exposure can prompt early recognition and appropriate action to reduce nephrotoxin exposure and possibly reduce AKI [31].
In mild CKD, cautious use of topical (rather than oral) NSAIDs for the shortest duration to avoid systemic accumulation may ameliorate kidney risks [32]. In severe CKD, alternative analgesic strategies such as non-pharmacologic therapy are recommended as initial management [33], but effective pharmacological pain control is complicated as safe alternatives are limited [34]. A prospective cohort of 3939 adults with CKD found that self-reported, time-updated opioid use was associated with a substantial risk for adverse kidney disease outcomes, death, and hospitalization, possibly due to reduced clearance in CKD [24]. Higher doses of gabapentinoids (more than 300 mg gabapentin or more than 75 mg pregabalin daily) were associated with increased risk of hospital visit with encephalopathy, fall or fracture, or hospitalization with respiratory depression in a population-based retrospective cohort study of 74,084 older adults with CKD in Canada [35]. Ultimately, prescribers will need to consider the risks and benefits of the various analgesics according to patients’ risk profiles and preferences for effective pain control and quality of life within an acceptable level of risk and risk modification, such as avoiding concurrent medications that potentiate risks and close surveillance to identify complications early.
This study has several limitations. While it was necessary to exclude those with missing laboratory values for serum creatinine and potassium to accurately define the study outcomes, we may have introduced selection bias, since those included may have had their biochemistry performed, because they were deemed by their physicians to be at-risk for kidney dysfunction. On the other hand, immortal time bias was introduced, since the included cohort was alive until the time of their laboratory tests, while loss to follow up bias may be present if the group who should have, but did not have biochemistry performed after NSAID prescription was significantly different in their risks for the outcome compared to those who did. In determining and classifying exposure to NSAIDs, we acknowledge that NSAID prescriptions may not equate to NSAID use and our study may not capture possible NSAID exposure from over-the-counter purchases or from other healthcare providers such as general practitioners in private practice. While there may be other unaccounted confounders such as conditions that cause hemodynamic instability that predispose to NSAID-induced AKI, we did not include NSAID prescribed during inpatient treatment for acute illnesses, so it was less likely that there was active untreated hypotension or decompensated congestive cardiac failure at the time of NSAID prescription. Instead, the known risk factors for NSAID-induced AKI that had been consistently reported in the literature were included in the multi-variate analyses as potential confounders, so that the regression analyses adjusted for the prognostic imbalance conferred by different prevalence of potential confounders in the systemic NSAID, topical NSAID, and no-NSAID groups in this observational study [36]. The specific indications for prescribing NSAIDs were not available from the de-identified data in this large medical records database study; hence, the analysis could not control for confounding by indication, where conditions that are treated with NSAIDs also predispose to kidney injury and need for nephrology consults (such as gouty arthritis with urate nephropathy) may over-estimate the kidney risks conferred by NSAIDs. It is recognized that the indication for treatment is usually difficult to characterize outside of randomized controlled trials, since prescribing decisions may be driven by multiple considerations, especially for NSAIDs that are used for their broad range of analgesic, anti-pyretic, and anti-inflammatory effects [37]. However, we utilized the “new user” method (excluded those with NSAID prescriptions during the pre-specified washout of 60 days) to reduce survivor bias [38]. While the indication for the nephrology consult was not available, Supplementary Table S3 shows that need for nephrology consult was associated with incident AKI, KDIGO Stage 2 or 3 AKI, and hyperkalemia and need for nephrology consult, thus lending support to its use as a pertinent measure of an adverse kidney outcome requiring healthcare utilization. The associations in this observational study do not necessarily indicate causal relationships. However, this study utilized a large medical records and pharmacy database, and reported findings according to the rigorous STROBE guidelines (Supplementary Table S4). The findings that both topical and systemic NSAIDs may be associated with acute adverse kidney events, albeit with different magnitude and severity, support concerns regarding their prescriptions in patients with impaired kidney function [10,11,12]. Future studies would need to account for the prescribing indication when evaluating the impact of both route and cumulative exposure of NSAIDs on adverse kidney and cardiovascular outcomes in individuals with chronic kidney disease.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available, because data sharing will be subject to institutional approval.
