Abstract
Elranatamab (elranatamab-bcmm; ELREXFIO™) is a bispecific B-cell maturation antigen (BCMA)-directed CD3 T cell engager being developed by Pfizer for the treatment of multiple myeloma (MM). Elranatamab bridges CD3 on T cells with BCMA expressed on multiple myeloma cells, thereby activating T cells to induce T cell-mediated cytotoxicity against myeloma cells. In August 2023, elranatamab received its first approval in the USA for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who have received at least four prior lines of therapy including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody. Elranatamab received accelerated approval for this indication based on response rate and durability of response, and continued approval may be contingent on the demonstration of clinical benefit in a confirmatory trial(s). Elranatamab has also received a positive opinion in the EU for RRMM and is under regulatory review in Japan and several other countries worldwide. Clinical studies of elranatamab are also underway in countries around the world. This article summarizes the milestones in the development of elranatamab leading to this first approval for the treatment of RRMM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.24201675. |
A bispecific BCMA-directed CD3 T cell engager is being developed by Pfizer for the treatment of MM |
Received its first approval on 14 August in the USA |
Approved for the treatment of adult patients with RRMM who have received at least four prior lines of therapy including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody |
1 Introduction
The treatment landscape of multiple myeloma has evolved significantly with the development of novel therapies such as chimeric antigen receptor (CAR) T cell therapy and bispecific antibodies. Bispecific antibodies are recombinant antibodies that combine two different epitopes of antigens or two different antigens [1]. They have the advantage of being directly available for use, are generally associated with lower rates of cytokine release syndrome (CRS) and neurotoxicity than with CAR T cell therapy, and treatment can be stopped immediately if toxicity does occur, allowing for their use in older patients and in patients with more advanced disease [1]. Several bispecific antibody formats have been developed, including bispecific T cell engagers that bridge T cells with tumour cells, resulting in T cell activation and T cell-mediated killing of tumour cells [2, 3].

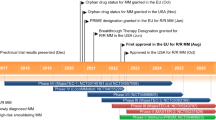
Key milestones in the development of elranatamab for the treatment of multiple myeloma. BLA biologics license application, MAA marketing authorisation application, NDA new drug application
Elranatamab (elranatamab-bcmm; ELREXFIO™) is one such bispecific B-cell maturation antigen (BCMA)-directed CD3+ T cell engager antibody that targets the human T cell surface antigen CD3 and human BCMA expressed on multiple myeloma cells, as well as plasma cells and plasmablasts. In August 2023 [4], elranatamab received its first approval in the USA for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who have received at least four prior lines of therapy including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody [5]. Elranatamab received accelerated approval for this indication based on response rate and durability of response, and continued approval may be contingent on demonstration of clinical benefit in a confirmatory trial(s) [5].
Elranatamab is administered subcutaneously with a step-up dosing schedule: 12 mg step-up dose 1 to be administered on day 1, 32 mg step-up dose 2 on day 4, followed by the first treatment dose of 76 mg on day 8, and 76 mg weekly thereafter through week 24 [5]. Patients who have received ≥ 24 weeks of elranatamab treatment and have achieved a response (partial or better) and maintained the response for ≥ 2 months, can be transitioned to a 2-week schedule, with treatment continued until disease progression or unacceptable toxicity. Recommended pretreatment medications [oral acetaminophen (or equivalent) 650 mg, oral or intravenous dexamethasone (or equivalent) 20 mg, oral diphenhydramine (or equivalent) 25 mg)] should be administered ≈ 1 h prior to dose 1, dose 2 and the first treatment dose. Dosage delays may be required to manage adverse events (AEs) associated with elranatamab; dosage reductions of elranatamab are not recommended. The US prescribing information for elranatamab carries a boxed warning regarding the risk of CRS and neurologic toxicity, including immune effector cell-associated neurotoxicity syndrome (ICANS), with elranatamab therapy [5].
