Abstract
Donanemab (donanemab-azbt; KisunlaTM) is an amyloid β-directed antibody developed by Eli Lilly and Company for the treatment of Alzheimer’s disease. Donanemab recently received approval in the USA for the treatment of adults with early symptomatic Alzheimer’s disease (patients with mild cognitive impairment or mild dementia stage of disease). This article summarizes the milestones in the development of donanemab leading to this first approval for Alzheimer’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.26409457. |
An amyloid β-directed antibody developed by Eli Lilly and Company for the treatment of Alzheimer’s disease |
Received its first approval on 2 July 2024 in the USA |
Approved for use in patients with early symptomatic Alzheimer’s disease (mild cognitive impairment or in the mild dementia stage of disease) |
1 Introduction
Donanemab (donanemab-azbt; KisunlaTM) is an amyloid β-directed antibody developed by Eli Lilly and Company for the treatment of Alzheimer’s disease. An early event in Alzheimer's disease is the amyloid cascade [1], where the accumulation of amyloid β in the brain into plaques is associated with cognitive and functional impairment [2]. Amyloid β is also linked to tau pathology and clinical progression to dementia [2]; patients with high tau pathology tend to have more neuropathologically advanced disease, which is expected to be more difficult to treat with amyloid β antibodies [1].
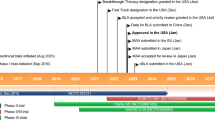
Donanemab received its first approval on 2 July 2024 in the USA for the treatment of early symptomatic Alzheimer’s disease [3]. Treatment should only be initiated in patients with mild cognitive impairment (MCI) or mild dementia stage of disease (the population in which treatment was initiated in the clinical trials) [4]. Any presence of amyloid β pathology must be confirmed prior to starting treatment. The approved dosage regimen is 700 mg administered by intravenous (IV) infusion over ≈ 30 m every 4 weeks for the first three doses, then 1400 mg every 4 weeks [4].
Donanemab has a boxed warning in the USA for amyloid-related imaging abnormalities (ARIA), which can be serious and life-threatening; increased monitoring for ARIA is recommended during the first 24 weeks of treatment [4]. A higher incidence of ARIA, including symptomatic and serious ARIA, is typically seen in patients who are apolipoprotein E (ApoE) ε4 homozygotes versus heterozygotes and non-carriers; ApoE ε4 status should be tested prior to initiation of treatment with donanemab, and the benefits of treatment weighed against the risk of developing ARIA. Patients may also experience hypersensitivity and infusion-related reactions (IRRs). In the event of an IRR, the infusion rate may be reduced or the infusion discontinued, and symptoms managed accordingly. Pre-treatment with antihistamines, acetaminophen or corticosteroids may be required prior to their next infusion [4].

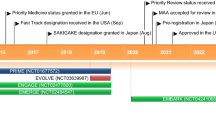
Key milestones in the development of donanemab. AD Alzheimer's disease, pts patients
2 Scientific Summary
2.1 Pharmacodynamics
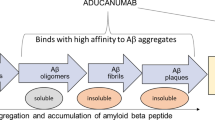
Donanemab, a humanized immunoglobulin γ1 (IgG1) monoclonal antibody, is expressed in a Chinese hamster ovary cell line and attaches to insoluble N-truncated pyroglutamate amyloid β (present only in amyloid β plaques formed in the brain [1]) [4]. Once attached, donanemab then aids in the removal of the plaque through microglial-mediated phagocytosis [1]. However, unlike other antibodies that target amyloid β, donanemab only binds to established plaque and not to the soluble form of amyloid β [5]. The molecular weight of donanemab is ≈ 145 kDa [4].
In clinical pharmacology studies, donanemab reduced amyloid β plaque levels in the brain in a dose- and time-dependent manner from week 12 of treatment onwards [4]. In an analysis of a phase Ib trial (NCT02624778) in patients with MCI or mild-to-moderate dementia due to Alzheimer’s disease and the phase II TRAILBLAZER-ALZ trial (Sect. 2.3.2), sustaining a donanemab serum concentration of ≥ 4.43 μg/mL was associated with reduced amyloid β plaque formation [5]. It was also noted that baseline amyloid β levels impacted the time taken for amyloid β plaque clearance (defined as < 24.1 Centiloids); fewer patients with higher baseline levels achieved amyloid β plaque clearance [5].
