Abstract
Background
Significant improvement in survival outcome with the programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) inhibitors has been shown in advanced non-small cell lung cancer (NSCLC) patients compared with chemotherapy. However, the full spectrum of toxic events of PD-1/PD-L1 inhibitors was not well characterized. We conducted a comprehensive meta-analysis to state the safety profile of PD-1/PD-L1 inhibitors in NSCLC, and identify the exact incidence and relative risk (RR) of both summary and detailed AEs.
Materials and methods
Electronic databases (PubMed, EMBASE and the Cochrane library databases) and major conference proceedings were systematically searched for all clinical trials in lung cancer using PD-1/PD-L1 inhibitors. Eligible studies included randomized controlled trials (RCTs) comparing PD-1/PD-L1 inhibitors with chemotherapy in NSCLC patients reporting all-grade (1–4) or high-grade (3–4) AEs [toxic symptoms, hematologic toxicities, and immune-related AEs (irAEs)], treatment discontinuation due to toxicities, or toxic deaths. The pooled incidence, RR, and corresponding 95% confidence interval (CI) of toxicity outcomes were calculated.
Results
A total of 4413 patients from 8 RCTs (3 with nivolumab; 2 with atezolizumab, and 3 with pembrolizuma) were included. In terms of summary toxic events, PD-1/PD-L1 inhibitors had a significantly lower risk of any all-grade AEs (66.20 vs. 86.08%; RR 0.77) and high-grade AEs (14.26 vs. 43.53%; RR 0.32), treatment discontinuation (5.94 vs. 13.92%; RR 0.44), and toxic deaths (0.48 vs. 1.12%; RR 0.45) than chemotherapy. With regard to detailed toxic events, the risk of toxic symptoms (including all-grade fatigue, nausea, constipation, diarrhea and peripheral sensory neuropathy; high-grade fatigue, anorexia, diarrhea and peripheral sensory neuropathy) and hematologic toxicities (including all-grade and high-grade neutropenia, thrombocytopenia, and anemia) from PD-1/PD-L1 inhibitors was significantly lower than from chemotherapy. However, there was a small but significantly increased risk of irAEs, including all-grade rash, pruritus, colitis, hypothyroidism, hyperthyroidism, ALT/AST elevations and pneumonitis, as well as high-grade pneumonitis.
Conclusion
PD-1/PD-L1 inhibitors are generally safer and better tolerated than chemotherapy for patients with NSCLC with regard to summary toxic events, detailed toxic symptoms and hematologic toxicities. However, PD-1/PD-L1 inhibitors can generate a unique spectrum of irAEs, and several of them can be severe and even life-threatening. Clinicians should be aware of the risk of these AEs, as they may have a potentially negative impact on the patients’ quality of life and survival outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) inhibitors have revolutionized the clinical management of multiple malignancies, including non-small cell lung cancer (NSCLC) over the past decade (Chae et al. 2018). PD-1 is an immune checkpoint protein expressed on the surface of T-lymphocytes. PD-L1, the ligand for PD-1, is expressed in tumor cells or tumor infiltrating immune cells at different levels. The binding of PD-L1 to PD-1 can leads to negative regulation of T-cell signaling (Buchbinder and Desai 2016). PD-1/PD-L1 inhibitors are antibodies that block immune checkpoint molecular (i.e., PD-1 and PD-L1), and serve to restore T-cell immune responses against tumor (Fay et al. 2016; Iwai et al. 2017).
To date, three PD-1/PD-L1 inhibitors have been approved by the US Food and Drug Administration (FDA) for treatment of advanced NSCLC. Pembrolizumab is the first FDA-approved PD-1 inhibitor for treatment of advanced NSCLC based on two randomized, open-label clinical trials (Pai-Scherf et al. 2017). In the phase III KEYNOTE-024 study, untreated patients with metastatic NSCLC receiving pembrolizumab had a significant improvement in progression-free survival [PFS, hazard ratio (HR) 0.50] and overall survival (OS, HR 0.60) compared with chemotherapy (Reck et al. 2016). In the KEYNOTE-010 study, patients with disease progression or following chemotherapy received pembrolizumab and also had a significantly better OS compared with chemotherapy (Herbst et al. 2016). Atezolizumab is the first FDA-approved PD-L1 inhibitor for treatment of advanced NSCLC (De Velasco et al. 2017). The approval was based on two randomized, open-label clinical trials (OAK and POPLAR), which have demonstrated that patients receiving atezolizumab had a significant improvement in OS compared with chemotherapy (Fehrenbacher et al. 2016; Rittmeyer et al. 2017).