Elranatamab received a positive opinion in the EU on 12 October 2023 for RRMM [6], and is under regulatory review for RRMM in Japan and several other countries worldwide. Clinical studies of elranatamab are also underway in countries around the world.
2 Scientific Summary
2.1 Pharmacodynamics
Elranatamab comprises an anti-BCMA monoclonal antibody domain and an anti-CD3 monoclonal antibody domain, each contributing a heavy and a light chain [5]. Elranatamab at dosages of ≥ 30 µg/kg (≥ 0.03 times the approved dose) led to transient increase in interleukin (IL)-2, IL-6, IL-8, IL-10, tumour necrosis factor-α, and interferon-γ levels [5]. Highest cytokine levels were generally seen within 72 h after administration of the day 1 dose of elranatamab 12 mg, with cytokine levels reducing to almost baseline levels before administration of the first full dose of elranatamab 76 mg [5]. In part 1 of the phase 1 MagnetisMM-1 study (Sect. 2.3.2), anti-myeloma activity was observed with elranatamab doses of 215–1000 µg/kg weekly [7]. At the recommended phase 2 dose (RP2D) of 1000 µg/kg every two weeks, elranatamab exposure levels achieved were associated with anti-myeloma activity [7]. T cell proliferation in peripheral blood was also increased with elranatamab therapy; disease response was associated with a decrease in soluble BCMA levels [7,8,9].
Genomic analysis of bone marrow aspirate samples collected at screening from patients in the pivotal MagnetisMM-3 study who were naïve to BCMA-targeted therapy (cohort A; Sect. 2.3.1) found that higher expression of TNFRSF17 (the gene encoding for BCMA) was associated with increased tumour burden, which may lead to poorer elranatamab response [10]. There was correlation between increasing TNFRSF17 expression levels and factors such as advancing disease stage (p = 0.014), presence of high-risk cytogenetics (p = 0.002) and plasma cell load (p < 10−10). Non-response to elranatamab correlated with increased plasma cells (p = 0.03), macrophages and monocytes in bone marrow aspirates, while response to elranatamab was most likely to be observed in patients who had both low plasma and low myeloid cells. Elranatamab non-response also correlated with amplification of the TNFRSF17 gene locus (p = 0.008), and chromosomal alterations associated with (e.g. 1q21+) and not associated with (e.g. 17q21+ and 6p21+) high-risk multiple myeloma [10].
2.2 Pharmacokinetics
The pharmacokinetics of subcutaneous elranatamab 6–76 mg (0.079 to 1 times the approved dose) increased in a dose proportional manner [5, 7]. A pooled analysis of four studies (MagnetisMM-1, -2, -3 and -9) correlated maximum elranatamab serum concentration at 24 h with the probability of all grade or grade ≥ 2 CRS and found that a two-dose step-up priming dosing regimen of elranatamab (12 mg on day 1, 32 mg on day 4 and first full dose of 76 mg on day 8) with premedications (Sect. 1) was optimal, based on the incidence, predictable timing and the manageable profile of CRS [11]. This regimen was subsequently approved for use in the USA for patients with RRMM.
After subcutaneous administration of elranatamab, the median time to peak concentration (Tmax) was 7 days (range 3–7) [5, 7], mean bioavailability was 56.2% and the volume of distribution at steady state was 7.76 L [5]. Catabolic pathways are thought to be involved in the metabolism of elranatamab into small peptides [5]. After administration of elranatamab 76 mg weekly, the half-life of elranatamab was 22 days, and clearance following 24 weeks of treatment was 0.324 L/day [5].