In the phase III TRAILBLAZER-ALZ 2 trial (Sect. 2.3.1.1), amyloid β levels were significantly (p < 0.0001) reduced in donanemab versus placebo recipients from week 24 through to week 76 of treatment, as evaluated using amyloid Positron Emission Tomography (PET) imaging (18F-florbetapir tracer) [1, 4]. Treatment with donanemab also resulted in reduced levels of plasma p-tau217 compared with placebo in this trial [1, 4].
The immunogenicity of donanemab was evaluated in 792 patients from TRAILBLAZER-ALZ 2; 87% of patients developed anti-drug antibodies (ADAs) following up to 18 months of treatment [4]. Of these patients, all tested positive for neutralizing antibodies against donanemab. Although patients who had higher titres of ADAs displayed less reduction in amyloid β plaque than patients with lower ADA titres, the efficacy of donanemab was not significantly affected by ADA presence during the study treatment period. However, the presence of ADAs in donanemab recipients was associated with an increased incidence of IRRs compared with placebo recipients (≈ 10% vs 2% of patients in TRAILBLAZER-ALZ 2) [4].
2.2 Pharmacokinetics
Donanemab exposures increased proportionally in a population pharmacokinetic analysis of 2131 patients with Alzheimer's disease who received single (10–40 mg/kg) or multiple (10–20 mg/kg) doses of donanemab [4]. Steady state was reached after a single dose of donanemab, and accumulation (< 1.3-fold) occurred with once every 4 weekly dosing. The central volume of distribution for donanemab is 3.36 L. Donanemab is expected to be degraded by proteolytic enzymes, similarly to endogenous IgG, and thus is not expected to be renally eliminated or metabolised by hepatic enzymes. The mean terminal half-life and clearance of donanemab are ≈ 12.1 days and 0.0255 L/h, respectively [4].
The pharmacokinetics of donanemab are not affected to any clinically significant degree by patient age, sex, race or weight [4]. The presence of ADAs in donanemab recipients was associated with increased clearance and lower mean serum trough concentrations of donanemab [4].
Features and properties of donanemab
Alternative names | Donanemab-azbt injection—Eli Lilly and Company; Kisunla; LY 3002813; LY-002813-SC; LY-3002813-IV; LY3002813-SC; N3pG-Ab MAb—Eli Lilly and Company; N3pG-AB-monoclonal antibody—Eli Lilly and Company; N3pG-amyloid-β monoclonal antibody—Eli Lilly and Company |
Class | Antidementias; monoclonal antibodies; amyloid-targeting treatment |
Mechanism of action | Pyroglutamyl(3)-amyloid β-protein (3-42) inhibitor |
Route of administration | Intravenous |
Pharmacodynamics | Aggregation of insoluble N-truncated pyroglutamate amyloid β into amyloid β plaques is a defining feature of Alzheimer’s disease; donanemab reduces amyloid β plaque levels in the brain in a dose- and time-dependent manner, slowing disease progression |
Pharmacokinetics | Steady state reached after a single dose; central volume of distribution 3.36 L; mean terminal half-life ≈ 12.1 days; clearance 0.0255 L/h |
Adverse events | |
Most frequent (≥ 5% and ≥ 2% higher incidence than placebo) | ARIA-H and microhaemorrhage, ARIA-E, ARIA-H and superficial siderosis, headache, infusion-related reaction |
Boxed warning in the USA | ARIA |
ATC codes | |
WHO ATC code | N06D-X05 (Donanemab) |
EphMRA ATC code | N7D9 (All other anti-Alzheimer products) |
2.3 Therapeutic Trials
2.3.1 Phase III trials
2.3.1.1 TRAILBLAZER-ALZ 2
Donanemab was more effective than placebo in reducing cognitive and functional decline in the multicentre, randomized, double-blind, parallel, placebo-controlled, phase III TRAILBLAZER-ALZ 2 trial (NCT04437511) in patients with early symptomatic Alzheimer's disease (n = 1736) [1]. Patients were randomized to receive IV donanemab (700 mg for the first three doses, then 1400 mg for subsequent doses) or placebo once every 4 weeks for up to 72 weeks, and stratified according to baseline tau category [low/medium tau or combined tau (low/medium and high)] and enrolment site. Following the double-blind period, patients were able to enter a 78-week extension period, for a maximum of 150 weeks of treatment. Donanemab recipients who qualified for dose-reduction criteria (evaluated using amyloid β plaque level on PET scans) at week 72 were switched to placebo in a blinded procedure [1].