Along with the impressive therapeutic efficacy, we have seen a wide range of adverse events (AEs) in NSCLC clinical trials. Different from chemotherapy or molecular targeted therapy, PD-1/PD-L1 inhibitors therapy can induce some special side effects termed immune-related adverse events (irAEs), which clinically manifest with autoimmune-like/inflammatory toxicities involving multiple organs and tissues (Cousin and Italiano 2016; O’Kane et al. 2017). Since the AEs may be severe and even fatal, it is important for clinicians to be familiar with these AEs to recognize them timely and manage them appropriately for patients. Therefore, we conducted a comprehensive meta-analysis to state the safety profile of PD-1/PD-L1 inhibitors in NSCLC, and identify the exact incidence and relative risk (RR) of both summary and detailed AEs.
Materials and methods
Literature search
The present meta-analysis was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (Panic et al. 2013). Potentially relevant studies were identified by searching PubMed, Embase, and the Cochrane library databases. The search dates were from the inception of each database to May 1, 2018. The search terms included: “lung neoplasm”, “non-small cell lung cancer”, “nivolumab”, “atezolizumab”, “pembrolizumab”, “PD-1”, “PD-L1”, “programmed death receptor 1”, “programmed death receptor ligand”, and “immune checkpoint inhibitor”. To identify additional studies, we also searched the reference lists of relevant studies.
Study selection
The following predefined inclusion criteria were used: (a) clinical trials in patients with lung cancer; (b) random assignment of patients to single-agent PD-1/PD-L1 inhibitor treatment or chemotherapy; and (c) reporting number or rate and sample size for any all-grade (1–4) or high-grade (3–4) AEs, detailed all-grade or high-grade AEs, treatment discontinuation due to AEs, and toxic deaths. The exclusion criteria were listed as follows: (a) no chemotherapy control arm; (b) PD-1/PD-L1 inhibitors in both arms; and (c) studies not published in English.
Data extraction
The following data were extracted by two authors independently: name of first author, year of publication, trial phase, masking, histology of lung cancer, treatment arms, number of patients available for analysis, age, follow-up duration, any all-grade or high-grade AEs, detailed all-grade or high-grade AEs, treatment discontinuation due to AEs, and toxic deaths. The AEs included clinical relevant symptoms (fatigue, anorexia, nausea, constipation diarrhea, and peripheral sensory neuropathy), hematologic AEs (neutropenia and anemia), and immune-related AEs (irAEs; rash, pruritus, colitis, hypothyroidism, hyperthyroidism, hypophysitis, alanine aminotransferase (ALT)/aspartate aminotransferase (AST) elevations, and pneumonitis) (Naidoo et al. 2015; Reeve et al. 2014). All the included studies evaluated and graded AEs according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) 4.0 (National Cancer Institute 2009). When multiple doses of PD-1/PD-L1 inhibitors were used for different cohort of patients in the same trial, they were extracted as separate studies. The third author assessed the data and resolved the disagreement.
Assessment of study quality and publication bias
The Cochrane Collaboration’s risk of bias tool was used to assess the methodological quality of each included study (Higgins 2011). This tool is based on the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. Each item was described as low risk of bias, high risk of bias, or unclear risk of bias by two independent authors. The publication bias was assessed using Funnel plot and Egger’s test (Begg and Mazumdar 1994; Egger et al. 1997).