The pharmacokinetics of elranatamab were not affected by age (range 36 to 89 years), sex, race (White, Asian, or Black), body weight (range 37 to 160 kg), mild or moderate renal impairment [estimated glomerular filtration rate (eGFR) 30–89 mL/min] or mild hepatic impairment [total bilirubin 1 to ≤1.5 times the upper limit of normal (ULN) or any aspartate transaminase (AST) levels greater than ULN] [5]. The pharmacokinetics of elranatamab in patients with severe renal impairment (eGFR 15–29 mL/min), end-stage renal disease (eGFR <15 mL/min) or moderate to severe hepatic impairment (total bilirubin > 1.5 times the ULN and any AST level) have not been assessed. It is unknown if anti-drug antibodies (ADA) affect the pharmacokinetics, pharmacodynamics, safety or efficacy of elranatamab. In the pivotal MagnetisMM-3 study in patients with RRMM (Sect. 2.3.1), ADA were detected in 8.9% of the 168 patients who had received elranatamab at the approved dosage and schedule for up to 24 months. Among the ADA-positive patients, neutralizing antibodies were detected in 60% of patients (9/15) [5].
Features and properties of elranatamab
Alternative names | Elranatamab-bcmm; ELREXFIO; PF-06863135; PF‑3135; RN-613 |
Class | Antineoplastics; bispecific antibodies; immunotherapies |
Mechanism of action | Bispecific BCMA-directed T cell engager bridges CD3-expressing T-cells and BCMA-expressing myeloma cells, resulting in the activation of T cells and induction of T cell-mediated cytotoxicity against myeloma cells |
Route of administration | Subcutaneous |
Pharmacodynamics | Demonstrated anti-myeloma activity in patients with relapsed or refractory multiple myeloma |
Associated with transient elevation in IL-2, IL-6, IL-8, IL-10, tumour necrosis factor-α, and interferon-γ levels | |
Increased T cell proliferation in peripheral blood and decreased soluble BCMA levels | |
Pharmacokinetics | Median time to peak concentration 7 days (range 3–7) |
Mean bioavailability 56.2%; steady state volume of distribution 7.76 L | |
Half-life after 76 mg weekly dosage 22 days, and clearance after 24 weeks therapy 0.324 L/day | |
Most common adverse reactions | |
Nonhaematologic | CRS, fatigue, injection-site reaction, diarrhoea, upper respiratory tract infection, musculoskeletal pain, pneumonia |
Serious | Pneumonia, sepsis, CRS, URTI, acute kidney injury, UTI, COVID-19, encephalopathy, pyrexia, febrile neutropenia |
ATC codes | |
WHO ATC code | L01F-X (Other monoclonal antibodies and antibody drug conjugates) |
EphMRA ATC code | L1G (Monoclonal antibody antineoplastics) |
2.3 Therapeutic Trials
2.3.1 MagnetisMM-3 Phase 2 Trial
Elranatamab was associated with deep and durable responses in patients with RRMM participating in the ongoing, registrational, open-label, multicentre, phase 2 MagnetisMM-3 trial (NCT04649359) [12]. Eligible patients had disease refractory to at least one proteasome inhibitor, one immunomodulatory drug and one anti-CD38 antibody and had measurable disease as defined by International Myeloma Working Group (IMWG) criteria. Patients were also required to have an Eastern Cooperative Oncology Group performance status of ≤ 2 and adequate baseline bone marrow (absolute neutrophil count ≥ 1.0 × 109/L, platelet count ≥ 25 × 109/L, haemoglobin level ≥ 8 g/dL), renal (creatinine clearance ≥ 30 mL/min), and hepatic (AST and alanine aminotransferase ≤ 2.5 times the ULN, total bilirubin ≤ 2 times ULN) function [12]. The study included 123 patients who had not been previously treated with BCMA-directed therapies (pivotal cohort A) and 64 patients with prior exposure to BCMA-directed therapies, antibody drug conjugate (ADC) and CAR-T cell therapy (cohort B) [5]. All but four patients received the elranatamab as per the approved dosing schedule [5, 12]. The primary endpoint was objective response rate (ORR), defined as partial response or better as assessed by blinded independent central review (BICR) per IMWG criteria [12].
