Abstract
Background
Recognition and management of adverse events (AEs) associated with immune checkpoint inhibitor (ICI) use by cancer patients requires expertise from multiple disciplines. Greater awareness of potential AEs may result in earlier recognition, appropriate management, and better patient outcomes.
Objective
The primary objective of this overview of systematic reviews was to synthesize and consolidate systematic review evidence describing the incidence proportion and severity of AEs associated with various ICI therapies across different cancers.
Methods
A systematic literature search of four databases was conducted to identify systematic reviews that describe the incidence proportion and severity of AEs related to ICI therapy in cancer patients. A systematic review was eligible if it included adults with cancer; on ICI alone or in combination with another ICI, chemotherapy, or targeted therapy; severity (graded according to the Common Terminology Criteria for Adverse Events) and incidence proportion of AEs and whether it reported its eligibility criteria. AEs of interest were identified through an iterative ranking exercise by key stakeholders and knowledge users. Extraction of PICOTTS elements and quality indicators (AMSTAR-2) were used to manage overlap of primary studies across systematic reviews at the outcome level. Cancer subtypes were mapped to drug class and AE severity.
Results
Overall, 129 systematic reviews met the inclusion criteria for data mapping. Systematic reviews reported incidence proportions for more than 76 AEs, of which 34 were identified as AEs of interest. After overlap assessment, 65 systematic reviews were chosen for data extraction. The three AEs with the highest median incidence were fatigue (18.3%, interquartile range [IQR] 15.0–28.0%), diarrhea (15.3%, IQR 9.7–29.2%) and rash (14.4%, IQR 10.3–19.2%). The three AEs (high-grade) with the highest median incidence were diarrhea (1.5%, IQR 1.2–6.0%), colitis (1.3%, IQR 0.6–6.1%) and neutropenia (1.2%, IQR 0.4–3.3%). Incidence proportions of high-grade AEs were often considerably lower than all-grade AEs and combination therapy (ICI combinations or combinations of ICI with chemotherapy or targeted therapy) was responsible for some of the highest incidence proportions regardless of AE. Rare AEs and certain cancer subtypes were not well reported.
Conclusions
Early recognition of AEs associated with ICIs requires expertise from diverse specialists, not just oncologists. Greater awareness of potential AEs may result in earlier recognition, appropriate management, and better patient outcomes.
PROSPERO Registration
CRD42021231593.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Incidence proportions were determined for 34 different adverse events (AEs) to immune checkpoint inhibitors (ICIs) when used for cancer treatment, from 65 systematic reviews. |
The most common all-grade AEs were fatigue (18.3%), diarrhea (15.3%) and rash (14.4%). |
The incidence proportions of high-grade AEs was almost always lower than all-grade events. |
Combinations of ICIs or ICI with chemotherapy were responsible for most upper limit outliers. |
Greater awareness may result in earlier recognition, appropriate management, and better patient outcomes. |
1 Introduction
A growing number of oncology patients are being treated with immunotherapy, a type of biologic therapy against cancer [1]. The use of immunotherapy is part of a shift away from traditional cytotoxic chemotherapy [2]. Immune checkpoint inhibitors (ICIs) are novel immunotherapy agents that use monoclonal antibodies to block the negative regulators of T cells [3, 4]. Regulatory agencies have approved several ICIs to treat a variety of different cancers, including programmed cell death protein 1 inhibitors (anti-PD-1; pembrolizumab, nivolumab, cemiplimab, camrelizumab), programmed death-ligand 1 inhibitors (anti-PD-L1; atezolizumab, durvalumab, avelumab) and anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4; ipilimumab) [5].
Although this class of medication is effective in enhancing the body’s immune system to target tumor cells, it can also lead to immune- or treatment-related toxicities that can affect multiple organ systems [4]. This significantly changes the type, frequency and severity of adverse events (AEs) associated with cancer treatment compared with cytotoxic therapies or molecularly targeted agents [6]. The Common Terminology Criteria for Adverse Events (CTCAE) provides standardized definitions for the categorization of AEs associated with these and other drugs as well as defined levels of severity ranging from one to five [4, 7]. The incidence of AEs varies by agent, exposure time, dose, tumor histology and patient population [8, 9].
Understanding ICI-related AEs is important for oncologists and non-oncology clinicians, given the growing use of ICIs and the multidisciplinary approach to the diagnosis and management of serious AEs. While the toxicity profile of these drugs may be familiar to oncologists, other healthcare providers may not be as familiar with these toxicities. A recent survey suggests that even medical oncologists experience some discomfort managing ICI-related AEs and rely on a multidisciplinary team approach for management [10]. Literature scoping exercises performed by our group identified an overwhelming number of systematic reviews that describe the incidence of ICI-related AEs, however there are no current, high-quality, comprehensive syntheses of AE incidence, and severity data that map different types of cancers and ICIs were found.
Overviews of systematic reviews use explicit methods to identify relevant systematic reviews, and extract and synthesize relevant systematic review-level data to address a research question. Overviews are a particularly useful method to use for research questions related to AEs, as in this case where many systematic reviews exist that focus on different AEs across differing populations (i.e., different cancer subtypes) with different interventions (i.e., different ICIs and their combinations) [11]. This overview of systematic reviews synthesizes and consolidates the current evidence on risk of AEs associated with ICI therapy across cancer indications in the hope of providing relevant and important data for oncology and non-oncology clinicians to increase earlier recognition and appropriate management of AEs.
2 Methodology
The primary objective of this overview of systematic reviews was to describe, map and assess the quality of systematic review evidence reporting AE rates and their severity with the use of ICIs grouped by class (ICI alone, ICI combinations, ICI with chemotherapy) across different cancer subtypes. The protocol was written in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) checklist along with guidance from Lunny et al. and Gates et al., and was registered in PROSPERO (CRD42021231593) [12,13,14].