Statistical analysis
All statistical analyses were done with R version 3.4.4. P value less than 0.05 was considered statistically significant. Pooled incidences and risk ratios (RRs) were used to evaluate the risk of AEs and 95% confidence intervals (CIs) were calculated for each estimate. Statistical heterogeneity among studies was examined using Cochran Q and I2 statistics. Heterogeneity was considered low, moderate or high for I2 values < 25, 25–50, and > 50%, respectively. In this analysis, the null hypothesis that the studies were homogenous would be rejected if P for heterogeneity is less than 0.10 or I2 > 50%. When there is significant heterogeneity among the results of included study, the random effects model was used to calculate summary estimate, reported using the DerSimonian and Laird method, assuming both within-study and between-study variations (DerSimonian and Laird 1986). Otherwise, the summary estimate was calculated based on the fixed effects model, reported using the inverse variance method, assuming that the studies included in the meta-analysis have the same effect size.
Results
Search results and characteristics of the included studies
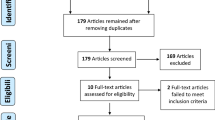
Our search retrieved 1380 potentially relevant publications from the PubMed, Embase, the Cochrane library and ASCO databases. The selection process and reasons for exclusion are presented in Fig. 1. A total of 4413 patients (2272 with PD-1/PD-L1 inhibitors; 2141 with chemotherapy) from 8 RCTs (3 with nivolumab; 2 with atezolizumab and 3 with pembrolizuma) were included. The characteristics of the included studies are shown in Table 1.
Quality of the included studies and publication bias
Most of the included studies had a high risk of selection bias, performance bias, and detection bias due to their open-label design (Supplementary Fig. 1). On the other hand, most studies had a low risk of attrition bias, reporting bias and other bias, because all the assessments of AEs were based on the CTCAE 4.0.
Funnel plots showed some evidence of publication bias for the RRs of any high-grade AEs and treatment discontinuation (Supplementary Fig. 2). When using Egger’s test to evaluate the asymmetry of funnel plots, the results suggested that there was no evidence for publication bias for the RRs of any high-grade AEs (t = − 1.585, P = 0.1641), as well as treatment discontinuation (t = − 1.001, P = 0.3554). This difference might be attributed to the small number of included studies, which would affect the statistical power of Egger’s test.
Incidence and relative risk of summary toxic events
The incidences of any all-grade AEs (66.20 vs. 86.08%), high-grade AEs (14.26 vs. 43.53%), discontinuation of treatment (5.94 vs. 13.92%), and toxic deaths (0.48 vs. 1.12%) from PD-1/PD-L1 inhibitors were lower than those from chemotherapy; PD-1/PD-L1 inhibitors also had a significantly lower risk of any all-grade AEs (RR 0.77; 95% CI: 0.74–0.80; P < 0.0001), high-grade AEs (RR 0.32; 95% CI: 0.25–0.41; P < 0.0001), treatment discontinuation (RR 0.44; 95% CI: 0.33–0.59; P < 0.0001), and toxic deaths (RR 0.45; 95% CI: 0.23–0.90; P = 0.0229) from PD-1/PD-L1 inhibitors (Fig. 2; Table 2).
Toxicity-related death was rare from PD-1/PD-L1 inhibitors and occurred in 0.48% (11/2272) of cases. The most common cause for treatment-related deaths was pneumonitis (36.4%, 4/11), other causes included: two from pneumonia, one from encephalitis, one from cardiac failure, one from myocardial infarction, one from multiorgan failure, and another from unknown cause.
Incidence and relative risk of toxic symptoms
Patients receiving PD-1/PD-L1 inhibitors had a significantly lower risk for five evaluated all-grade toxic symptoms when compared with chemotherapy (Table 3): fatigue (18.75 vs. 30.83%; RR 0.61; 95% CI: 0.55–0.68; P < 0.0001), nausea (12.54 vs. 25.69%; RR 0.45; 95% CI: 0.31–0.65; P < 0.0001), constipation (6.34 vs. 8.08%; RR 0.49; 95% CI: 0.26–0.94; P = 0.031), diarrhea (10.61 vs. 19.85%; RR 0.51; 95% CI: 0.37–0.72; P < 0.0001), and peripheral sensory neuropathy (1.32 vs. 6.31%; RR 0.13; 95% CI: 0.05–0.34; P < 0.0001). The risk of four high-grade toxic symptoms was significantly lower from PD-1/PD-L1 inhibitors therapy than chemotherapy: fatigue (1.58 vs. 4.06%; RR 0.39; 95% CI: 0.27–0.57; P < 0.0001), anorexia (0.35 vs. 1.26%; RR 0.30; 95% CI: 0.14–0.64; P = 0.0018), diarrhea (0.75 vs. 1.77%; RR 0.44; 95% CI: 0.25–0.76; P = 0.0034), and peripheral sensory neuropathy (0.00 vs. 0.61%; RR 0.10; 95% CI: 0.02–0.53; P = 0.0068).