Patients in cohort A had a median age of 68 years and had received a median of 5 prior lines of therapy [12]. After a median follow-up of 14.7 months (data cutoff 14 March 2023), the confirmed ORR was 61% [15.4% stringent complete response (sCR), 19.5% complete response (CR), 21.1% very good partial response (VGPR) and 4.9% PR], indicating that the primary endpoint was met. Response rates were generally consistent across subgroups based on demographics and disease characteristics, including in patients with ≥ 50% bone marrow plasma cells at baseline and in those with high-risk cytogenetics. Among patients evaluable for minimal residual disease (MRD) and who had achieved CR or better, MRD negativity (sensitivity of 1 × 10−5 by next generation sequencing from bone marrow aspirates) was achieved by 89.7% of the patients. Median progression-free survival (PFS) and median overall survival (OS) were not reached and the 15-month PFS and OS rates were 50.9% and 56.7%, respectively. Among responders in cohort A (n = 75), the median time to first response (TTR) was 1.2 months and median duration of response (DOR) was not reached. The Kaplan-Meier-estimated probability of maintaining response at 15 months was 71.5% and responses improved over time [12].
Of the 123 patients in cohort A, 97 (median age 69 years) had received ≥ 4 prior lines of therapy [5]. The median time to first response in these patients was 1.22 months, the ORR was 57.7% (25.8% CR or better, 25.8% VGPR, 6.2% PR) and the median DOR was not reached. After a median follow-up of 11.1 months in responders, the 6- and 9-month DOR rates were 90.4% and 82.3%, respectively [5].
In cohort B, 63 of 64 patients had received ≥ 4 prior lines of therapy, including 73% of patients with prior exposure to BCMA-directed antibody drug conjugate (ADC) and 32% of patients who had previously received CAR T cell therapy [5]. After a median follow-up of 10.2 months, the confirmed ORR in these patients was 33.3%, the median DOR was not reached and the 9-month DOR rate was 84.3% [5].
2.3.2 MagnetisMM-1 Phase 1 Trial
Elranatamab provided durable clinical responses in patients with RRMM in the ongoing, first-in-human, phase 1, MagnetisMM-1 trial (NCT03269136) [7]. Patients received elranatamab 80–1000 µg/kg weekly or every 2 weeks and a subset of pts received a single priming dose (600 μg/kg or 44 mg equivalent) followed 1 week later by the RP2D of 1000 μg/kg or 76 mg equivalent [7]. As of 30 September 2022, 55 patients had received elranatamab at a dose of ≥ 215 µg/kg (the minimum effective dose) [7]. Patients had a median age of 64 years and a median of five previous regimens, with 24% of patients having received prior BCMA-targeted therapy. After a median follow-up of 12.0 months, objective responses were achieved in 64% of patients, including VGPR or better in 56% of patients and CR or better in 38% of patients. In patients who had previously received BCMA-targeted therapy (n = 13), the ORR was 54%, with 46% of patients achieving VGPR or better. MRD negativity was 100% in MRD evaluable patients (i.e. patients with confirmed CR or better and a dominant clone sequence at baseline); two patients had ongoing sCR after 2 years of treatment. The median DOR was 17.1 months, median PFS was 11.8 months and the median OS was 21.2 months [7].