2.1 Systematic Review Eligibility Criteria
A systematic review was included if it met the following criteria: (1) the population of interest was adult patients with cancer; (2) the intervention was any ICI alone or in combination with another ICI, chemotherapy or targeted therapy; (3) incidence proportions of any AEs are reported (in aggregate, meta-analyzed, or could be calculated from raw numbers) for those receiving ICI therapy; (4) AE severity is reported using the CTCAE framework; and (5) systematic review authors reported their eligibility criteria.
While systematic reviews of case reports and case series focusing on specific rare AEs were excluded from data mapping and extraction of incidence proportions, they were retained and described, as we anticipated that rare AEs would not be well represented in other systematic reviews.
2.2 Literature Search and Citation Screening
The initial search strategy was developed by an experienced medical information specialist and was peer-reviewed using the Peer-Review of Electronic Search Strategies (PRESS) guideline [15]. We searched OVID Medline®, Embase®, the International Health Technology Assessment Database and the Cochrane Database of Systematic Reviews from January 2011 to January 2021, and then updated the search again in September 2021 (Online Resource 1). The search date was limited to 2011 onwards, as the first ICI, ipilimumab, was approved for use in the US in 2011. To supplement the search, reference lists of included systematic reviews were manually searched for additional relevant systematic reviews. Grey literature searching was limited to Google Scholar’s first 10 pages [16]. There was no language restriction to the search. After deduplication, search results were imported into COVIDENCE® (Veritas Health Innovation, Melbourne, VIC Australia; http://www.covidence.org), a systematic review management software program. Study selection was conducted in two stages, in duplicate, by three reviewers (SM, KA, PT) and discrepancies were resolved by another reviewer (SK). In the first stage, potentially relevant systematic reviews were identified from title and abstract review, after which eligible systematic reviews were confirmed from full-text review in the second stage.
2.3 Data Mapping and Extraction
The data mapping went through several stages. First, all systematic reviews with AE incidence proportion and severity data were mapped across all included systematic reviews in Excel® (version 16.54).
Second, a ranking process with 12 experts in the field was conducted. AEs reported in three or more systematic reviews were listed and shared with local knowledge users, content experts and key stakeholders (five medical oncologists, three oncology pharmacists, two intensive care physicians, one endocrinologist, one internal medicine specialist). These experts were purposefully selected from our institution based on their expertise. Two rounds of an iterative ranking process were used to systematically select AEs of interest to be included for data extraction. In the first round, all experts were given a list of all AEs reported in three or more eligible systematic reviews and were asked to identify AEs of clinical importance and rank the top 10. AEs identified by more than one expert were included as AEs of interest, as were all AEs ranked in the top 10 regardless of confirmation. The second round underwent the same process after disclosing the findings of the first round to all experts. The ranking process identified 34 AEs of interest from 76 unique AEs reported in systematic reviews. These 34 AEs of interest, from 12 CTCAE categories, included anemia, thrombocytopenia, neutropenia, hypothyroid, hyperthyroid, hypophysitis, adrenal insufficiency, hyperglycemia, diabetes, thyroiditis, hypopituitarism, diarrhea, colitis, nausea/vomiting, hepatitis, pancreatitis, increased ALT, increased AST, increased bilirubin, increased lipase, rash, pruritis, vitiligo, arthralgia, myalgia, myositis, arthritis, pneumonitis, acute kidney injury, nephritis, peripheral neuropathy, uveitis, fatigue and pyrexia.
Third, for each selected AE of interest, the incidence proportion and 95% confidence interval (CI) was collected for each class or combination of drugs (PD-1/PD-L1, CTLA4, PD-1/PD-L1 and CTLA4, any ICI and cytotoxic chemotherapy or targeted therapy), for all cancers and the predetermined cancer subtypes (melanoma, lung cancer, renal cell cancer, urothelial cancer, digestive system cancer [including esophageal, gastric, colorectal and hepatobiliary cancers], head and neck cancer, gynecological cancer and lymphoma) and separately for all severities (CTCAE 1–5) and high severity (CTCAE 3 or higher). For simplicity, all incidence proportion data extracted were rounded to one decimal place when possible. Data were extracted in Microsoft Excel® (version 16.54; Microsoft Corporation, Redmond, WA, USA) by one reviewer and verified by another.
2.4 Approach to Managing Overlap in Primary Studies Across Systematic Reviews
‘Overlap’ describes the scenario where multiple included systematic reviews contain same primary study data for the same comparison and outcome [17]. Using primary study results multiple times in the same analysis overstates its sample size and number of events, falsely leading to greater precision in the analysis [18]. This may impact both narrative description of the results or a statistical synthesis (e.g., including the results from a primary study twice in the same meta-analysis). Significant overlap in primary study AE data between systematic reviews was anticipated since selected AEs were reported in at least three systematic reviews.
Overlap in primary study AE data was assessed and managed for each combination of AE, cancer type and severity at the outcome level. The systematic review with the greatest relevance to our research objectives and highest quality, as per AMSTAR-2, was selected for data extraction when overlap in primary study AE data for the same outcome (AE, severity, cancer subtype and ICI class or combination) was identified [19, 20]. Relevance of systematic reviews was determined using the following criteria in order of importance: (1) relevance of the systematic review’s research question to our own overview objectives; (2) publication recency; (3) number of included trials and patients enrolled; (4) availability of both high-grade and all-grade severity; (5) meta-analysed or weighted incidence proportions preferred over aggregate data. We assessed systematic review relevance first, and when two or more systematic reviews were deemed equally relevant, the higher quality (per AMSTAR-2) was chosen.
2.5 Quality Assessment of Included Systematic Reviews
The methodological quality of the systematic reviews was assessed using AMSTAR-2 by one study member and verified by another. Individual items in the AMSTAR-2 tool were tabulated, described, and integrated into our results and conclusions. Overall quality ratings (high, moderate, low, and critically low) were determined using critical domains and the method described by Shea et al. [19]. The methodological quality of individual trials within the included systematic reviews was not assessed.