Incidence and relative risk of hematologic toxicities
Patients receiving PD-1/PD-L1 inhibitors were at a significantly lower risk of all-grade neutropenia (0.70 vs. 18.68%; RR 0.03; 95% CI: 0.01–0.08; P < 0.0001), thrombocytopenia (0.09 vs. 2.57%; RR 0.04; 95% CI: 0.01–0.16; P < 0.0001), and anemia (5.59 vs. 23.26%; RR 0.19; 95% CI: 0.10–0.34; P < 0.0001) when compared with chemotherapy. A significantly lower risk of high-grade neutropenia (0.13 vs. 14.53%; RR 0.02; 95% CI: 0.01–0.04; P < 0.0001), thrombocytopenia (0.04 vs. 1.40%; RR 0.05; 95% CI: 0.01–0.25; P = 0.0003), and anemia (1.01 vs. 6.03%; RR 0.17; 95% CI: 0.07–0.42; P = 0.0001) was also observed in PD-1/PD-L1 inhibitors (Table 3).
Incidence and relative risk of immune-related AEs
The most frequently reported all-grade irAEs from PD-1/PD-L1 inhibitors therapy included rash (5.77%), hypothyroidism (4.89%), and pneumonitis (3.21%), while the most frequently observed high-grade irAE was pneumonitis (1.45%), ALT/AST elevations (0.57%) and colitis (0.40%).
Compared to chemotherapy, PD-1/PD-L1 inhibitors therapy was associated to a significantly increased risk of seven all-grade irAEs: rash (5.77 vs. 2.76%; RR 2.07; 95% CI: 1.54–2.80; P < 0.0001), pruritus (2.16 vs. 0.51%; RR 4.15; 95% CI: 2.20–7.81; P < 0.0001), colitis (0.70 vs. 0.00%; RR 5.44; 95% CI: 1.42–20.80; P = 0.013), hypothyroidism (4.89 vs. 0.23%; RR 17.59; 95% CI: 7.74–39.98; P < 0.0001), hyperthyroidism (2.11 vs. 0.37%; RR 5.27; 95% CI: 2.56–10.86; P < 0.0001), ALT/AST elevations (1.85 vs. 0.89%; RR 2.15; 95% CI: 1.31–3.51; P = 0.002), and pneumonitis (3.21 vs. 0.65%; RR 3.83; 95% CI: 2.20–6.68; P < 0.0001). There was also a small, but significantly increased risk of high-grade pneumonitis from PD-1/PD-L1 inhibitors compared with chemotherapy (1.45 vs. 0.19%; RR 3.78; 95% CI: 1.43–10.03; P = 0.007) (Table 3).
Subgroup analysis
There was no significant heterogeneity among studies for RRs of any all-grade AEs (P 0.5215; I2 0.0%) and toxic deaths (P = 0.9858; I2 0.0%). However, significant heterogeneity existed among studies for RRs of any high-grade AEs (P = 0.0001; I2 76.2%) and treatment discontinuation (P = 0.067; I2 47.0%).
We conducted subgroup analysis with regard to summary AEs stratified by types of PD-1/PD-L1 inhibitors (nivolumab vs. atezolizumab vs. pembrolizumab) (Supplementary Fig. 3; supplementary Table 1). The results showed that only the difference in RR of any high-grade AEs between patients treated with nivolumab and pembrolizumab reached statistically significance (0.21 vs. 0.43; P = 0.0217). In addition, we performed subgroup analyses with regard to detailed AEs based on different PD-1/PD-L1 inhibitors and found the RRs of several detailed AEs among these three subgroups were statistically different (Supplementary Table 2).