Key clinical trials of elranatamab in patients with multiple myeloma
Drug(s) | Phase | Status | Location(s) | Identifier | Sponsor |
|---|---|---|---|---|---|
Elranatamab, daratumumab, pomalidomide, dexamethasone | 3 | Recruiting | Multinational | MagnetisMM-5; NCT05020236; C1071005; 2021-000044-22 | Pfizer |
Elranatamab, daratumumab, lenalidomide, dexamethasone | 3 | Recruiting | Multinational | MagnetisMM-6; NCT05623020; C1071006; 2021-000803-20 | Pfizer |
Elranatamab, lenalidomide | 3 | Recruiting | Multinational | MagnetisMM-7; NCT05317416; C1071007; 2021-006052-14 | Pfizer |
Elranatamab | 2 | Active not recruiting | Multinational | MagnetisMM-3; NCT04649359; C1071003; 2020-004533-21 | Pfizer |
Elranatamab, nirogacestat, lenalidomide, dexamethasone | 1/2 | Recruiting | Canada, USA | MagnetisMM-4; NCT05090566; C1071004; 2021-003885-11 | Pfizer |
Elranatamab | 1/2 | Active not recruiting | China | MagnetisMM-8; NCT05228470; C1071008 | Pfizer |
Elranatamab, dexamethasone | 1/2 | Active, not recruiting | Japan, Taiwan, UK, USA | MagnetisMM-9; NCT05014412; C1071009 | Pfizer |
Elranatamab, carfilzomib, maplirpacept | 1b | Recruiting | USA | MagnetisMM-20; NCT05675449; C1071020 | Pfizer |
Elranatamab, cevostamab, tocilizumab | 1b | Recruiting | Australia, Republic of Korea | NCT05927571; GO43979; 2022-501724-15-00 | Genentech |
Elranatamab | 1 | Active not recruiting | Japan | MagnetismMM-2; NCT04798586; C1071002 | Pfizer |
Elranatamab, dexamethasone, lenalidomide, pomalidomide | 1 | Active not recruiting | Canada, USA | MagnetismMM-1; NCT03269136; C1071001; 2019-000822-24 | Pfizer |
Elranatamab | Expanded access | Available | USA, Canada | MagnetismMM-17; NCT05462639 | Pfizer |
Elranatamab | Expanded access | Available | Not available | NCT05238311; C107 | Pfizer |
Elranatamab, standard of care | Observational | Not yet recruiting | USA | MagnetisMM-31; NCT05932290 | Pfizer |
Elranatamab, standard of care | Observational | Completed | USA | MagnetisMM-24; NCT05565391; C1071024 | Pfizer |
2.3.3 Pooled Analysis of Patients with Prior BCMA Exposure
Elranatamab also demonstrated efficacy in patients with RRMM and prior exposure to BCMA-targeted therapies (ADC and CAR-T cell therapy) in a pooled analysis of data from patients in the MagnetisMM-1 (n = 13), MagnetisMM-3 (n = 64) and the phase 1/2 MagnetisMM-9 (NCT05014412; n = 9) trials [13]. Patients from MagnetisMM-1 received elranatamab 215–1000 µg/kg or the RP2D (Sect. 2.3.2) and patients from MagnetisMM-3 and MagnetisMM-9 received the approved elranatamab dose at the recommended dosing schedule. The median age of patients was 66 years and patients had received a median of seven prior lines of therapy, including 67.4% of patients who had prior BCMA-targeted ADC, 41.9% with previous CAR T cell therapy and 9.3% who had both BCMA ADC and CAR T cell therapies. After a median follow-up of 10.3 months, 45.3% of patients achieved objective responses, including CR or better in 17.4% of patients. Among patients previously treated with BCMA-targeted ADC, 42.4% had objective responses and in those previously treated with CAR T cell therapy, 52.8% had objective responses. The median DOR was not reached and the 9-month median DOR rate was 57.5% (67.3% and 78.9% in patients with prior BCMA-targeted ADC and CAR T cell therapy, respectively). In the overall population, the median PFS was 4.8 months and the median OS was not reached; the 9-month OS rate was 60.1% [13].