2.6 Reporting of Findings
Reporting of findings followed the Preferred Reporting Items for Overviews of Systematic Reviews Including Harms (PRIO-HARMS) checklist [21] [Online Resource 1, eTable 1]. For each AE, the incidence proportions were extracted by AE severity, drug class or combination and cancer subtype. When only raw numbers (i.e., cases of AEs and total number of patients at risk are reported but incidence proportions are not calculated) were available, incidence proportions and 95% CIs were calculated in aggregate (i.e., without meta-analysis). In cases where two systematic reviews were identified as the best available evidence with no overlap of primary studies, and raw numbers were available from both reviews, we calculated and reported the aggregate. Incidence proportions of AE for all cancers are reported in tabular form as well as in forest plots and box and whisker plots for comparison by cancer subtype, AE severity and anticancer therapy used. Within each AE and severity grouping (all-grade or high-grade), median incidence proportions and interquartile ranges (IQRs) were calculated across values extracted for different cancer subtypes and drug classes or combinations, and reflected in box and whisker plots, while forest plots were report for all data points without measures of central tendency. When medians and IQRs were calculated, minimum and maximum values are exclusive of outliers, where outliers are defined as data points outside of the interval: Q1 − (1.5 × IQR) to Q3 + (1.5 × IQR). It should be noted that in this context, outliers may identify high- or low-risk groups for each AE defined by their cancer, treatment or both.
With the goal of broader readability for multiple audiences, we have utilized focused appendices for descriptive tables to provide detailed results at the outcome level, organized by AE. The “Results” and “Discussion” sections within this manuscript are limited to a higher-level summary of findings.
3 Results
3.1 Search Results, Data Mapping and Overlap Assessment
Our search identified 2255 unique records, 652 of which met the eligibility criteria at the title/abstract stage and went on to the full-text selection stage. After inspection of full texts, 129 systematic reviews were eligible for inclusion (Online Resource 1, eFig. 1). Data mapping exercises revealed that incidence proportions of 76 unique AEs were reported across the 129 eligible systematic reviews. Most systematic reviews did not attempt to differentiate between immune-mediated and treatment-related AEs.
Following the management of primary study overlap, 65 of 129 systematic reviews were selected for data extraction [9, 22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85]. Generally, systematic reviews assessed either a variety of AEs in a specific cancer or a specific type of AE across a variety of cancers. The 65 included systematic reviews varied by population, intervention, and outcome (Table 1). Characteristics of eligible systematic reviews that were not chosen for data extraction (n = 64) and systematic reviews of case reports (n = 21) are provided in Online Resource 1, eTables 2 and 3. Chosen systematic reviews were generally of critically low (20/65), low (12/65) or moderate quality (29/65), as assessed using AMSTAR-2 (Online Resource 1, eTables 4 and 5).
3.2 Incidence Proportions of Immune Checkpoint Inhibitor (ICI)-Related Adverse Events (AEs)
Incidence proportions for each individual AE were reported by as many as 74 (diarrhea; from which 14 non-overlapping systematic reviews were chosen) and as few as 3 (uveitis; from which all 3 were chosen) unique but overlapping systematic reviews. Across all cancer subtypes and ICI classes and combinations, the three AEs (all-grade) with the highest median incidence proportion were fatigue (18.3%, IQR 15.0–28.0%), diarrhea (15.3%, IQR 9.7–29.2%) and rash (14.4%, IQR 10.3–19.2%). Across all cancer subtypes and ICI classes and combinations, the three AEs (high-grade) with the highest median incidence proportions were diarrhea (1.5%, IQR 1.2–6.0%), colitis (1.3%, IQR 0.6–6.1%) and neutropenia (1.2%, IQR 0.4–3.3%). A more detailed description (and graphical depiction with forest plots) of the incidence proportion of each AE by drug class/combination and between cancers is provided in Online Resource 2.
Briefly, among blood and lymphatic system all-grade AEs (anemia, thrombocytopenia, neutropenia), anemia (median 5.5%, IQR 3.8–9.2%) was most common. For high-grade AEs in this group, neutropenia was most common (median 1.2%, IQR 0.4–3.3%). Among endocrine AEs (hypothyroidism, hyperthyroidism, hypophysitis, adrenal insufficiency, hyperglycemia, diabetes, thyroiditis, hypopituitarism), hypothyroid was the most common all-grade AE (median 7.7%, IQR 4.6–10.9%), while hypopituitarism was the most common high-grade AE (median 1.0%, IQR 0.6–1.3%). Among gastrointestinal and hepatobiliary AEs (diarrhea, colitis, nausea/vomiting, hepatitis, pancreatitis, increased ALT/AST/bilirubin/lipase), diarrhea was the most common all-grade AE (median 15.3%, IQR 9.7–29.2%) and high-grade AE (median 1.5%, IQR 1.2–6.0%). Among skin and subcutaneous tissue AEs (rash, pruritis, vitiligo), rash was the most common all-grade AE (median 14.4%, IQR 10.3–19.2%) and high-grade AE (median 0.8%, IQR 0.5–0.8%). Among musculoskeletal and connective tissue AEs (arthralgia, myalgia, myositis, arthritis), arthralgia was the most common all-grade AE (median 6.3%, IQR 5.0–10.6%) and high-grade AE (median 0.2%, IQR 0.0–0.4%). Among respiratory, renal, nervous system and ocular AEs (pneumonitis, acute kidney injury, nephritis, peripheral neuropathy, uveitis), pneumonitis was the most common all-grade AE (median 3.7%, IQR 2.3–6.3%), while acute kidney injury was the most common high-grade AE (median 1.1%, IQR 0.6–1.3%). Among general AEs (pyrexia and fatigue), fatigue was the most common all-grade AE (median 18.3%, IQR 15.0–28.0%) and high-grade AE (median 1.1%, IQR 0.9–2.0%). Acknowledging that median incidence proportion rates represent a wide range of cancer subtypes and therapeutic combinations, more granular data are also provided, by cancer subtype and therapeutic regimens (i.e., monotherapy, combination therapy), in Online Resource 2. Readers with specific queries (i.e., what is the incidence proportion of high-grade colitis in melanoma with CTLA4 monotherapy?) will find more granular descriptions of incidence proportions here.