Discussion
In the present meta-analysis of all published RCTs comparing PD-1/PD-L1 inhibitors therapy and chemotherapy in patients with NSCLC, we found PD-1/PD-L1 inhibitors therapy had a significantly lower risk of summary toxic events. More specifically, a significantly lower risk of toxic symptoms and hematologic toxicities was observed in patients treated with PD-1/PD-L1 inhibitors therapy. There was a small, but significantly increased rise in the risk of several irAEs from PD-1/PD-L1 inhibitors therapy. To our knowledge, this is the most comprehensive meta-analysis to provide an evaluation of the full spectrum of toxic events from the PD-1/PD-L1 inhibitors in patients with NSCLC.
For decades, cytotoxic chemotherapy has been used as the first-line therapy for advanced NSCLC; however, toxic events are frequently reported in patients receiving chemotherapy (Li et al. 2017). Our analysis of summary toxic events revealed that compared to chemotherapy, PD-1/PD-L1 inhibitors was associated with a lower risk of any all-grade AEs, any high-grade AEs, treatment discontinuation due to AEs, and toxic deaths. Notably, the absolute difference in risk of any high-grade AEs (14.26 vs. 43.53%; RR 0.32) was more substantial than for any all-grade AEs (66.20 vs. 86.08%; RR 0.77). Furthermore, through subgroup analysis stratified by types of PD-1/PD-L1 inhibitors (nivolumab vs. atezolizumab vs. pembrolizumab), we found only the difference in the risk of any high-grade AEs between patients treated with nivolumab and pembrolizumab reached statistically significance, indicating that the risk of summary toxic events appeared to be consistent among these three PD-1/PD-L1 inhibitors, except for any high-grade AEs between nivolumab and pembrolizumab. Overall, PD-1/PD-L1 inhibitors therapy is safer than chemotherapy for NSCLC patients.
With regard to detailed toxic symptoms and hematologic toxicities, PD-1/PD-L1 inhibitors also appeared to be better tolerated than traditional chemotherapy according to our analysis. However, PD-1/PD-L1 inhibitors can lead to a unique spectrum of irAEs, included dermatologic (rash, pruritus), gastrointestinal (colitis), hepatic (ALT/AST elevations), endocrine (hypothyroidism, hyperthyroidism, and hypophysitis), and pulmonary events (pneumonitis) (Michot et al. 2016). In our analysis, the use of PD-1/PD-L1 inhibitors was associated with a significantly increased risk of several irAEs, including all-grade rash, pruritus, colitis, hypothyroidism, hyperthyroidism, ALT/AST elevations, and high-grade pneumonitis, compared with chemotherapy.
The irAEs are considered as a possible cause for treatment discontinuation and treatment-related deaths in PD-1/PD-L1 inhibitor treatment. Immune-mediated pneumonitis, which is defined as a focal or diffuse inflammation of the lung parenchyma, can be serious and even life-threatening (O’Kane et al. 2017). Our analysis showed that high-grade immune-related pneumonitis occurred in 1.45% (33/2272) of patients treated with PD-1/PD-L1 inhibitors, and accounted for the most frequently reported cause for treatment-related deaths (36.4%, 4/11). It is reported that immune-related pneumonitis occurs more commonly in NSCLC than with other malignancies (O’Kane et al. 2017). Immune-related hepatic toxicity, which is most frequently observed as asymptomatic ALT/AST elevations in serum, is another uncommon but potentially severe irAE of PD-1/PD-L1 inhibitors. The patients can then develop severe hepatitis if they are not recognized and managed properly (O’Kane et al. 2017). In our analysis, ALT/AST elevations are the second most frequently reported high-grade irAE (0.57%). Clinicians should be aware of the risk of these unique AEs, recognize them timely, and manage them appropriately (Thompson 2018).