2.4 Adverse Events
Elranatamab had a manageable tolerability profile in patients with RRMM who were participating in the phase 2 MagnetisMM-3 study. As of 14 March 2023, 48.0% of the BCMA-naïve patients (cohort A) had received elranatamab for ≥ 6 months, 35.8% for ≥ 12 months and 33.3% of patients remained on elranatamab therapy. The median treatment duration was 5.6 months. Treatment-emergent AEs occurred in all patients receiving elranatamab, including grade 3 or 4 events in 70.7% of patients. The most common (incidence ≥ 30%) any-grade nonhaematologic treatment-emergent adverse events (AEs) with elranatamab were CRS (58%), diarrhoea (42%), fatigue (37%), decreased appetite (33%) and pyrexia (30%) [12]. The most common grade 3 or 4 treatment-emergent haematologic AEs with elranatamab were neutropenia (49%), anaemia (37%), lymphopenia (25%) and thrombocytopenia (24%). Infections occurred in 69.9% of patients, including grade 3 or 4 infections in 39.8% of patients and fatal infections in 6.5% of patients. The most common infections were coronavirus disease 2019-related occurring in 29.3% of patients. Peripheral neuropathy was defined as motor dysfunction and sensory neuropathy, which occurred in 17.1% and 13.8% of patients, respectively. ICANS was reported in four (3.4%) of the 119 patients who received the approved dose and schedule of elranatamab in cohort A [12].
Treatment-emergent AEs resulted in elranatamab dose reductions in 28.5% of patients, with the most common (incidence ≥ 15%) reasons being haematologic AEs (17.1%), including neutropenia (15.4%) [12]. Treatment-emergent AEs resulted in elranatamab dose interruptions in 77.2% of patients, with the most common (incidence ≥ 20%) reasons for interruptions being infections in 50.4% of patients (most common COVID-19-related in 25.2%) and haematologic AEs in 40.7% (most-common neutropenia in 35.0%) of patients [12].
Elranatamab also demonstrated a manageable tolerability profile in a safety analysis of all patients (BCMA-naïve and -experienced) who received the approved dosage of elranatamab in the MagnetisMM-3 study (n = 183) [5]. Of the 183 patients who received elranatamab at the approved dosage and recommended schedule (safety population), 42% of patients had received the drug for ≥ 6 months and 9% for ≥ 1 year [5]. The most common any-grade nonhaematologic adverse reactions (incidence >30%) with elranatamab were CRS (58%), fatigue (43%), injection site reaction (37%), diarrhoea (36%), upper respiratory tract infection (URTI; 34%), musculoskeletal pain (34%) and pneumonia (32%). The most common (incidence >3%) grade 3 or 4 nonhaematologic adverse reactions with elranatamab were pneumonia (19%), sepsis (11%), URTI (4.9%) and urinary tract infection (UTI; 4.4%). In terms of grade 3 or 4 laboratory abnormalities, decreased lymphocytes (84%), decreased neutrophils (51%), decreased haemoglobin (43%), decreased white blood cells (40%), and decreased platelets (32%) occurred most frequently (incidence >30%) in patients receiving elranatamab [5].
Among patients receiving elranatamab, 68% reported serious adverse reactions, with the most common adverse reactions (incidence > 2%) being pneumonia (25%), sepsis (13%), CRS (13%), URTI (4.4%), acute kidney injury (3.8%), UTI (3.3%), COVID-19 (3.3%), encephalopathy (3.3%), pyrexia (2.2%), and febrile neutropenia (2.2%) [5]. Other clinically relevant adverse reactions with elranatamab reported in < 10% of patients included ICANS (3.3% of patients receiving the approved schedule), febrile neutropenia (2.2%) and Guillain-Barré syndrome (0.5%). Adverse reactions resulted in fatalities in 10% of patients, with pneumonia (3.3%), sepsis (2.7%) and acute respiratory distress syndrome being the most common reasons. Adverse reactions led to treatment discontinuation in 17% of patients (most commonly because of septic shock in 2.2% of patients) and dosage interruptions in 73% of patients (most common reasons included neutropenia, pneumonia, COVID-19 and URTI) [5].