Key clinical trials of donanemab in patients with Alzheimer’s disease (sponsored by Eli Lilly and Company)
Drug (s) | Phase | Status | Location(s) | Identifiers |
|---|---|---|---|---|
Donanemab | III | Active, no longer recruiting | Global | NCT04437511, EudraCT2020-000077-25, TRAILBLAZER-ALZ 2 |
Donanemab | III | Recruiting | Japan, Puerto Rico, USA | NCT05026866, TRAILBLAZER-ALZ 3 |
Donanemab | III | Recruiting | Global | NCT05508789, EudraCT2021-006395-17, TRAILBLAZER-ALZ 5 |
Donanemab | III | Recruiting | England, USA | NCT05738486, EudraCT2022-502268-18-00, TRAILBLAZER-ALZ 6 |
Donanemab | III | Completed | USA | NCT05108922, TRAILBLAZER-ALZ 4 |
Donanemab | II | Completed | Canada, USA | NCT04640077, TRAILBLAZER-EXT |
Donanemab, LY 3202626 | II | Completed | Canada, USA | NCT03367403, TRAILBLAZER-ALZ |
The least-squares mean (LSM) change in the Integrated Alzheimer's Disease Rating Scale (iADRS; range 0–144, where a lower score indicates greater cognitive and functional impairment) score from baseline to 76 weeks (primary efficacy endpoint) were significantly lower in donanemab than placebo recipients in both the low/medium tau and combined tau populations [1]. In the low/medium tau population, the LSM change was − 6.02 in donanemab recipients and − 9.27 in placebo recipients [between-group difference (BGD) 3.25; p < 0.001]; in the combined population, the LSM change was − 10.19 and − 13.11, respectively (BGD 2.92; p < 0.001). This corresponds to a 35.1% and 22.3% slowing of disease progression. Sensitivity analyses of the iADRS score confirm a 33.4–39.6% slowing of clinical progression [1].
Subgroup analyses of the iADRs score generally favoured donanemab across the baseline characteristics evaluated (age, sex, race, ethnicity, clinical stage of disease, ApoE ε4 genotype, medication use, body mass index and tau PET category), although subgroups with a smaller number of patients were more likely to have a lower bound of the 95% confidence interval that was below zero [1]. Post hoc analysis of the high tau population (n = 552) showed the LSM change in iADRS score in donanemab recipients was not statistically significantly different compared with placebo recipients at week 76 (BGD 1.26; p = 0.42) [1].
A subpopulation analysis of Japanese patients (n = 88) in the study showed a smaller LSM change in iADRS score from baseline to week 76 in donanemab than placebo recipients [6]. In the low/medium tau population (when evaluated using the natural cubic spline model with two degrees of freedom), the BGD was 3.99, corresponding to a 40.2% slowing of disease progression; in the combined population, the BGD was 4.43, corresponding to a 38.8% slowing of disease progression. Results were consistent when evaluated using a mixed model for repeat measures [6].
Statistically significant differences were seen in most of the 24 gated outcomes evaluated, including the secondary outcome of change in the Clinical Dementia Rating scale Sum of Boxes (CDR-SB; range 0–18, where a higher score indicates greater impairment) [1]. In the low/medium tau population, the LSM change in CDR-SB from baseline to week 76 was 1.20 and 1.88 in donanemab and placebo recipients (BGD – 0.67; p < 0.001); in the combined tau population, the LSM change was 1.72 and 2.42 (BGD – 0.70; p < 0.001), respectively [1].
Eligible patients in TRAILBLAZER-ALZ 2 were aged 60–85 years with early symptomatic Alzheimer's disease (MCI or Alzheimer’s disease with mild dementia); Mini-Mental State Examination score 20–28; amyloid pathology (≥ 37 Centiloids) assessed with 18F-florbetapir13 or 18F-florbetaben14 PET; and presence of tau pathology assessed by 18F-flortaucipir PET imaging with central image evaluation [1]. Patients were excluded from the study if they had ARIA of oedema/effusion (ARIA-E), > 4 cerebral microhaemorrhages, > 1 area of superficial siderosis, and any intracerebral haemorrhage > 1 cm or severe white matter disease on magnetic resonance imaging. Baseline patient demographics and disease characteristics were generally similar between donanemab and placebo recipients in the low/medium tau population and similarly between the two treatment groups in the combined tau population [1].