References
Tuttle KR, Alicic RZ, Duru OK et al (2019) Clinical characteristics of and risk factors for chronic kidney disease among adults and children: an analysis of the CURE-CKD registry. JAMA Netw Open 2(12):e1918169–e1918169
Davison SN, Rathwell S, George C, Hussain ST, Grundy K, Dennett L (2020) Analgesic use in patients with advanced chronic kidney disease: a systematic review and meta-analysis. Can J Kidney Health Dis 7:2054358120910329
Lefebvre C, Hindié J, Zappitelli M, Platt RW, Filion KB (2019) Non-steroidal anti-inflammatory drugs in chronic kidney disease: a systematic review of prescription practices and use in primary care. Clin Kidney J 13(1):63–71
Zhang X, Donnan PT, Bell S, Guthrie B (2017) Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol 18(1):256
Orlandi PF, Fujii N, Roy J et al (2018) Hematuria as a risk factor for progression of chronic kidney disease and death: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. BMC Nephrol 19(1):150
Heyneman CA, Lawless-Liday C, Wall GC (2000) Oral versus topical NSAIDs in rheumatic diseases. Drugs 60(3):555–574
Lin TC, Solomon DH, Tedeschi SK, Yoshida K, Kao Yang YH (2017) Comparative risk of cardiovascular outcomes between topical and oral nonselective NSAIDs in Taiwanese patients with rheumatoid arthritis. J Am Heart Assoc. https://doi.org/10.1161/JAHA.117.006874
Makris UE, Kohler MJ, Fraenkel L (2010) Adverse effects of topical nonsteroidal antiinflammatory drugs in older adults with osteoarthritis: a systematic literature review. J Rheumatol 37(6):1236
Lim C, Choo J, Kwek JL, Abdul Kadir H, Tan NC (2021) Non-steroidal anti-inflammatory drug prescription in the young-old and very-old: type, route and burden of cardiovascular risk factors. Nephrol Dialysis Transplant. 36(Supplement_1):gfab092.0065
Terrill M, Soden M, Srivastava V (2020) Survey of Australasian Renal and Rheumatology Specialists investigating topical NSAID use and adverse renal outcomes. Musculoskeletal Care 18(2):134–139
Fernando AH, Thomas S, Temple RM, Lee HA (1994) Renal failure after topical use of NSAIDs. BMJ (Clin Res ed) 308(6927):533–533
Andrews PA, Sampson SA (1999) Topical non-steroidal drugs are systemically absorbed and may cause renal disease. Nephrol Dialysis Transplant 14(1):187–189
Lim CC, Ang ATW, Kadir HBA et al (2020) Short-course systemic and topical non-steroidal anti-inflammatory drugs: impact on adverse renal events in older adults with co-morbid disease. Drugs Aging 38:147–156
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Nash DM, Markle-Reid M, Brimble KS et al (2019) Nonsteroidal anti-inflammatory drug use and risk of acute kidney injury and hyperkalemia in older adults: a population-based study. Nephrol Dialysis Transplant. 34:1145–1154
Kellum JA, Lameire N, Aspelin P et al (2012) Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2(1):1–138
Schneider V, Lévesque LE, Zhang B, Hutchinson T, Brophy JM (2006) Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol 164(9):881–889
Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S (2013) Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ (Clin Res ed). 346:e8525
Chou C-I, Shih C-J, Chen Y-T et al (2016) Adverse effects of oral nonselective and cyclooxygenase-2-selective NSAIDs on hospitalization for acute kidney injury: a nested case–control cohort study. Medicine 95(9):e2645
Horl WH (2010) Nonsteroidal anti-inflammatory drugs and the kidney. Pharmaceuticals (Basel, Switzerland) 3(7):2291–2321
Williams AW, Dwyer AC, Eddy AA et al (2012) Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol CJASN 7(10):1664–1672
Szeto C-C, Sugano K, Wang J-G, et al (2020) Non-steroidal anti-inflammatory drug (NSAID) therapy in patients with hypertension, cardiovascular, renal or gastrointestinal comorbidities: joint APAGE/APLAR/APSDE/APSH/APSN/PoA recommendations. Gut
Ingrasciotta Y, Sultana J, Giorgianni F et al (2015) Association of individual non-steroidal anti-inflammatory drugs and chronic kidney disease: a population-based case control study. PLoS ONE 10(4):e0122899