Within each AE grouping, incidence proportions were highly variable between ICI class or combination, and between cancer subtypes with no obviously notable pattern other than in every instance the incidence proportion of high-grade AEs was lower than the all-grade incidence proportion; a summary of the incidence proportion of all selected AEs with ICI treatments of all cancers is presented in Fig. 1 and again graphically in Online Resource 1, eFig. 2. Median incidence proportions across cancer subtypes and ICI groups are presented in Fig. 2a, b, and Online Resource 1, eTables 7a and 7b. Maximum outliers were identified for 22 of 34 AEs (Fig. 2a, b, and Online Resource 1, eTables 7a and 7b). Outliers were most often attributed to combinations of PD-1/PD-L1 and CTLA-4 inhibitors (47%) followed by combinations of any ICI with chemotherapy (26%). Maximum outliers in blood and lymphatic system AEs (anemia, thrombocytopenia, and neutropenia) were all attributed to combinations of ICI and chemotherapy. Maximum outliers were distributed across most cancer subtypes, with melanoma being the most common (42%).
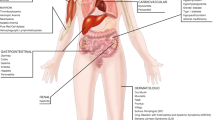
Incidence proportions and severity of adverse events associated with ICI use in all cancers. ICI immune checkpoint inhibitor. Artwork Credit: Artist is tigatelu via www.vectorstock.com
a Distribution of incidence proportions (%) for all-grade adverse events associated with any ICI use across all cancers. Colored boxes represent median and interquartile ranges, while the lines extending beyond the boxes represent the minimum and maximum incidence proportion values. Dots represent individual data points and dots beyond the vertical lines are outliers. b Distribution of incidence proportions (%) for high-grade adverse events associated with any ICI use across all cancers. Colored boxes represent median and interquartile ranges, while the lines extending beyond the boxes represent the minimum and maximum incidence proportion values. Dots represent individual data points and dots beyond the vertical lines are outliers.
Rare AEs identified from systematic reviews of case reports included sarcoidosis like-granulomas, sclerosing cholangitis, lupus, Stevens–Johnson syndrome/toxic epidermal necrolysis, scleroderma, bullous disorders, polymyalgia rheumatica, glomerular disease, encephalitis, myasthenia gravis, neuro-ophthalmic AEs, cardiac AEs, and vasculitis (Online Resource 1, eTable 3 and Online Resource 2).
4 Discussion
Immunotherapy has changed the landscape of cancer therapy over the last decade with the introduction of ICIs. It has provided treatment options alone or in combination as first- or second-line treatments for more than 50 cancer types, and there are more than 3000 ongoing active clinical trials [86]. Clinical success is largely due to its different mechanism of action, cancer destruction by activating the host’s immune system rather than targeting cancer cells directly, such as traditional chemotherapy. Not only has this resulted in improved clinical outcomes compared with traditional chemotherapy alone but it has also come with a profound change in the type of AEs associated with cancer treatment. Since the efficacy of ICIs is related to its manipulation of the immune system, the pathophysiology of AEs is presumably also mediated by manipulation of the immune system. Immune-mediated or immune-related AEs appear as auto-immune diseases that can affect any organ system with a wide range of severity and are not always reversible. Delayed recognition and inappropriate management results in negative outcomes, including death [87]. This new spectrum of AEs requires rapid recognition and appropriate management, however due to the diverse range of severity and organ systems affected, a multidisciplinary team of organ system specialists and internists in addition to medical oncologists need to be aware and involved in both the diagnosis and management of AEs.
There is currently an overwhelming quantity of systematic reviews available in the literature that report incidence proportions of a wide range of AEs in various cancers, using ICIs alone or in combination [88]. The purpose of this overview of systematic reviews was to map all the available evidence related to ICI AEs across subpopulations of cancer patients to provide a comprehensive synthesis of relevant data for oncologists and non-oncology clinicians alike. Creating awareness of the incidence and types of AEs beyond the field of oncology will hopefully lead to earlier recognition of these AEs and subsequently earlier, appropriate treatment. We are only aware of one other overview of reviews related to ICI toxicity [89]. Raschi et al. conducted an overview of reviews to characterize immune-related AEs for the purpose of comparison with postmarketing surveillance using the FDA’s Adverse Event Reporting System. These authors identified 32 systematic reviews published before October 2018 from a search of a single database and focused on the comparative risk of AEs between ICI-based therapies and chemotherapy alone rather than incidence proportions as we have in this overview.
In this overview, we identify a set of AEs that are meaningful to clinicians and report their incidence proportions in a variety of clinically relevant settings. AEs occur commonly in all patient populations and in all contexts in which ICIs are prescribed, but compared with all presentations of AEs, high-grades are considerably less common. Combination therapy, whether it is combinations of ICI drugs or combinations of ICI drugs with traditional chemotherapy (or targeted therapy), accounts for more than half of the highest incidence proportion estimates identified for all AEs. It is interesting to note that most systematic reviews did not differentiate between immune-related and treatment-related AEs. This differentiation is difficult to make and perhaps not clinically important (i.e., diarrhea may not only be immune-mediated but may also be a precursor to colitis, which is considered immune-mediated) if the link is made between the AEs and the ICI. More obvious treatment-related AEs, such as infusion-related reactions, were not ranked high enough by our panel of experts to be included in this review.
Rare AEs were not well represented across cancer subtypes in the systematic reviews identified. For this reason, we systematically excluded AEs that were only reported in fewer than three systematic reviews. Although not a main objective of this study, we did collate 21 systematic reviews of case reports and case series of rare AEs. During the AE ranking process by which we chose the AEs of interest for this overview, there were several AEs that were excluded because they were reported in fewer than three eligible systematic reviews or they were not deemed to be of interest by our expert panel. Because our search strategy was not specifically designed for systematic reviews of case reports and case series, it is possible that more exist in the literature. It is worth mentioning that pharmacovigilance, particularly through spontaneous reporting databases, may be a practical way to characterize rare AEs [90]. It is also worth noting that certain cancers were also not well represented across systematic reviews (e.g., lymphoma breast cancer, gynecological cancers). This is most likely because indications for ICI therapy for these cancers are not as well established (and thus there are fewer primary trials) as other cancers (e.g., melanoma, renal cell carcinoma).