2.3.1.2 TRAILBLAZER-ALZ 4
Following 6 months of treatment in the randomized, open-label, parallel-group, phase III TRAILBLAZER-ALZ 4 trial (NCT05108922) in patients with early symptomatic Alzheimer’s disease (n = 148), the amyloid β plaque clearance rate (≤ 24.1 Centiloids; primary endpoint) was significantly (p < 0.001) higher in donanemab (37.9% of patients; n = 71) than aducanumab recipients (1.6%; n = 69) [7]. Consistent with the initial analysis, significantly more donanemab than aducanumab recipients achieved amyloid β plaque clearance at 12 months of treatment (70.2% of and 21.9% of patients) [8]. In a subpopulation analysis of patients with low/medium tau (n = 27 and 28 in donanemab and aducanumab recipients), amyloid β plaque clearance was achieved in significantly (p < 0.01) more donanemab than aducanumab recipients; 38.5% and 3.8% at 6 months [7], and 80.0% and 9.6% at 12 months of treatment [8].
Patients were randomized to receive IV donanemab (700 mg once every 4 weeks for the first three doses, then 1400 mg for subsequent doses; n = 71) or IV aducanumab (1 mg/kg once every 4 weeks for two doses, then 3 mg/kg for two doses, then 6 mg/kg for two doses, then 10 mg/kg for subsequent doses; n = 69) for up to 18 months of treatment [7]. Baseline patient demographics and disease characteristics were generally similar between donanemab and aducanumab recipients [7].
2.3.2 Phase II trial
2.3.2.1 TRAILBLAZER-ALZ
Treatment with donanemab resulted in significantly less cognitive and functional decline, as evaluated using the primary endpoint of change from baseline in iADRS score at week 76 (− 6.86 in donanemab recipients vs − 10.06 in placebo recipients; BGD 3.20; p = 0.04), in the randomized, double-blind, placebo-controlled, multicentre, phase II TRAILBLAZER-ALZ trial (NCT03367403) in patients with early symptomatic Alzheimer’s disease (n = 272) [2]. Patients received IV donanemab (700 mg for the first three doses then 1400 mg thereafter) or placebo once every 4 weeks for up to 72 weeks. At weeks 24 and 52 patients were switched to either a lower dose of donanemab or placebo, dependent on florbetapir scan results. At week 24, amyloid β clearance (< 24.1 Centiloids) was achieved in 40% vs 0% of donanemab and placebo recipients, respectively [2].
Eligible patients were aged 60–85 years with early symptomatic Alzheimer’s disease (prodromal Alzheimer’s disease or mild Alzheimer’s disease with dementia) with tau and amyloid β deposition, with a tau standardized uptake value ratio between 1.10 and 1.46 (patients with a ratio < 1.10 with a topographic deposition pattern consistent with advanced Alzheimer’s disease were also included) [2]. Baseline patient and disease characteristics were generally similar between donanemab and placebo recipients; the mean iADRS scores at baseline were 106.2 and 105.9, respectively [2].
2.4 Adverse Events
The safety and tolerability of IV donanemab were evaluated in 2885 patients with Alzheimer’s disease who received donanemab in clinical trials; the most common (incidence > 5% of patients in either group) adverse reactions included ARIA with hemosiderin deposition (ARIA-H) and microhaemorrhage (25% of donanemab recipients vs 11% of placebo recipients), ARIA-E (24% vs 2%), ARIA-H and superficial siderosis (15% vs 3%), headache (13% vs 10%) and IRRs (9% vs 0.5%) [4]. Treatment was discontinued due to an adverse reaction in 13% vs 4% of patients, respectively; this was most commonly due to IRRs (4% vs 0%) [4].
In the TRAILBLAZER-ALZ 2 trial, treatment-emergent adverse events (AEs) occurred in 89.0% and 82.2% of donanemab and placebo recipients, respectively; these were most commonly (> 10% of patients in either group) ARIA-E (24.0% vs 1.9%), ARIA-H (19.7% vs 7.4%), Covid-19 infection (15.9% vs 17.6%), headache (14.0% vs 9.8%) and falls (13.4% vs 12.6%) [1]. Serious AEs occurred in 17.4% and 15.8% of patients. AEs led to treatment discontinuation in 13.1% and 4.3% of patients, most commonly due to IRRs, ARIA-E, ARIA-H and hypersensitivity. AEs led to withdrawal from the study in 8.1% and 3.7% of patients. Death was reported in 1.9% and 1.1% of patients in each group; three deaths (0.4%) in the donanemab group and one death (0.1%) in the placebo group were considered related to treatment [1].