Zhan M, Doerfler RM, Xie D, et al (2020) Association of opioids and nonsteroidal anti-inflammatory drugs with outcomes in CKD: findings from the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis
Katsuno T, Togo K, Ebata N et al (2021) Burden of renal events associated with nonsteroidal anti-inflammatory drugs in patients with osteoarthritis and chronic low back pain: a retrospective database study. Pain Ther 10:443–455
Dreischulte T, Morales DR, Bell S, Guthrie B (2015) Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin–angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int 88(2):396–403
Schjerning A-M, McGettigan P, Gislason G (2020) Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat Rev Cardiol 17:1–11
Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Nally JV (2015) Cause-specific deaths in non–dialysis-dependent CKD. J Am Soc Nephrol 26(10):2512–2520
Chertow GM, Lee J, Kuperman GJ et al (2001) Guided medication dosing for inpatients with renal insufficiency. JAMA 286(22):2839–2844
Yamamoto T, Nakayama I, Kawakatsu Y et al (2019) Effects of pharmacist participation in chronic kidney disease (CKD) network and CKD manual distribution on drug-related kidney injury. Pharmacoepidemiol Drug Saf 28(6):887–896
Martin M, Wilson FP (2019) Utility of electronic medical record alerts to prevent drug nephrotoxicity. Clin J Am Soc Nephrol 14(1):115–123
Pham PC, Khaing K, Sievers TM et al (2017) 2017 update on pain management in patients with chronic kidney disease. Clin Kidney J 10(5):688–697
Persons O (2009) Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 57(8):1331–1346
Novick TK, Surapaneni A, Shin J-I et al (2018) Prevalence of opioid, gabapentinoid, and NSAID use in patients with CKD. Clin J Am Soc Nephrol 13(12):1886–1888
Muanda FT, Weir MA, Ahmadi F et al (2022) Higher-dose gabapentinoids and the risk of adverse events in older adults with CKD: a population-based cohort study. Am J Kidney Dis 80(1):98-107.e101
Agoritsas T, Merglen A, Shah ND, O’Donnell M, Guyatt GH (2017) Adjusted analyses in studies addressing therapy and harm: users’ guides to the medical literature. JAMA 317(7):748–759
Signorello LB, McLaughlin JK, Lipworth L, Friis S, Sørensen HT, Blot WJ (2002) Confounding by indication in epidemiologic studies of commonly used analgesics. Am J Ther 9(3):199–205
Secrest MH, Platt RW, Dormuth CR et al (2020) Extreme restriction design as a method for reducing confounding by indication in pharmacoepidemiologic research. Pharmacoepidemiol Drug Saf 29:26–34
Acknowledgements
We thank the SingHealth Health Services Research Center for providing de-identification service and the SingHealth Research Office for administrative support.
Funding
This research was supported by the SHF-Foundation Research Grant (SHF/HSRHO014/2017).
Author information
Authors and Affiliations
Contributions
All authors contributed to and approved the final version of this manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no relevant conflict of interest.
Ethics approval
For manuscripts that report the results of a clinical trial, a statement confirms that the study was approved (or granted exemption) by the appropriate institutional and/or national research ethics committee (including the name of the ethics committee) and certifying that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent
Waiver of informed consent for use of de-identified electronic medical record data was approved by the SingHealth Centralized Institutional Review Board (2018/2567).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teo, S.H., Tan, N.C., Choo, J.C.J. et al. Non-steroidal anti-inflammatory drugs in chronic kidney disease and risk of acute adverse kidney events according to route of administration. Int Urol Nephrol 55, 679–686 (2023). https://doi.org/10.1007/s11255-022-03344-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03344-9