The quality of the included systematic reviews ranges mainly from critically low to moderate according to our AMSTAR-2 assessments. While this does speak to the overall quality of the systematic reviews we included, we feel that this does not reflect the quality of incidence proportion data that we extracted. The most relevant AMSTAR-2 questions for this overview pertain to the conduct of meta-analyses. While efficacy outcomes were typically meta-analysed appropriately, AE incidence proportion data were often reported simply in aggregate. Due to resource limitations, we did not extract AE rates from primary studies for the purpose of meta-analysis; rather, we elected to report them as described by systematic review authors, with identification of those that were meta-analysed and those that are reported in aggregate. We acknowledge this as a limitation to our methods as some estimates may be over- or underestimated without appropriate weighting. For transparency, we have identified how each incidence proportion estimate was derived. It should also be recognized that given the time span of our search, different versions of the CTCAE were used in some studies. As the definitions of some AEs may change slightly between versions, it is possible that older studies may have used outdated definitions of some AEs. To minimize the impact of this, we considered publication recency when assessing the relevance of systematic reviews when selecting systematic reviews for data extraction.
One of the strengths of our overview is our management of primary study overlap across systematic reviews. The nature of our research question inevitably identified systematic reviews with overlapping or duplicated primary studies. We employed a reproducible strategy for selecting the single best systematic review for each data point that we extracted based on quality and relevance. With this strategy, we maximized the use of published data without any overlap of primary studies. This would not have been possible without a comprehensive data mapping exercise. Another strength of this overview is the identification of outliers. In this context, outliers may represent high- or low-risk groups for each AE. For example, the median incidence proportion of high-grade pneumonitis across all cancers and treatments was 1.0% (IQR 0.7–1.3%), but one systematic review identified that when ICI therapy was combined with traditional chemotherapy for lung cancer, the incidence proportion of pneumonitis was much higher (6.8%, 95% CI 4.9–9.5). As expected, outliers (above the median) were more likely to involve combination ICI therapy or combinations of ICI and traditional chemotherapy. Melanoma was the most common cancer subtype that was associated with outliers, which may be related to the fact that ICI therapy has been used in melanoma for longer than other cancer subtypes. It must be acknowledged that in order to identify outliers, we calculated median incidence proportions across all cancer subtypes and therapeutic regimens (including monotherapy and combination therapies). These median estimates are based on incidence proportions from a heterogenous selection of populations receiving ICI therapy. Although this type of analysis allows us to identify outliers, readers must understand that the median incidence proportions may be skewed by combination therapies where incidence proportions are high, or cancer subtypes where incidence proportions are low. For this reason, we provide granular data from data mapping exercises to allow the reader to see the data from different perspectives, acknowledging that readers may come from different disciplines with specific queries.
Creating awareness of the types and incidence of AEs with ICI therapy in cancer is the first step but only partly addresses the clinical problem we have identified. Important topics such as AE rates for individual drugs, the effect of dose, and timing were beyond the scope of this overview and should be the topics of future systematic reviews and overviews. Diagnosis and treatment of the included AEs were also not addressed in this overview but will be an important part of the overall management of AEs related to ICI therapy. In accordance with recent guidelines [91, 92] creating awareness of ICI-related AEs will lead to earlier diagnostic and treatment interventions.
5 Conclusion
Early recognition of AEs associated with ICIs requires expertise from various specialists, not just oncologists. We hope that readers will develop a greater awareness of potential AEs and that this results in earlier recognition, appropriate management, and better patient outcomes. This overview synthesizes and maps the current evidence on AEs associated with ICI therapy across cancer types with the aim of increasing awareness among oncology and non-oncology clinicians. In this overview, we characterize the incidence of AEs across an extensive variety of clinical conditions defined by type of cancer, severity, and therapeutic combinations. Incidence proportions of AEs varied between cancer subtypes, but combination therapy, including combinations with traditional chemotherapy or targeted therapy, were responsible for most upper limit outliers. Considering the number of ongoing trials with ICIs in cancer, there will be a considerable increase in the volume of new data that will requiring ongoing monitoring to further enhance our understanding of the risks and benefits of these therapies.
References
Schirrmacher V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment. Int J Oncol. 2019;54(2):407–19.
Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30(6):507–19.
Hui E. Immune checkpoint inhibitors. J Cell Biol. 2019;218(3):740–1.
Cancer Care Ontario. Immune Checkpoint Inhibitor Toxicity Management Clinical Practice Guideline. Updated March 2018 [cited 10 Dec 2020]. https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/52976. Accessed 10 Dec 2020.
Horrow JC, Digregorio GJ, Barbieri EJ, Rupp E. Intravenous infusions of nitroprusside, dobutamine, and nitroglycerin are compatible. Crit Care Med. 1990;18(8):858–61.
Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–85.
Kirschenbaum HL, Aronoff W, Perentesis GP, Plitz GW, Cutie AJ. Stability and compatibility of lidocaine hydrochloride with selected large-volume parenterals and drug additives. Am J Hosp Pharm. 1982;39(6):1013–5.
Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–80.
Xing P, Zhang F, Wang G, Xu Y, Li C, Wang S, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer. 2019;7(1):341.
Reynolds KL, Cohen JV, Ryan DP, Hochberg EP, Dougan M, Thomas M, et al. Severe immune-related adverse effects (irAE) requring hospital admission in patients treated with immune checkpoint inhibitors for advanced malignancy: Temporal trends and clinical significance. J Clin Oncol. 2018;36(15):3096.
Pollock M FR, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews: Cochrane; 2020 [updated September 2020. http://www.training.cochrane.org/handbook. Accessed 10 Dec 2020.