In a pooled analysis of patients with RRMM and prior exposure to BCMA-targeted therapies from the MagnetisMM-1 (n = 13), MagnetisMM-3 (n = 64) and MagnetisMM-9 (n = 9) trials, the most common treatment-emergent AEs were CRS (65%; 1.2% grade 3), anaemia (59%; 46.5% grade 3 or 4), neutropenia (44%; 41% grade 3 or 4), thrombocytopenia (41%; 29% grade 3 or 4), diarrhoea (34%; no grade 3 or 4 AEs) and lymphopenia (33%; 30% grade 3 or 4) [13]. ICANS occurred in 6% (2% grade 3 AEs) of patients [13].
2.5 Ongoing Clinical Trials
In addition to the ongoing phase 2 MagnetisMM-3 and phase 1 MagnetisMM-1 trials (Sect. 2.3), several trials are ongoing, including the randomized, open-label, two-part, phase 3 MagnetisMM-5 trial (NCT05020236) is recruiting patients to evaluate the efficacy and safety of elranatamab monotherapy or elranatamab plus daratumumab combination therapy versus daratumumab plus pomalidomide and dexamethasone in ≈ 854 patients with RRMM [14]. Recruitment is also underway for the randomized, open-label phase 3 MagnetisMM-6 trial (NCT05623020) that will evaluate the efficacy and safety of elranatamab plus daratumumab and lenalidomide versus daratumumab plus lenalidomide and dexamethasone in ≈ 966 patients with newly diagnosed multiple myeloma who are not candidates for transplant. The randomized, open-label phase 3 MagnetisMM-7 trial (NCT05317416) is recruiting patients to evaluate the efficacy and safety of elranatamab versus lenalidomide in ≈ 760 patients with newly diagnosed multiple myeloma after undergoing autologous stem-cell transplantation [15, 16].
The non-randomized, open-label, phase 1/2 MagnetisMM-4 umbrella study (NCT05090566) is recruiting patients to determine the recommended phase 2 dose and clinical benefit of elranatamab in combination with other anti-cancer therapies in ≈ 105 patients with multiple myeloma [17]. The open-label, two-part, phase 1/2 MagnetisMM-9 study (NCT05014412) is underway to evaluate an alternative two-step dosing regimen and longer dosing intervals of elranatamab in ≈ 76 patients with RRMM [18, 19]. Also underway is the open-label, phase 1/2 MagnetisMM-8 study (NCT05228470) that will evaluate the safety, pharmacokinetics and efficacy of elranatamab in 39 Chinese patients with RRMM.
In addition, an observational study (MagnetisMM-31; NCT05932290) is planned that will compare the effectiveness of elranatamab in MagnetisMM-3 versus standard-of-care in the real world.
3 Current Status
Elranatamab received its first approval on 14 August 2023 in the USA [4] for the treatment of adult patients with RRMM who have received ≥ 4 prior lines of therapy including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody [5]. Elranatamab received accelerated approval for this indication based on response rate and durability of response, and continued approval may be contingent on demonstration of clinical benefit in a confirmatory trial(s) [5]. Elranatamab received a positive opinion in the EU on 12 October 2023 for RRMM [6], and is under regulatory review in Japan and several other countries worldwide.
References
van de Donk NWCJ, Zweegman S. T-cell-engaging bispecific antibodies in cancer. Lancet. 2023;402(10396):142–58.
Wang Q, Chen Y, Park J, et al. Design and production of bispecific antibodies. Antibodies (Basel). 2019;8(3):43.
Wei J, Yang Y, Wang G, Liu M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front Immunol. 2022;13:1035276.
US FDA. BLA accelerated approval (elranatamab-bcmm). 2023. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/761345Orig1s000ltr.pdf. Accessed 28 Aug 2023.
Pfizer Inc. ELREXFIOTM (elranatamab-bcmm): US prescribing information. 2023. https://labeling.pfizer.com/ShowLabeling.aspx?id=19669. Accessed 28 Aug 2023.