Our study had several limitations. For calculating the RR of toxic events, we only included RCTs and excluded several single-arm trials. As a result, the number of studies for the present meta-analysis was limited. In addition, there was some heterogeneity among studies for RRs of high-grade AEs and treatment discontinuation. We minimized the heterogeneity using the random effects model to calculate RRs and conducted exploratory subgroup analysis stratified by the types of PD-1/PD-L1 inhibitors.
Our meta-analysis has demonstrated that PD-1/PD-L1 inhibitors are generally safer and better tolerated than chemotherapy for patients with NSCLC with regard to summary toxic events, detailed toxic symptoms and hematologic toxicities. However, PD-1/PD-L1 inhibitors can generate a unique spectrum of irAEs, and several of them can be severe and even life-threatening. Clinicians should be aware of the risk of these AEs, as they may have a potentially negative impact on the patients’ quality of life and survival outcome.
Abbreviations
- AEs:
-
Adverse events
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ASCO:
-
American Society of Clinical Oncology
- CTCAE:
-
The Common Terminology Criteria for Adverse Events version
- CI:
-
Confidence interval
- FDA:
-
Food and Drug Administration
- irAEs:
-
Immune-related AEs
- PD-1:
-
Programmed death 1
- PD-L1:
-
Programmed death ligand 1
- RR:
-
Relative risk
- RCTs:
-
Randomized controlled trials
References
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Buchbinder EI, Desai A (2016) CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 39(1):98–106
Chae YK, Arya A, Iams W et al (2018) Immune checkpoint pathways in non-small cell lung cancer. Ann Transl Med 6(5):88
Cousin S, Italiano A (2016) Molecular pathways: immune checkpoint antibodies and their toxicities. Clin Cancer Res 22(18):4550–4555
De Velasco G, Je Y, Bosse D et al (2017) Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res 5(4):312–318
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Fay AP, Moreira RB, Nunes Filho PRS et al (2016) The management of immune-related adverse events associated with immune checkpoint blockade. Expert Rev Qual Life Cancer Care 1(1):89–97
Fehrenbacher L, Spira A, Ballinger M et al (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387(10030):1837–1846
Herbst RS, Baas P, Kim DW et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
Higgins JP (2011) The Cochrane Collaboration. 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. http://www.cochrane-handbook.org. Accessed 21 Jun 2018
Iwai Y, Hamanishi J, Chamoto K et al (2017) Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci 24(1):26
Li A, Wei ZJ, Ding H et al (2017) Docetaxel versus docetaxel plus cisplatin for non-small-cell lung cancer: a meta-analysis of randomized clinical trials. Oncotarget 8(34):57365–57378
Michot JM, Bigenwald C, Champiat S et al (2016) Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54:139–148
Naidoo J, Page DB, Li BT et al (2015) Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26(12):2375–2391
National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) 4.0 Available from https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Accessed 21 Jun 2018
O’Kane GM, Labbe C, Doherty MK et al (2017) Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist 22(1):70–80
Pai-Scherf L, Blumenthal GM, Li H et al (2017) FDA approval summary: pembrolizumab for treatment of metastatic non-small cell lung cancer: first-line therapy and beyond. Oncologist 22(11):1392–1399
Panic N, Leoncini E, de Belvis G et al (2013) Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PloS One 8(12):e83138
Reck M, Rodriguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833
Reeve BB, Mitchell SA, Dueck AC et al (2014) Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst 106(7)
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389(10066):255–265
Thompson JA (2018) New NCCN guidelines: recognition and management of immunotherapy-related toxicity. J Natl Compr Cancer Netw: JNCCN 16(5 s):594–596
Funding
This study was supported by the Science and Technology Support Program of Sichuan Province (No.2016CZYD0001 to Wemin Li and No.2016SZ0073 to PanwenTian).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding the present study.
Ethical approval
The article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luo, W., Wang, Z., Tian, P. et al. Safety and tolerability of PD-1/PD-L1 inhibitors in the treatment of non-small cell lung cancer: a meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol 144, 1851–1859 (2018). https://doi.org/10.1007/s00432-018-2707-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2707-4