Lunny C, Brennan SE, Reid J, McDonald S, McKenzie JE. Overviews of reviews incompletely report methods for handling overlapping, discordant, and problematic data. J Clin Epidemiol. 2020;118:69–85.
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6.
CADTH. Grey Matters: a practical tool for searching health-related grey literature. 2014. https://www.cadth.ca/resources/finding-evidence/grey-matters. Accessed 10 Dec 2020.
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 2-risk of bias assessment; synthesis, presentation and summary of the findings; and assessment of the certainty of the evidence. Syst Rev. 2018;7(1):159.
Lunny C, Pieper D, Thabet P, Kanji S. Managing overlap of primary study results across systematic reviews: practical considerations for authors of overviews of reviews. BMC Med Res Methodol. 2021;21(1):140.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18.
Bougioukas KI, Liakos A, Tsapas A, Ntzani E, Haidich AB. Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. J Clin Epidemiol. 2018;93:9–24.
Abdel-Rahman O. Toxicity patterns associated with chemotherapy/immune checkpoint inhibitor combinations: a meta-analysis. Immunotherapy. 2019;11(6):543–54.
Almutairi AR, McBride A, Slack M, Erstad BL, Abraham I. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: a systematic review and meta-analysis. Front Oncol. 2020;10:91.
Arnaud-Coffin P, Maillet D, Gan HK, Stelmes J-J, You B, Dalle S, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer. 2019;145(3):639–48.
Balasubramanian A, Onggo J, Gunjur A, John T, Parakh S. Immune checkpoint inhibition with chemoradiotherapy in stage III non-small-cell lung cancer: a systematic review and meta-analysis of safety results. Clin Lung Cancer. 2021;22(2):74–82.
Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173–82.
Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
Bishay K, Tandon P, Bourassa-Blanchette S, Laurie SA, McCurdy JD. The risk of diarrhea and colitis in patients with lung cancer treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Curr Oncol. 2020;27(5):e486–94.
Chen K, Wang X, Yang L, Chen Z. The anti-PD-1/PD-L1 immunotherapy for gastric esophageal cancer: a systematic review and meta-analysis and literature review. Cancer Control. 2021;28:1073274821997430.
Da L, Teng Y, Wang N, Zaguirre K, Liu Y, Qi Y, et al. Organ-specific immune-related adverse events associated with immune checkpoint inhibitor monotherapy versus combination therapy in cancer: a meta-analysis of randomized controlled trials. Front Pharmacol. 2019;10:1671.
de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51(3):145–56.
El Osta B, Hu F, Sadek R, Chintalapally R, Tang SC. Not all immune-checkpoint inhibitors are created equal: Meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol. 2017;119:1–12.
Facchinetti F, Di Maio M, Tiseo M. Adding PD-1/PD-L1 inhibitors to chemotherapy for the first-line treatment of extensive stage small cell lung cancer (SCLC): a meta-analysis of randomized trials. Cancers. 2020;12(9):2645.
Fu J, Li W-Z, McGrath NA, Lai CW, Brar G, Xiang Y-Q, et al. Immune checkpoint inhibitor associated hepatotoxicity in primary liver cancer versus other cancers: a systematic review and meta-analysis. Front Oncol. 2021;11:650292.
Grunwald V, Voss MH, Rini BI, Powles T, Albiges L, Giles RH, et al. Axitinib plus immune checkpoint inhibitor: evidence- and expert-based consensus recommendation for treatment optimisation and management of related adverse events. Br J Cancer. 2020;123(6):898–904.
Gu Y, Zhang H, Liu Z, Xia Y, Liang B, Liang L. Different patterns of treatment-related adverse events of programmed cell death-1 and its ligand-1 inhibitors in different cancer types: a meta-analysis and systemic review of clinical trials. Asia Pac J Clin Oncol. 2020;16(5):e160–78.
Guo X, Li W, Hu J, Zhu EC, Su Q. Hepatotoxicity in patients with solid tumors treated with PD-1/PD-L1 inhibitors alone, PD-1/PD-L1 inhibitors plus chemotherapy, or chemotherapy alone: systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76(10):1345–54.
Huang Y-F, Xie W-J, Fan H-Y, Du J. Comparative risks of high-grade adverse events among FDA-approved systemic therapies in advanced melanoma: systematic review and network meta-analysis. Front Oncol. 2020;10:571135.
Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. Medicine. 2018;97(33):e11936.
Li H, Xu J, Bai Y, Zhang S, Cheng M, Jin J. Nephrotoxicity in patients with solid tumors treated with anti-PD-1/PD-L1 monoclonal antibodies: a systematic review and meta-analysis. Invest New Drugs. 2021;39(3):860–70.
Li L, Xu F, Chen Y, Ren X, Liu Y, Chen Y, et al. Indirect comparison between immunotherapy alone and immunotherapy plus chemotherapy as first-line treatment for advanced non-small cell lung cancer: a systematic review. BMJ Open. 2020;10(11):e034010.
Li Z-Q, Yan H-C, Gu J-J, Yang Y-L, Zhang M-K, Fang X-J. Comparative efficacy and safety of PD-1/PD-L1 Inhibitors versus platinum-based chemotherapy for the first-line treatment of advanced non-small cell lung cancer: a meta analysis of randomized controlled trials. Pharmacol Res. 2020;160:105194.
Lin L-L, Lin G-F, Yang F, Chen X-Q. A systematic review and meta-analysis of immune-mediated liver dysfunction in non-small cell lung cancer. Int Immunopharmacol. 2020;83:106537.
Liu H, Xu D, Wang W, Sun F, Zhang S, Yang X, et al. Systematic assessment of risk of fever in solid tumor patients treated with PD-1/PD-L1 inhibitors: a systematic review and meta-analysis. Front Oncol. 2020;10:570080.
Liu Q, Fang Z, Liu M, Xu R, Yi F, Wei Y, et al. The benefits and risks of CTLA4 inhibitor plus PD1/PDL1 inhibitor in stage IIIB/IV non-small cell lung cancer: a systematic analysis and meta-analysis based on randomized controlled trials. J Clin Pharm Ther. 2021;46(6):1519–30.