European Medicines Agency. CHMP summary of positive opinion for Elrexfio (elranatamab). 2023. https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-elrexfio_en.pdf. Accessed 18 Oct 2023.
Bahlis NJ, Costello CL, Raje NS, et al. Elranatamab in relapsed or refractory multiple myeloma: the MagnetisMM-1 phase 1 trial. Nat Med. 2023. https://doi.org/10.1038/s41591-023-02589-w.
Dalovisio A, Bahlis N, Raje N, et al. Updated results from the ongoing phase 1 study of elranatamab, a BCMA targered T-cell redirecting immunotherapy, for patients with relapsed or refractory multiple myeloma [abstract no. P897]. HemaSphere. 2022;6(S3):1509–10.
Raje N, Bahlis NJ, Costello C, et al. Elranatamab, a BCMA targeted T-cell engaging bspecific antibody, induces durable clinical and molecular responses for patients with relapsed or refractory multiple myeloma [abstract]. Blood. 2022;140(Suppl 1):388–90.
Rodriguez-Otero P, Quach H, Weisel K, et al. Genomic analysis to identify determinants of inherent response and resistance to elranatamab in MagnetisMM-3 cohort A [abstract no. P906]. HemaSphere. 2023;7(S3):1709–10.
Elmeliegy M, Viqueira A, Vandendries E, et al. Dose optimization to mitigate the risk of CRS with elranatamab in multiple myeloma [abstract]. Blood. 2022;140(Supplement 1):7174–5.
Lesokhin AM, Tomasson MH, Arnulf B, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023. https://doi.org/10.1038/s41591-023-02528-9.
Manier S, Lesokhin A, Mohty M, et al. Efficacy and safety of elranatamab in patients with relapsed/refractory multiple myeloma and prior B-cell maturation antigen (BCMA)-directed therapies: a pooled analysis from MagnetisMM studies [abstract no. P870 plus poster]. HemaSphere. 2023;7(S3):1633–34.
Leleu X, Iida S, Landgren CO, et al. Elranatamab monotherapy or in combination with daratumumab vs daratumumab + pomalidomide + dexamethasone for patients with relapsed/refractory multiple myeloma: phase 3 MagnetisMM-5 Study, Part 2 [abstract no. P930]. HemaSphere. 2023;7(S3):1758.
Manteca MVM, Grosicki S, Kim K. Magnetismm-7: an open-label, multicenter, randomized phase 3 study of elranatamab versus lenalidomide in post-transplant patients with newly dagnosed multiple myeloma [abstract no. PB2131]. HemaSphere. 2023;7(S3):4084.
Manteca MVM, Grosicki S, Kim K. MagnetisMM-7: an open-label, multicenter, randomized phase 3 study of elranatamab versus lenalidomide in post-transplant patients with newly diagnosed multiple myeloma [abstract no. TPS8066]. J Clin Oncol. 2023;41(16 Suppl).
Landgren O, Kazandjian D, O’Connell A, et al. Magnetismm-4: an open label, phase 1b/2 umbrella study of elranatamab in combination with other anti-cancer treatments for patients with multiple myeloma. Blood. 2022;140(Suppl 1):10172–3.
Fonseca R, Kuroda J, Ishida T, et al. MagnetisMM-9: an open-label, multicenter, non-randomized phase 1/2 study of elranatamab in patients with relapsed/refractory multiple myeloma [abstract no. TPS8068]. J Clin Oncol. 2022;40(16 Suppl).
Fonseca R, Kuroda J, Ishida T, et al. MafnetisMM-9: an open-label, multicenter, non-randomized phase 1/2 study of elranatamab in patients with relapsed/refractory multiple myeloma [abstract no. PB2032]. HemaSphere. 2022;6(S3):3518.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sohita Dhillon is a contracted employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhillon, S. Elranatamab: First Approval. Drugs 83, 1621–1627 (2023). https://doi.org/10.1007/s40265-023-01954-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01954-w