ARIA (including ARIA-E and ARIA-H) developed in 36.8% and 14.9% of donanemab and placebo recipients, respectively, in TRAILBLAZER-ALZ 2 [1]. ARIA-E occurred in 24.0% and 2.1% of patients, which were mostly mild-to-moderate in severity, and was serious in 1.5% of donanemab recipients. ARIA-E was symptomatic in 6.1% of patients, and most (86.5%) cases resolved. Patients who were ApoE ε4 carriers had a numerically higher risk of developing ARIA-E than non-carriers, as with patients who were homozygotes rather than heterozygotes. ARIA-H and microhaemorrhages developed in 31.4% and 13.6% of donanemab and placebo recipients. The proportion of patients with ARIA-H with microhaemorrhages and no ARIA-E was similar between the two groups (12.7% vs 12.4%) [1].
IRRs occurred in 8.7% and 0.5% of donanemab and placebo recipients, respectively [1]. Most were mild-to-moderate in severity, occurred within 30 m of the end of an infusion, and developed most frequently between the second to fifth infusions. Serious IRRs and anaphylactic reactions (mild-to-moderate) were reported in donanemab recipients only (both 0.4% of patients) [1].
In TRAILBLZER-ALZ 4, AEs were reported in 78.9% and 82.6% of donanemab and aducanumab recipients, respectively [8]. ARIA developed in 29.6% and 40.6% of patients, and was serious in 1.4% and 2.9% of patients. A higher incidence of ARIA was seen in patients who were ApoE ε4 carriers [8].
2.5 Ongoing Clinical Trials
The efficacy and tolerability of donanemab are being investigated in several ongoing randomized, double-blind, placebo-controlled, parallel-group, phase III clinical trials: TRAILBLAZER-ALZ 2, NCT05026866 (TRAILBLAZER-ALZ 3), NCT05508789 (TRAILBLAZER-ALZ 5) and NCT05738486 (TRAILBLAZER-ALZ 6).
3 Current Status
Donanemab received its first approval on 2 July 2024 in the USA for Alzheimer’s disease in patients with mild cognitive impairment or mild dementia stage of disease [3].
References
Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512–27.
Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691–704.
Eli Lilly and Company. Lilly's Kisunla™ (donanemab-azbt) approved by the FDA for the treatment of early symptomatic Alzheimer's disease [media release]. 2 Jul 2024. https://investor.lilly.com/news-releases/news-release-details/lillys-kisunlatm-donanemab-azbt-approved-fda-treatment-early.
Eli Lilly and Company. KISUNLA (donanemab-azbt) injection, for intravenous use: US prescribing information. 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761248s000lbl.pdf. Accessed 15 Aug 2024.
Gueorguieva I, Willis BA, Chua L, et al. Donanemab population pharmacokinetics, amyloid plaque reduction, and safety in participants with Alzheimer’s disease. Clin Pharmacol Ther. 2023;113(6):1258–67.
Sato S, Hatakeyama N, Fujikoshi S, et al. Donanemab in Japanese patients with early Alzheimer’s disease: subpopulation analysis of the TRAILBLAZER-ALZ 2 randomized trial. Neurol Ther. 2024;13(3):677–95.
Salloway S, Lee E, Papka M, et al. TRAILBLAZER-ALZ 4: topline study results directly comparing donanemab to aducanumab on amyloid lowering in early, symptomatic Alzheimer’s disease [abstract]. BJPsych Open. 2023;9(Suppl. 1):S67.
Pain A, Ferguson MB, Wang H, et al. TRAILBLAZER-ALZ 4: directly comparing donanemab to aducanumab on amyloid lowering in early, symptomatic Alzheimer’s disease - results from 12-months [abstract]. Alzheimer’s Dement. 2023;19(Suppl. 24): e082529.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Connie Kang is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, C. Donanemab: First Approval. Drugs (2024). https://doi.org/10.1007/s40265-024-02087-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s40265-024-02087-4