Lu J, Li L, Lan Y, Liang Y, Meng H. Immune checkpoint inhibitor-associated pituitary-adrenal dysfunction: a systematic review and meta-analysis. Cancer Med. 2019;8(18):7503–15.
Lu J, Yang J, Liang Y, Meng H, Zhao J, Zhang X. Incidence of immune checkpoint inhibitor-associated diabetes: a meta-analysis of randomized controlled studies. Front Pharmacol. 2019;10:1453.
Luo W, Wang Z, Tian P, Li W. Safety and tolerability of PD-1/PD-L1 inhibitors in the treatment of non-small cell lung cancer: a meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol. 2018;144(10):1851–9.
Miyashita H, Mikami T, Satoi S, Cruz C, Galsky MD. Incidence and risk of colitis with programmed death 1 versus programmed death ligand 1 inhibitors for the treatment of cancer. J Immunother. 2020;43(9):291–8.
Narayan V, Kahlmeyer A, Dahm P, Skoetz N, Risk MC, Bongiorno C, et al. Pembrolizumab monotherapy versus chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum-containing chemotherapy. A Cochrane Rapid Review. Cochrane Database Syst Rev. 2018;7(7):CD012838.
Ni X, Xing Y, Sun X, Suo J. The safety and efficacy of anti-PD-1/anti-PD-L1 antibody therapy in the treatment of previously treated, advanced gastric or gastro-oesophageal junction cancer: a meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol. 2020;44(2):211–22.
Ornstein MC, Garcia JA. Toxicity of checkpoint inhibition in advanced RCC: a systematic review. Kidney cancer. 2017;1(2):133–41.
Park R, Lopes L, Cristancho CR, Riano IM, Saeed A. Treatment-related adverse events of combination immune checkpoint inhibitors: systematic review and meta-analysis. Front Oncol. 2020;10:258.
Petrelli F, Ardito R, Borgonovo K, Lonati V, Cabiddu M, Ghilardi M, et al. Haematological toxicities with immunotherapy in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2018;103:7–16.
Shi Y, Duan J, Guan Q, Xue P, Zheng Y. Effectivity and safety of PD-1/PD-L1 inhibitors for different level of PD-L1-positive, advanced NSCLC: a meta-analysis of 4939 patients from randomized controlled trials. International immunopharmacology. 2020;84:106452.
Si Z, Zhang S, Yang X, Ding N, Xiang M, Zhu Q, et al. The association between the incidence risk of peripheral neuropathy and PD-1/PD-L1 inhibitors in the treatment for solid tumor patients: a systematic review and meta-analysis. Front Oncol. 2019;9:866.
Sonpavde GP, Grivas P, Lin Y, Hennessy D, Hunt JD. Immune-related adverse events with PD-1 versus PD-L1 inhibitors: a meta-analysis of 8730 patients from clinical trials. Future Oncol. 2021;17(19):2545–58.
Su Q, Sun Z, Zhang C, Hou Y, Cao B. PD-1/PD-L1 antibodies efficacy and safety versus docetaxel monotherapy in advanced NSCLC patients after first-line treatment option: systems assessment. Oncotarget. 2017;8(35):59677–89.
Su Q, Zhu EC, Wu J-B, Li T, Hou Y-L, Wang D-Y, et al. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: a systematic review and meta-analysis. Front Immunol. 2019;10:108.
Tandon P, Bourassa-Blanchette S, Bishay K, Parlow S, Laurie SA, McCurdy JD. The risk of diarrhea and colitis in patients with advanced melanoma undergoing immune checkpoint inhibitor therapy: a systematic review and meta-analysis. J Immunother. 2018;41(3):101–8.
Tian Y, Zhang Z, Yang X, Li D, Zhang L, Li Z, et al. The risk ratio of immune-related colitis, hepatitis, and pancreatitis in patients with solid tumors caused by PD-1/PD-L1 inhibitors: a systematic review and meta-analysis. Front Oncol. 2020;10:261.
Tong Z-Q, Wu D-Y, Liu D, Dong M. Incidence risk of PD-1/PD-L1-related pneumonia and diarrhea in non-small cell lung cancer (NSCLC) patients: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2021;77(8):1079–88.
Tun AM, Thein KZ, Thein WL, Guevara E. Checkpoint inhibitors plus chemotherapy for first-line treatment of advanced non-small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Future Sci OA. 2019;5(9):FSO421.
Voutsadakis IA. PD-1 inhibitors monotherapy in hepatocellular carcinoma: meta-analysis and systematic review. Hepatobil Pancreat Dis Int. 2019;18(6):505–10.
Voutsadakis IA. A systematic review and meta-analysis of PD-1 and PD-L1 inhibitors monotherapy in metastatic gastric and gastroesophageal junction adenocarcinoma. Euroasian J Hepato-gastroenterol. 2020;10(2):56–63.
Wang B-C, Cao R-B, Li P-D, Fu C. The effects and safety of PD-1/PD-L1 inhibitors on head and neck cancer: a systematic review and meta-analysis. Cancer Med. 2019;8(13):5969–78.
Wang P-F, Chen Y, Song S-Y, Wang T-J, Ji W-J, Li S-W, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730.
Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008–19.
Xiao BY, Lin GH, Zhao YX, Wang BC. The efficacy and safety of PD-1/PD-L1 inhibitors in breast cancer: a systematic review and meta-analysis. Transl Cancer Res. 2020;9(6):3804–18.
Xu D, Liu H, Xiang M, Feng A, Tian M, Li D, et al. The relationship between pneumonitis and programmed cell death-1/programmed cell death ligand 1 inhibitors among cancer patients: a systematic review and meta-analysis. Medicine. 2020;99(41):e22567.
Xu H, Tan P, Ai J, Zhang S, Zheng X, Liao X, et al. Antitumor activity and treatment-related toxicity associated with nivolumab plus ipilimumab in advanced malignancies: a systematic review and meta-analysis. Front Pharmacol. 2019;10:1300.
Xu H, Tan P, Zheng X, Huang Y, Lin T, Wei Q, et al. Immune-related adverse events following administration of anti-cytotoxic T-lymphocyte-associated protein-4 drugs: a comprehensive systematic review and meta-analysis. Drug Des Dev Ther. 2019;13:2215–34.
Xu M, Nie Y, Yang Y, Lu Y-T, Su Q. Risk of neurological toxicities following the use of different immune checkpoint inhibitor regimens in solid tumors: a systematic review and meta-analysis. Neurologist. 2019;24(3):75–83.
Yang L, Dong X-Z, Xing X-X, Cui X-H, Li L, Zhang L. Efficacy and safety of anti-PD-1/anti-PD-L1 antibody therapy in treatment of advanced gastric cancer or gastroesophageal junction cancer: a meta-analysis. World J Gastrointest Oncol. 2020;12(11):1346–63.
Yang Y, Pang P, Xie Z, Wang N, Liang H, Zhao L. The safety of first and subsequent lines of PD-1/PD-L1 inhibitors monotherapy in non-small cell lung cancer patients: a meta-analysis. Transl Cancer Res. 2020;9(5):3231–41.
Zhang B, Wu Q, Zhou YL, Guo X, Ge J, Fu J. Immune-related adverse events from combination immunotherapy in cancer patients: a comprehensive meta-analysis of randomized controlled trials. Int Immunopharmacol. 2018;63:292–8.
Zhang B, Zhou YL, Chen X, Wang Z, Wang Q, Ju F, et al. Efficacy and safety of CTLA-4 inhibitors combined with PD-1 inhibitors or chemotherapy in patients with advanced melanoma. Int Immunopharmacol. 2019;68:131–6.
Zhang C, Zhang S, Xu D, Liu R, Zhu Q, Zhao Y, et al. Incidence risk of PD-1/PD-L1 related diarrhea in non-small cell lung cancer (NSCLC) patients: a systematic review and meta-analysis. Cancer Manag Res. 2019;11:3957–69.
Zhang H, Shen J, Yi L, Zhang W, Luo P, Zhang J. Efficacy and safety of ipilimumab plus chemotherapy for advanced lung cancer: a systematic review and meta-analysis. J Cancer. 2018;9(23):4556–67.
Zhang Q, Huo G-W, Zhang H-Z, Song Y. Efficacy of pembrolizumab for advanced/metastatic melanoma: a meta-analysis. Open Med. 2020;15(1):447–56.
Zhang S, Zhou Z, Wang L, Li M, Zhang F, Zeng X. Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis. Therap Adv Chron Dis. 2021;12:2040622320976996.
Zhang X, Chen L, Zhao Y, Yin H, Ma H, He M. Safety and efficacy in relapsed or refractory classic hodgkin’s lymphoma treated with PD-1 inhibitors: a meta-analysis of 9 prospective clinical trials. Biomed Res Int. 2019;2019:9283860.
Zhao L, Yu J, Wang J, Li H, Che J, Cao B. Risk of immune-related diarrhea with PD-1/PD-L1 inhibitors in different cancer types and treatment regimens. J Cancer. 2020;11(1):41–50.
Zhou H, Fu X, Li Q, Niu T. Safety and efficacy of anti-PD-1 monoclonal antibodies in patients with relapsed or refractory lymphoma: a meta-analysis of prospective clinic trails. Front Pharmacol. 2019;10:387.
Ziogas IA, Evangeliou AP, Giannis D, Hayat MH, Mylonas KS, Tohme S, et al. The role of immunotherapy in hepatocellular carcinoma: a systematic review and pooled analysis of 2,402 patients. Oncologist. 2021;26(6):E1036–49.
Xin YuJ, Hubbard-Lucey VM, Tang J. Immuno-oncology drug development goes global. Nat Rev Drug Discov. 2019;18(12):899–900.
Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–8.
Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801.
Raschi E, Mazzarella A, Ippazio CA, Bendinelli N, Forcesi E, Tuccori M, et al. Toxicities with immune checkpoint inhibitors: emerging priorities from disproportionality analysis of the FDA adverse event reporting system. Targ Oncol. 2019;14:205–21.
Raschi E, Gatti M, Gelsomino F, Ardizzoni A, Poluzzi E, De Ponti F. Lessons to be learnt from real-world studies on immune-related adverse events with checkpoint inhibitors: a clinical perspective from pharmacovigilance. Target Oncol. 2020;15:449–66. https://doi.org/10.1007/s11523-020-00738-6.
Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9(6): e002435. https://doi.org/10.1136/jitc-2021-002435.
Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073–126. https://doi.org/10.1200/JCO.21.01440.
Acknowledgements
The authors would like to acknowledge Risa Shorr, MLS, for her assistance and expertise in developing their search strategy and conducting the literature search.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: SK, PT, BH, CL, DB, XW. Abstract screening: SM, DP, KA, PT and SK. Data collection: SM, DP and KA. Overlap management, data synthesis and interpretation: SK, PT, SM, DP, KA. Drafting the article: SM and SK. Critical revision of the article: All authors. Final approval of the version to be published: All authors.
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript
Conflicts of interest/competing interests
Brian Hutton has received honoraria from Eversana for the provision of scientific advice on methods for evidence synthesis. Dominick Bosse has received honoraria for consultations or presentations from Pfizer, BMS, AstraZeneca, AMGEN, IPSEN, Bayer, AbbVie, Eisai and Merck. Salmaan Kanji, Sydney Morin, Kyla Agtarap, Debanjali Purkayastha, Pierre Thabet, Xiang Wang and Carole Lunny declare they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanji, S., Morin, S., Agtarap, K. et al. Adverse Events Associated with Immune Checkpoint Inhibitors: Overview of Systematic Reviews. Drugs 82, 793–809 (2022). https://doi.org/10.1007/s40265-022-01707-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01707-1